Study on Viscosity Reducing and Oil Displacement Agent for Water-Flooding Heavy Oil Reservoir
Qin Bing; Zhao Lin; Jiang Jianlin
(Research Institute of Petroleum Processing, SINOPEC, Beijing 100083)
Abstract: In the process of water-flooding development of heavy oil reservoir, due to the high viscosity and oil-water mobility ratio of heavy oil, there are some problems such as poor fluidity, high residual oil saturation and low recovery efficiency, which seriously restrict the efficient development of heavy oil. The molecular structure characteristics of asphaltenes and resins in heavy oil were analyzed. Based on the three most concerned properties of chemical agents,including the emulsification performance, the interface performance and the oil washing performance, three chemical oil displacement agents for heavy oil reservoirs were developed, and the structure of the chemical agents were characterized by high resolution mass spectrometry. The performance evaluation of chemical agents and core displacement experiments show that there is no obvious correlation between the properties of chemical agents, including interfacial tension, emulsifying ability and oil washing ability. For heavy oil reservoirs, the emulsification and viscosity reduction performance of chemical agents was more important than the oil washing capacity, and the oil washing capacity was more important than the interface performance. Viscosity reduction performance was the key parameter of oil displacement agent suitable for heavy oil reservoir. The composite binary system consisting of the viscosity reducer and the polymer had better oil recovery than using viscosity reducer alone.
Key words: heavy oil, viscosity reducer, emulsion, surfactant, enhanced oil recovery
1 Introduction
With the continuous development of conventional reservoirs, how to improve the recovery of heavy oil reservoirs has been attracting more and more attention[1-3].The main contradictions of water-flooding development of ordinary heavy oil reservoir are that heavy oil has high viscosity, poor fluidity, high water oil mobility ratio and low sweep coefficient of water-flooding. Chemical flooding is an important technical method to improve oil recovery[4-6]. The method of reducing viscosity by emulsification is one of the common methods in chemical recovery of heavy oil reservoir[7-8]. The emulsion viscosity reduction method aims at changing the W/O emulsion of heavy oil into O/W emulsion under the action of surfactant, so as to achieve the purpose of viscosity reduction. Surfactant can also reduce the interfacial tension and improve the fluidity of heavy oil[9-10]. In addition, surfactant can strip the oil film and improve heavy oil recovery by oil washing[11-12].
With the deepening of the research on viscosity reduction mechanism of heavy oil, the relationship between interfacial tension, emulsifying ability, oil washing ability and oil recovery is an urgent problem to be discussed[13].If we can clarify which role is more important for enhancing the heavy oil recovery, we can better guide the development of chemical agents.
Aiming at the problems of high viscosity and poor fluidity in heavy oil reservoir with high temperature and high salt content, the chemical components of heavy oil were analyzed, and the chemical viscosity reduction and oil displacement technology were studied. Three types of chemical agents were developed, while focusing on emulsification, reducing interfacial tension and oil washing. The relationships between interfacial tension,emulsification and viscosity reduction ability, oil washing ability and oil displacement efficiency of chemical agents used in heavy oil chemical flooding were studied.
2 Experimental
2.1 Analysis of heavy oil composition
Aiming at the crude oil of an oil field, the asphaltenes and resins in the crude oil were separated, and the mass fraction of asphaltenes and resins was measured. Through elemental analysis, high-resolution mass spectrometry,X-ray photoelectron spectroscopy and nuclear magnetic resonance analyses of asphaltenes and resins, the average molecular structure diagram of asphaltenes and resins was put forward.
2.2 Development of oil displacement agents
2.2.1 Materials
Among the raw materials used in the experiment, except alkyl naphthol, which was self-made in the laboratory,other chemicals including ethylene oxide, sodium hydroxide, N,N-dimethyl ethylenediamine, DL malic acid dimethyl ester, 1-bromododecane, sodium bisulfate,epichlorohydrin, dodecyl dimethyl tertiary amine, etc.,were purchased from the Beijing InnoChem Science& Technology Co., Ltd., and were analytically pure reagents.
2.2.2 Viscosity reducer JN-1
Focusing on emulsifying properties, the viscosity reducer JY-1 was synthesized. The reaction equations are shown in Equations (1)-(3). The synthesis reaction was divided into three steps: firstly, alkyl naphthol reacted with ethylene oxide to form alkyl naphthol polyoxyethylene ether (Equation 1). Then alkyl naphthol polyoxyethylene ether reacted with sulfuric acid to form alkyl naphthol polyoxyethylene ether sulfate (Equation 2). Finally, the system was neutralized with alkali to obtain the viscosity reducer JN-1 (Equation 3).

2.2.3 Surfactant JM-1
Focusing on reducing interfacial tension properties,the oligomeric cationic surfactants were synthesized.The reaction equation is shown in Equation 4. The intermediate product was prepared via the reaction ofN,Ndimethyl ethylenediamine with DL malic acid dimethyl ester under reflux for 3 hours. The intermediate product and 1-bromododecane were dissolved in a methanol/acetone mixed solvent and reacted on each other at 40 °C for 72 h to prepare the oligomeric surfactant JM-1.

2.2.4 Oil washing agent JY-1
Focusing on oil washing performance,the hydroxysulfobetaine JY-1 was synthesized. The reaction equation is shown in Equation 5. 3-Chloro-2-hydroxypropyl sulfonic acid was prepared by the reaction of sodium bisulfite and epichlorohydrin in the presence of distilled water at 85 °C for 2 h, and then the reaction mixture was cooled and filtered. In the presence of solvent(withV(isopropanol):V(water) =1:2), sodium 3-chloro-2-hydroxypropyl sulfonate reacted with dodecyl dimethyl tertiary amine at 80 °C for 5 hours. The reaction product was the oil washing agent JY-1.
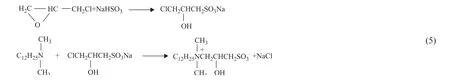
The above three chemical products were analyzed by the high resolution mass spectrometry.
2.3 Performance evaluation of oil displacement agents
2.3.1 Interfacial tension test
For studying three kinds of surfactants, the surfactant solutions of different concentrations were prepared with injection water. The salinity of injection water was 35200 mg/L. At 70 °C, the oil-water interfacial tension values of different surfactant solutions and experimental oil were measured by a TX-500D rotating drop interfacial tension meter. The viscosity and density of the experimental oil were 1780 mPa·s and 0.967 g/cm3at 70 °C, respectively.
2.3.2 Emulsion viscosity reduction test
Chemical agent solutions with a mass fraction of 0.3%were prepared with injection water. In the emulsion viscosity reduction test, 7 g of heavy oil sample were mixed with 3 g of chemical solution and shaken for 5 min to prepare emulsion. The emulsion viscosityV1was measured by a HAAKE Mars III rheometer. Compared with the viscosity of crude oil V0, the viscosity reduction rate achieved by chemical agent was calculated. The viscosity reduction rate formula is shown in Equation 6:

2.3.3 Oil washing ability test
The 40-70 mesh quartz sand was washed with water and dried. The oil and sand were mixed according to a mass ratio of 1:5, and were then put into an oven for aging at 60 °C for 24 hours on standby. Three chemical solutions with a mass concentration of 0.3% were prepared. The oil and sand mixture was added with chemical solution at a mass ratio of 1:2 under stirring prior to being kept at 70 °C for 20 hours. After having been taken out, the washed oil was poured out, and then the water on the quartz sand was dried and weighed, with the oil washing efficiency calculated.
2.4 Core displacement experiment
Through the displacement experiment, the changes of injection pressure, water content and oil recovery before and after injecting chemical solutions into cores were measured. The gas permeability of the four cores used in the experiment was similar, which was about 1 μm2. And the size of cores was equal to 4.5 cm × 4.5 cm × 30 cm.The experimental temperature was 70 °C, and the specific experimental steps were as follows.
(i) The core was dried and weighed, and the core was evacuated with a vacuum pump prior to being saturated with injection water. The pore volume of core could be calculated.
(ii) The cavity of the core was displaced by the injected water at a flow rate of 1 mL/min until the pressure reached equilibrium, and the water phase permeability was calculated.
(iii) The core was saturated with experimental oil, and the volume of saturated oil in the core was recorded.
(iv) The oil in the core was displaced by the injected water at a flow rate of 1 mL/min until the pressure was stable and no oil could be produced at the core outlet.
(v) The cavity of the core was displaced by 0.4 PV of chemical solution.
(vi) The water was injected into the core at a flow rate of 1 mL/min again until the pressure was stable and no oil was produced at the core outlet.
(vii) The cavity of the core was displaced by 0.4 PV of binary composite system.
(viii) The water was injected into the core at a flow rate of 1 mL/min for the third time until the pressure was stable and no oil was produced at the core outlet.
The injection pressure, water content and oil production at different time duration were recorded, and the oil recovery was calculated. The relationship between the performance of chemical agents and the result of enhanced oil recovery(EOR) was analyzed.
3 Results and Discussion
3.1 Structure characterization of asphaltenes and resins
The viscosity of heavy oil was measured by a rheometer.The viscosity of heavy oil at 50 °C was 6645 mPa•s.The elemental analysis of crude oil showed that the mass fraction of carbon, hydrogen, oxygen, nitrogen,and sulfur was 80.20%, 10.16%, 0.31%, 0.25%, and 0.66%, respectively. Asphaltenes and resins in the crude oil were separated. It was found that the mass fraction of asphaltenes was 7.8%, and the mass fraction of resins was 22.1%. Through elemental analysis, high-resolution mass spectrometry, X-ray photoelectron spectroscopy and nuclear magnetic resonance analyses of asphaltenes and resins, the average molecular structure diagram of asphaltenes and resins was put forward. And the natural active components of heavy oil were analyzed,with the inferred chemical structure formulas shown in Figure 1.

Figure 1 Molecular structure diagrams
Through the structural analysis of asphaltenes and resins, it was found that asphaltene molecules were mainly condensed aromatics surrounded by alkyl chains, containing a certain amount of heteroatoms such as sulfur, oxygen and nitrogen. The proportion of aromatics in resins was less than that of asphaltenes, and the proportion of alkyl chains was greater than that of asphaltenes. Sulfur in heavy oil was mainly contained in asphaltenes. Asphaltene and resin molecules were associated with each other mainly through the π-π force,the hydrogen bonding, the acid-base action, and the van der Waals force, which increased the viscosity and fluidity of heavy oil, and increased the difficulty of heavy oil recovery.
3.2 Mass spectrometric analysis results of chemical agents
The high resolution mass spectrometry analyses of three chemical agents including JN-1, JM-1, and YJ-12 are shown in Figure 2, Figure 3, and Figure 4, respectively.The theoretical value of MS-ESI (m/z) of JM-1 was 772.82. The test result of JM-1 was 306.5 ([M-2Br]2+/2).The results of mass spectrometry analyses were consistent with those of the target products, indicating that the expected target products were obtained.
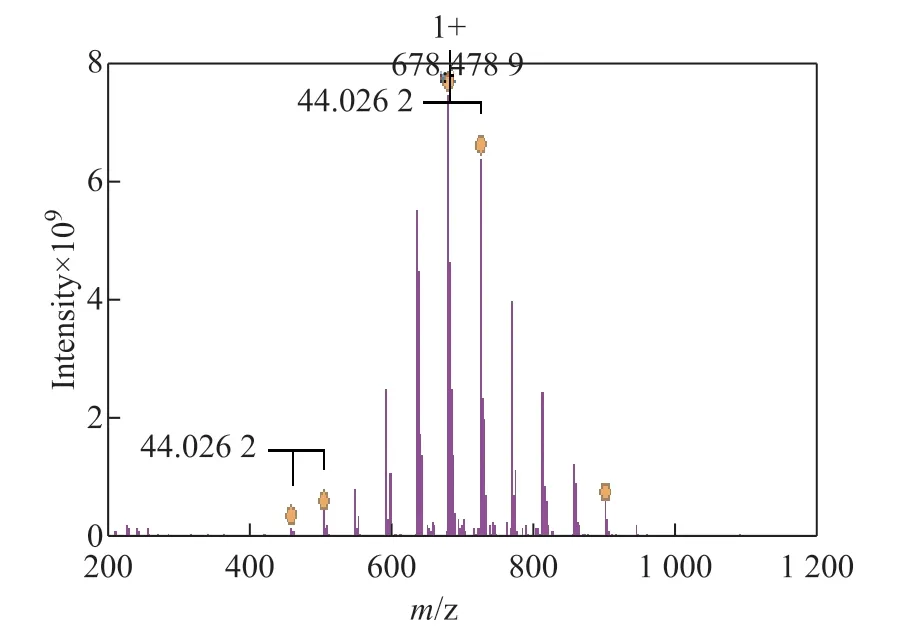
Figure 2 Mass spectrogram of JN-1

Figure 3 Mass spectrogram of JM-1
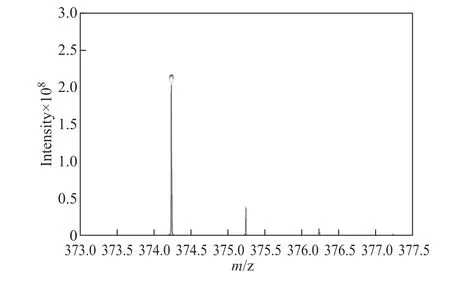
Figure 4 Mass spectrogram of YJ-1
3.3 Surface tension
The results of oil-water interfacial tension test of three chemical agents are shown in Figure 5.
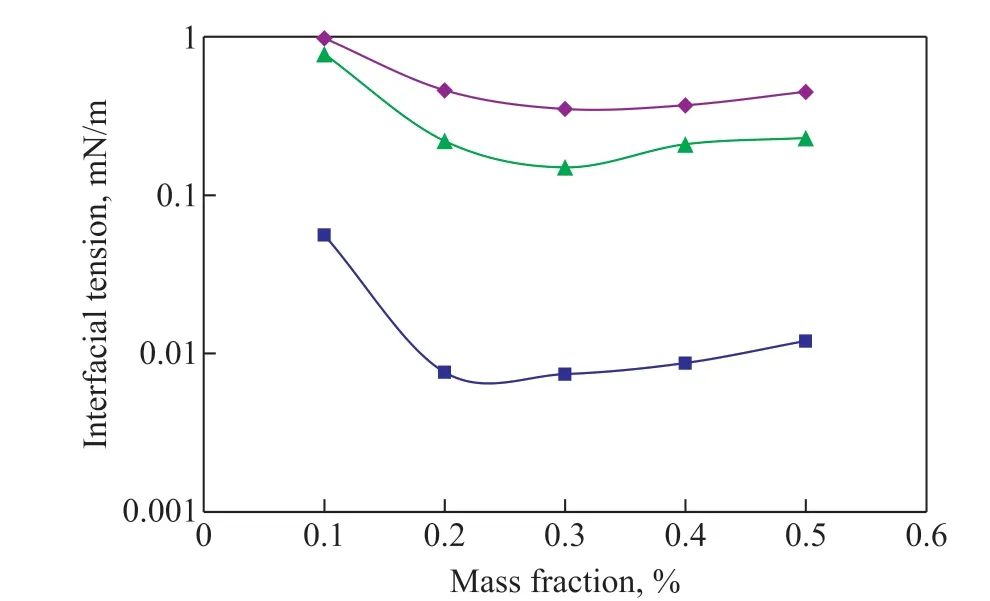
Figure 5 Interfacial tensions of different chemical solutions
It can be seen from Figure 5 that with the increase of the mass fraction of chemical agent, the interfacial tension at first decreased and then slightly rose. The minimum interfacial tension of JN-1, JM-1, and JY-1 solution was 0.35 mN/m, 0.0074 mN/m, and 0.15 mN/m, respectively.The lowest interfacial tension of JM-1 surfactant solution was by two orders of magnitude lower than that of ordinary surfactants.
3.4 Emulsion viscosity reduction test
At 70 °C,the results of viscosity reduction test of three chemical agents are shown in Table 1. The mass ratio of oil to water was 7:3 in the experiment.
It can be seen from Table 1 that when the oil-water ratio was 7:3, the surfactant JM-1 could not form emulsion at a mass fraction of 0.2%. And when the mass fraction of JM-1 was 0.3%, W/O emulsion was formed. The viscosity of W/O emulsion was greater than that of crude oil, and could not reduce the oil viscosity. The surfactant JN-1 could produce stable emulsion at a lower mass fraction of 0.2%, and the viscosity reduction rate could reach 99.6%.Although the surfactant JY-1 could form O/W emulsion when its mass fraction was 0.3%, the O/W emulsion was very unstable, and demulsification occurred soon. The viscosity reduction rate of JY-1 for heavy oil was less than that of JN-1. Figure 6 shows the photos of the O/W emulsion formed by JN-1 and JY-1 with experimental oil under an optical microscope at the same magnification.The emulsion particles formed by JY-1 were larger, and the emulsion formed by JN-1 was uniform and fine. The emulsifying performance of surfactant JM-1 with lower interfacial tension was not ideal.
3.5 Oil washing ability test
At 70 °C,the results of oil washing ability test of three chemical agents are shown in Figure 7.
It can be seen from Figure 7 that JY-1 had the best oil washing ability. When the mass fraction of JY-1 was 0.3%,the oil washing efficiency could reach 72.5%.
3.6 Core displacement experiment
The core displacement experiments involving three chemical agents were studied. The mass fraction of injected chemical agent was 0.3%, the injection volume of chemical agent was 0.4 PV, and the injection speed was 1 mL/min.

Table 1 Emulsifying effect of chemical agents on heavy oil

Figure 6 The O/W emulsion formed by JN-1 and JY-1

Figure 7 Oil washing ability of chemical agents
After the core J-1 saturated with oil was subjected to primary water-flooding, the liquid in the core was displaced by 0.4 PV of JN-1 solution. Then the core was subjected to secondary water-flooding. After water content at the core outlet was 98%, the remaining fluid in the core was displaced by 0.4 PV of composite system containing 0.3% of HPAM and 0.3% of JN-1. At last it was subjected to tertiary water-flooding. The experimental results are shown in Figure 8.

Figure 8 Experimental results of enhanced oil recovery from core J-1 by JN-1
It can be seen from Figure 8 that after injection of JN-1, the water content decreased significantly, with its lowest value equating to 63.1%, and the oil recovery increased by 7.9%. After injecting the JN-1 and polyacrylamide composite system, the lowest water content was 31.5%, and the oil recovery was increased by 13.8%. It shows that JN-1 can significantly improve the recovery of heavy oil, and the recovery effect of composite system is better.
Using the same experimental method, the displacement experiment of surfactant JM-1 was carried out on the core J-2, with the results shown in Figure 9.

Figure 9 Experimental results of enhanced oil recovery from core J-2 by JM-1
It can be seen from Figure 9 that the water content decreased to 81.1% and the oil recovery increased by 6.0% after injection of JM-1. After injecting the composite system comprising 0.3% of JM-1 and 0.3% of polyacrylamide, the lowest water content was reduced to 71.1%, and the oil recovery was increased by 11.8%.
During the study on core J-3, the core displacement test results achieved by JY-1 are shown in Figure 10.
It can be seen from Figure 10 that after injection of JY-1,the water content decreased to 93.6%, and the oil recovery increased by 4.2%. When the injected composite system included 0.3% of JY-1 and 0.3% of polyacrylamide, the lowest water content was reduced to 65.5%, and the oil recovery was greatly increased by 14.8%.

Figure 10 Experimental results of enhanced oil recovery from core J-3 by JY-1
Upon comparing the results of the above three groups of experiments, it can be seen that JN-1 had achieved a highest EOR, and the EOR effect of the composite system of chemical agent and polyacrylamide was significantly higher than that of a single chemical agent. Because the addition of polymer could increase the viscosity of displacement fluid, reduce the oil-water mobility ratio and increase the swept volume of fluid, hence the oil recovery was improved.
In order to compare the oil displacement effect between the chemical agent and the binary composite system, the experimental method for testing the core J-4 was modified as follows. After the primary water-flooding, the liquid in the core J-4 was at first displaced by 0.4 PV of the composite system comprising 0.3% of HPAM and 0.3%of JN-1. After the secondary water-flooding, the liquid in the core J-4 was once again displaced by 0.4 PV of the composite system consisting of 0.3% of HPAM and 0.3%of JN-1, and finally the tertiary water-flooding was carried out. The data changes obtained during oil displacement tests are shown in Figure 11.
It can be seen from Figure 11 that after the injection of composite system, the water content decreased to 38.8%,and the oil recovery increased by 28.7%. After the second injection of the composite system, the oil recovery was increased by 11.1%.

Figure 11 Experimental results of enhanced oil recovery from core J-4
The enhanced oil recovery amplitude achieved by the chemical agents and the composite system was compared between the above-mentioned four experiments, with the results shown in Figure 12.
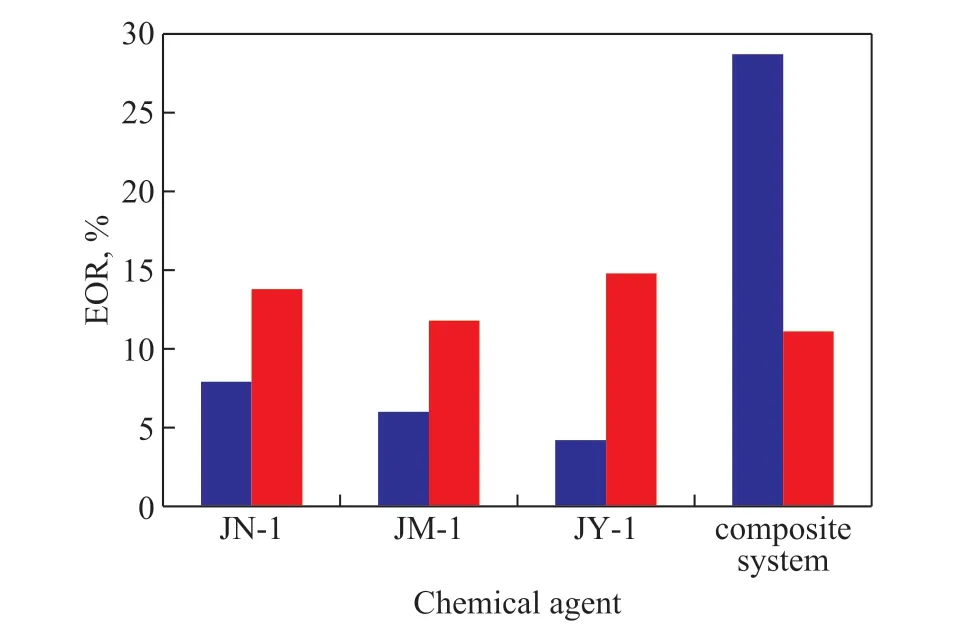
Figure 12 Comparison of enhanced oil recovery achieved by chemical agents
It can be seen from Figure 12 that after primary waterflooding, the liquid in the core was displaced by the binary composite system, which could greatly improve the crude oil recovery. The synergistic effect of viscosity reducer and polymer was very obvious. In addition, the contribution of chemical agent performance to EOR of heavy oil decreased in the following order: emulsification and viscosity reduction > interfacial activity > oil washing ability.
4 Conclusions
(1) Through the structural analysis of asphaltenes and resins, it was found that asphaltene molecules were mainly condensed aromatics surrounded by alkyl chains,containing a certain amount of heteroatoms such as sulfur, oxygen and nitrogen. And the proportion of aromatics in resins was less than that of asphaltenes, and the proportion of alkyl chains was greater than that of asphaltenes. Sulfur in heavy oil was mainly contained in asphaltenes.
(2) Anionic-nonionic surfactants similar to the natural active components of heavy oil have good emulsifying properties for heavy oil, and the oligomeric surfactants have good oil-water interface properties, while the betaine amphoteric surfactants have good oil washing ability.
(3) For chemical injection schemes employed in heavy oil reservoirs, the emulsification and viscosity reduction performance of chemical agents was more important than the oil washing capacity, and the oil washing capacity was more important than the interface performance. The viscosity reduction performance is the key parameter of oil displacement agent suitable for heavy oil reservoir.
(4) The EOR effect of the composite system consisting of chemical agent and polyacrylamide was significantly higher than that of chemical agent.
Acknowledgement: This work was supported by the 13th Five-Year Plan National Key Project of China (NO. 2016ZX0511-003-004 and No. 2017ZX05049-003-008).
- 中国炼油与石油化工的其它文章
- Electrospinning Nanofiber Membrane Reinforced PVA Composite Hydrogel with Preferable Mechanical Performance for Oil-Water Separation
- Preparation of Solid Waste-Based Activated Carbon and Its Adsorption Mechanism for Toluene
- Antibacterial and Corrosion Inhibition Properties of SA-ZnO@ODA-GO@PU Super-Hydrophobic Coating in Circulating Cooling Water System
- Investigation of Nitrite Production Pathway in Integrated Partial Denitrification/Anammox Process via Isotope Labelling Technique and the Relevant Microbial Communities
- Heteroatom-Doped Carbon Spheres from FCC Slurry Oil as Anode Material for Lithium-Ion Battery
- Study on Low-Temperature Properties of the Asphalt Modified by Carbon Nanotubes (CNTs) and Crumb Rubber (CR)

