Synthesis of Axially Coordinated Cobalt Porphyrin/graphene Oxide Nanocomposite for Enhanced Electrocatalytic CO2 Reduction to CO①
YOU Wei-Feng XU Xiao CAO Ai-Hui TAO Zhi-Jie KANG Long-Tian②
a (Key Laboratory of Design and Assembly of Functional Nanostructures,and Fujian Provincial Key Laboratory of Nanomaterials, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China)
b (Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou 350108, China)
c (University of Chinese Academy of Sciences, Chinese Academy of Sciences, Beijing 100049, China)
ABSTRACT The electrocatalysts containing cobalt-pyrrolic nitrogen-carbon (Co-N4-C) moiety for CO2 reduction reaction (CO2RR) have caught much attention. However, the effects of Co valence state and its synergy with graphene substrate are not clear yet. In this work, cobalt porphyrin (CoTPP) molecule with the intrinsic Co-N4-C moiety is successfully combined with graphene oxide (GO) via three kinds of liquid-phase methods. The ratio of CoTPP to GO and the valence state of Co atom are studied to explore their catalysis for CO2RR to CO. It is found that axially-coordinated Co(III)TPPCl/GO nanocomposites synthesized via a chemical method exhibit better ability for CO2RR, as compared with Co(II)TPP+GO and/or Co(III)TPPCl+GO nanocomposites obtained via a physically mixing way. After optimizing the ratio of CoTPP to GO, the Faradaic efficiency (FE) is more than 90% for CO2RR to CO between -0.7 and -0.8 V vs. reversible hydrogen electrode (RHE) in Co(III)TPPCl/GO75. The synergy between CoTPP and GO and the effect of Co valence state are systematically investigated, indicating that their strong interaction plays the key role in electrocatalytic CO2RR.
Keywords: cobalt porphyrin, graphene oxide, axial coordination, carbon dioxide reduction, synergistic catalysis; DOI: 10.14102/j.cnki.0254-5861.2011-3247
1 INTRODUCTION
The increasing concentration of carbon dioxide in the atmosphere has caused many environmental problems, such as climate change and desertification[1,2]. Therefore, in order to reduce carbon dioxide emissions and achieve globalcarbon neutralityas soon as possible[3], scientific methods must be carried out to reduce or convert carbon dioxide into valuable chemical substances. The electrocatalytic CO2RR is considered to be a relatively economical, green and feasible method[3-5]. However, due to the diversity of reduction products accompanied with the competition of hydrogen evolution reaction, the electrocatalytic CO2reduction reaction process still face many problems to be solved urgently, such as poor selectivity and high overpotential[4,6-8]. At present, the various materials, such as metals, metal oxides, carbon-based composite and organometallic compounds etc., have been used for the development and design for high efficiency and selectivity electrocatalysis[9-11].
When designing CO2reduction catalysts, Co element is considered to be one of the ideas for replacing the precious materials of platinum (Pt), ruthenium (Ru) and so on[5,6,12-14].In particular, when cobalt exists in small organic molecules like porphyrin[14,15]and phthalocyanine[16,17]to form Co-N4-C moiety, it exhibits excellent CO2RR catalytic activity. For example, after immobilization onto graphene simply throughπ-πinteraction, tetraphenylporphyrin cobalt showed enhanced electron transfer for CO2RR[18]. In the heterogeneous state, the electron transport between the metal active center and the substrate also greatly affects the electrocatalytic reaction of the complex[19]. Graphene is a suitable substrate because of its high electrical conductivity, stability in a reducing environment and good reactivity, thus enabling multi-purpose connections[20,21]. The planar structures of porphyrin and phthalocyanine were suitable for surface attachment with a substrate[14-17,22]. The electron communication ability and catalytic activity may be promoted while the cobalt site was coordinated to bond with other atoms in the axial direction of the cobalt porphyrin or cobalt phthalocyanine molecular plane. This was attributed to the change of the valence state of the central atom in the molecule and the stiffness and the degree ofπconjugation[12,23]. Cobalt porphyrin has a high degree ofπ-conjugation, allowingπ-πto stack with graphene[15,24]. Therefore, cobalt porphyrin complexes may have the potential for high CO2RR activity/selectivity[25-28].
The wet chemical reaction has exhibited its obvious advantages on preparing nanostructures of organic molecules with controllable size, morphology and self-organization[29-31].In this work, through a simple graphene-assisted wet chemical route, the nanostructure of graphene-based axially coordinated cobalt porphyrin was successfully constructed. The transmission electron microscope (TEM), scanning electron microscope (SEM), X-ray diffraction (XRD) etc. were used to analyze the effect of synthesis method and whether coordination occurred of catalysts. Its CO2RR catalytic performance and mechanism were systematically studied, as compared with the physical mixing one and the non-axial coordination one. The effects of the graphene amount and Co valence state in different cobalt porphyrin-graphene composites on the electrocatalytic CO2RR were investigated. The electrocatalytic mechanism was carefully analyzed through the characterization of Raman, Fourier transform infrared spectroscopy (FT-IR) and X-ray photoelectron spectroscopy(XPS). The synergy between Co porphyrin and graphene and the positive role of CoTPPCl were observed and can be attributed to the strong interaction between porphyrin and graphene.
2 EXPERIMENTAL
2. 1 Materials and methods
Anhydrous silver perchlorate (AgClO4), acetonitrile(CH3CN) and dichloromethane (CH2Cl2) were bought from J&K Chemicals Co. Ltd. Cobalt tetraphenylporphyrin (CoTPP)was purchased from Aladdin Industrial Incorporation. Nafion membrane solution (20%, Dupont), Nafion membrane (211,Dupont) and carbon paper (HP020) were used. Potassium bicarbonate (KHCO3), iron(II) chloride tetrahydrate(FeCl2·4H2O), N,N-dimethylformamide (DMF) and other reagents were gotten from Sinopharm Chemical Reagent Co.Ltd. Ultrapure water with a resistivity of 18.25 MΩ·cm-1was prepared from a Water Purifier apparatus (WP-UP-IV-20). All reagents were directly applied without purification.
High-resolution field emission SEM of all samples was measured using the Hitachi SU8010. Their structure and element distribution were characterized by TEM (FEI F20). XPS characterization was accomplished through an ESCALAB 250Xi instrument using an AlKαradiation as exciting source.XRD data were collected on a MiniFlex diffractometer(MiniFlex600, Rigaku) with CuKαradiation (λ= 1.54 Å).FT-IR spectra were obtained with a VERTEX70 spectrometer.Raman spectra were measured via the Lab RAM HR spectrometer (Horiba Jobin Yvon) with a 532 nm laser.
2. 2 Synthesis of CoTPP precursor (CoTPP+·ClO4-)
Synthesis of CoTPP·ClO4through reaction (1)[32]:2CoTPP + 2AgClO4+ I2= 2CoTPP+·ClO4-+ 2AgI (1)First, 0.6854 g (1.0 mmol) CoTPP, 0.2073 g (1.0 mmol)AgClO4and 0.1269 g (0.5 mmol) iodine (I2) were added into 50 mL of CH2Cl2solution, 50 mL of a CH3CN solution and 8.0 mL of a CH2Cl2solution, respectively. Then the solutions were rapidly mixed together. After the reaction was stirred for 5 hours, the precipitation of silver iodide (AgI) was separated by centrifugation at 8,000 rpm for 10 min. The as-obtained filtrate was added to 200 mL of petroleum, and then was kept for 50 min. After centrifugation, washing and drying, CoTPP+·ClO4-powder can be obtained. The powder was collected and stored in a dry and dark environment for further use.
2. 3 Synthesis of CoTPPCl/GO, CoTPP+GO and CoPPCl+GO composites
According to the modified Hummer’s method, we obtained graphene oxide (GO) by oxidizing natural graphite[33]. The sample was dispersed in a certain amount of ultrapure water to form a GO dispersion, and then the content of the dispersion was calibrated to 2.0 mg/mL for subsequent use. The calibrated GO dispersion was continuously dispersed in 360 mL of water. When GO content was 45, 60, 75, 90 and 105 mg, the aqueous dispersions were named GO45, GO60,GO75, GO90 and GO105, respectively.
CoTPPCl/GO nanocomposite was synthesized via a chemical method. Under vigorous stirring, the GO dispersion was quickly added to 120 mL 1.0 mM CoTPP·ClO4acetonitrile solution. After 5 hours, the product was collected after centrifugation, washing and drying, and labeled as CoTPPCl/GO.
For further research, CoTPP+GO and CoTPPCl+GO nanocomposites were obtained via a physically mixed way.1.0 mL of 1.0 mM CoTPP·ClO4/acetonitrile solution was added into 2 mL of 10.0 mM FeCl2solution, and then was aged for 5 hours. After centrifugation, the precipitate was collected for further characterization and was named as CoTPPCl. Finally, 10 mL of 1.0 mM CoTPPCl/DMF solution was mixed with 30 mL of GO75 aqueous dispersion to prepare CoTPPCl + GO samples. In addition, CoTPP + GO samples were prepared through mixing the 1.0 mM CoTPP raw materials/DMF solution with the dispersed GO75 dispersion.
2. 4 Electrochemical measurements
Electrochemical performance was characterized with the electrochemical workstation. The reference and counter electrodes of the three electrodes were Ag/AgCl electrode and Pt net, respectively. 5.0 mg catalyst was ultrasonically added into a mixed solution of 0.9 mL isopropanol and 0.1 mL Nafion. 80 μL of the catalyst ink was coated on 1×1 cm-2carbon paper as a working electrode. The CO2RR was carried out in 140 mL 0.1 M KHCO3solution. Carbon dioxide flows through the cathode chamber at a speed of 50 sccm, and then flows into the chromatograph (GC-2014) for detection. In order to remove other gases before the reaction, carbon dioxide was circulated for more than 20 minutes. Then, the linear sweep voltammetry was used for LSV test, sweeping at 10 mV·s-1from 0 V to -1.0 V. To measure the Faraday efficiency (FE) and i-t curve of the product, the reaction started under 90% IR compensation and was held for 15 min. In order to study the charge transfer resistance, the electrochemical impedance spectroscopy (EIS) was also measured with the voltage amplitude of 5 mV and a frequency range between 0.1 Hz and 100 KHz.
3 RESULTS AND DISCUSSION
3. 1 Morphology and phase structure
A series of CoTPP-graphene composite materials was synthesized by adding cobalt porphyrin raw material or CoTPP perchlorate (CoTPP+·ClO4-) to the aqueous dispersion of GO, as shown in Scheme 1, and the detail can be found in the experimental section. The as-obtained products were firstly characterized through SEM, TEM and XRD in order to investigate their size, shape and structure. The SEM image in Fig. 1a shows the flexible and smooth 2D structure of graphene oxide. Fig. 1b exhibits CoTPP raw material with a widely-distributed size. After the reaction between precursor CoTPP·ClO4and FeCl2, a large number of nanowires (NWs)with a diameter of ~80 nm can be synthesized, as shown in Fig. 1c. Basing on our previous study, it means the formation of porphyrin molecule with the axially coordinated Cl and H2O[32]. Here, it was named as CoTPPCl. After raw CoTPP in DMF was mixed with aqueous GO75, many octahedral particles attached to 2D GO in the sample of CoTPP+GO75 can be clearly seen in Fig. 1d. When raw CoTPP was replaced with CoTPPCl, the 1D CoTPPCl nanostructure can be still observed in the sample of CoTPPCl+GO75 (Fig. 1e). Similarly, the CoTPPCl/GO75 sample in Fig. 1f may be synthesized when CoTPP·ClO4in acetonitrile solution was used instead of CoTPP or CoTPPCl in DMF. CoTPPCl/GO75 sample was seemly the same as CoTPPCl+GO75. The most obvious difference in morphology was from the length of 1D CoTPPCl nanostructure. The 1D CoTPPCl becomes shorter in CoTPPCl/GO75 (~500 nm) than CoTPPCl+GO75 (> 1 μm), indicating more enough mixture between CoTPPCl and GO through the chemical method rather than the physical one.
The structure change of CoTPPCl/GO with GO content was also investigated to explore the formation mechanism of CoTPPCl/GO nanocomposites when the CoTPP precursor was kept, as displayed in Fig. 1g-i. When the solid content of GO was decreased to GO45, more and longer 1D NWs were obtained in Fig. 1g. However, both the amount and size of 1D NWs in Fig. 1h-i obviously become smaller, even gradually disappear with the GO increase to GO90 and GO105. The above phenomena prove the existence of strong interaction between CoTPPCl and GO, which results in the preferential nucleation of CoTPP molecules on the surface of GO and then the growth to 1D NWsviaself-assembly. That is to say,the reaction product of CoTPP molecules should firstly cover on GO rather than self-assembly[34]. As a result, higher ratio of GO/CoTPP induces the gradual disappearance of 1D CoTPP NWs.
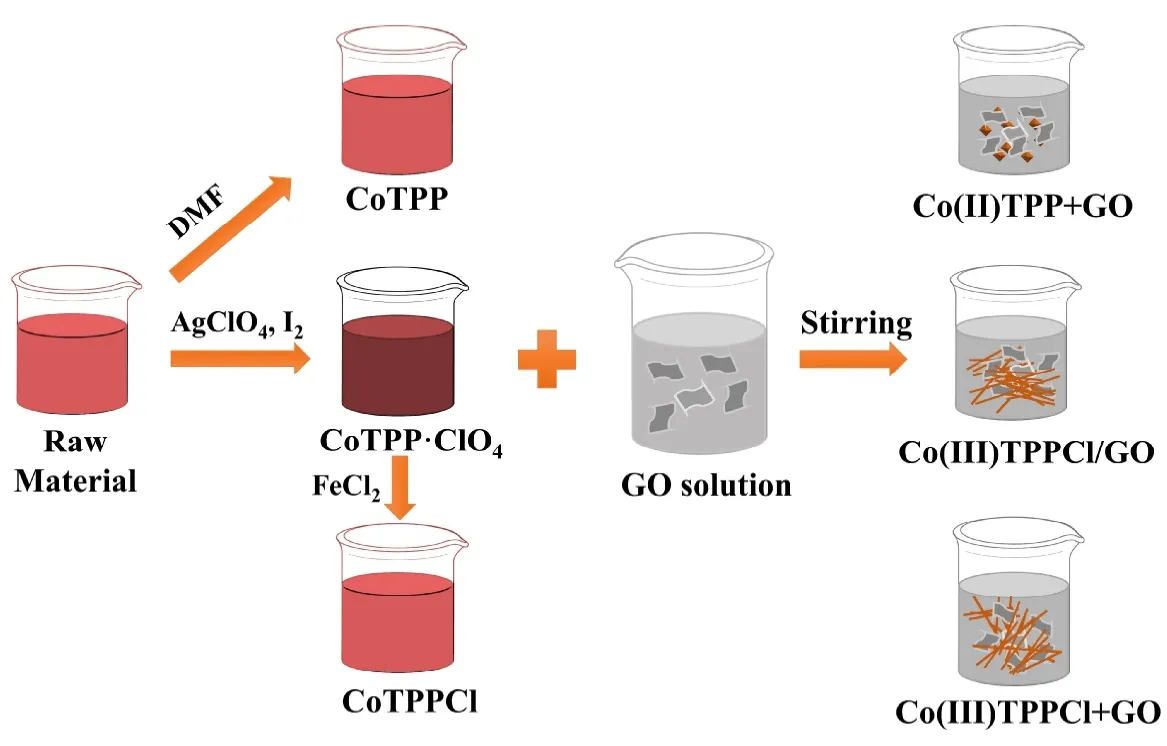
Scheme 1. Schematic diagram of preparation composites of cobalt porphyrin and GO composite material through different methods
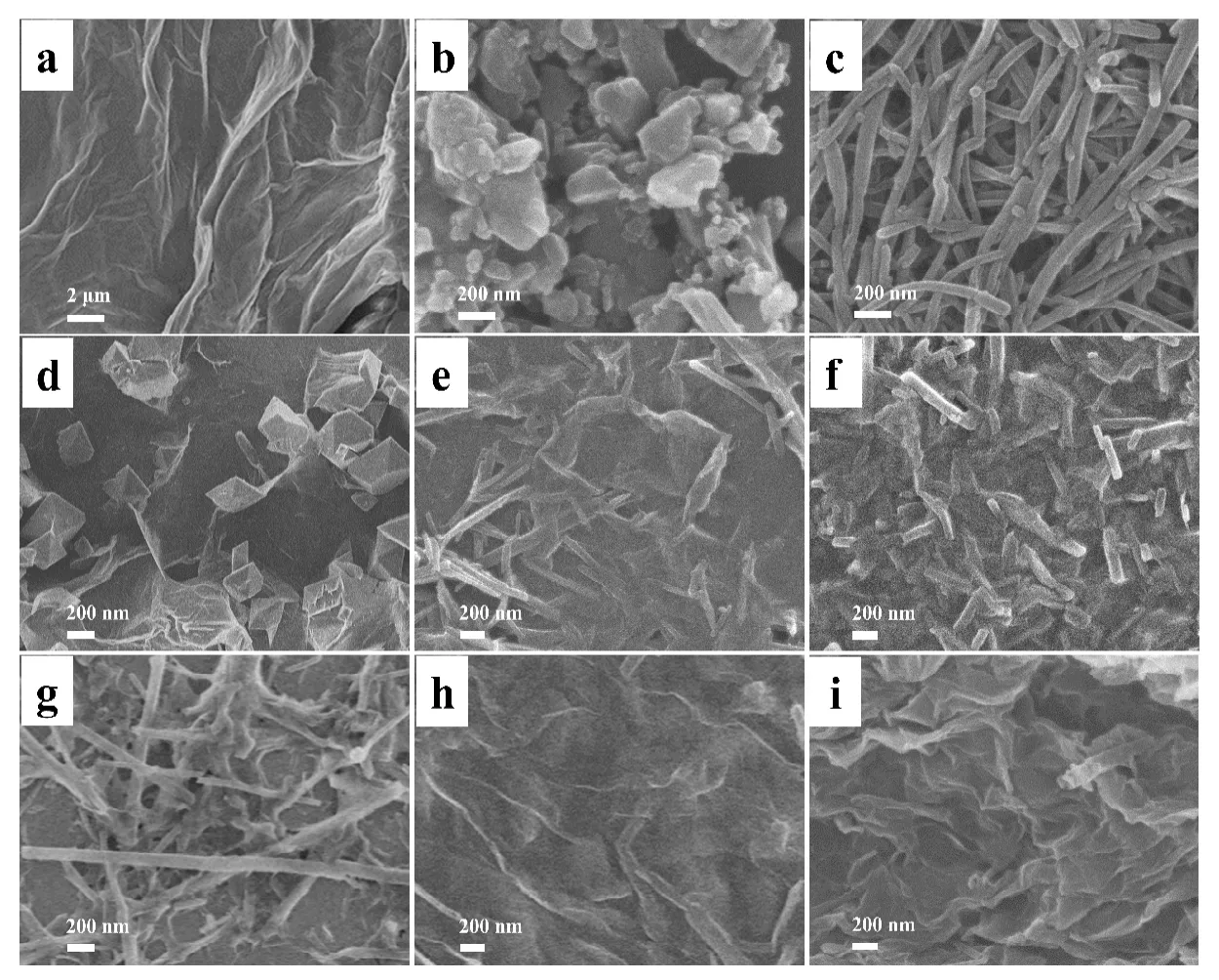
Fig. 1. (a-f) SEM images of GO, CoTPP, CoTPPCl, CoTPP+GO75, CoTPPCl+GO75 and CoTPPCl/GO75.(g-i) SEM images of CoTPPCl/GO45, CoTPPCl/GO90 and CoTPPCl/GO105
Fig. 2a and Fig. 2b show the XRD patterns of samples, in which GO exhibits a typical characteristic diffraction peak at 10°[35,36]. Indeed, there are two different forms of CoTPP crystal in our samples. The crystal structure of CoTPP in CoTPP + GO75 sample is the same as that of CoTPP raw materials, which may be indexed to the single crystal of CoTPP (C44H28CoN4, CCDC No. TPORCP11)[37]. The CoTPP crystal in CoTPPCl and CoTPPCl/GO75 can be assigned to C44H30ClCoN4O (CCDC No. GAMTAP)[38]. Here,the central Co atom was axially coordinated with Cl atom and H2O molecule. Fig. 2c and Fig. 2d exhibit the corresponding molecular structures of raw CoTPP and CoTPPCl. The single crystal octahedrons with an edge-length of 200 nm can be observed in Fig. 2e. Its selected area electron diffraction(SAED) proves that the Co porphyrin octahedra axially grow along the [001] direction, and should be surrounded by±(101), ±(011), ±(10-1) and ±(01-1) facets. And the d-spacing of 1.06 nm can be assigned to ±(101) facets of Co porphyrin crystal. The selected area electron diffraction (SAED, Fig. 2f)proves that the axially-coordinated CoTPPCl NWs preferentially grow along the [001] direction[32]. Hence, four side facets of Co porphyrin nanowires should be ±(020) and±(100). The d-spacing of 1.44 nm can be assigned to ±(100)facets of CoTPPCl crystal. The existence of strong intermolecular hydrogen bond of Cl-H easily results in the formation 1D nanostructure, in which Co-N-C moieties are actually sealed[32,39]. To further explore the component distribution of C, O and Co atoms in CoTPPCl/GO75 composite, the energy dispersive X-ray spectroscopy (EDS) mappings were also measured. Fig. 2g displays that the atoms of C and O were widely dispersed, indicating the existence of GO. Co atoms from cobalt porphyrin not only exist in 1D CoTPP nanostructures, but also can be found on the surface of GO, which agrees with the structure evolution of CoTPPCl/GO with the increase ratio of GO/CoTPP in Fig. 1. It means that the active sites of CoTPP for CO2RR may be enough exposed on GO when CoTPP and GO have been successfully compounded rather than simply mixed.
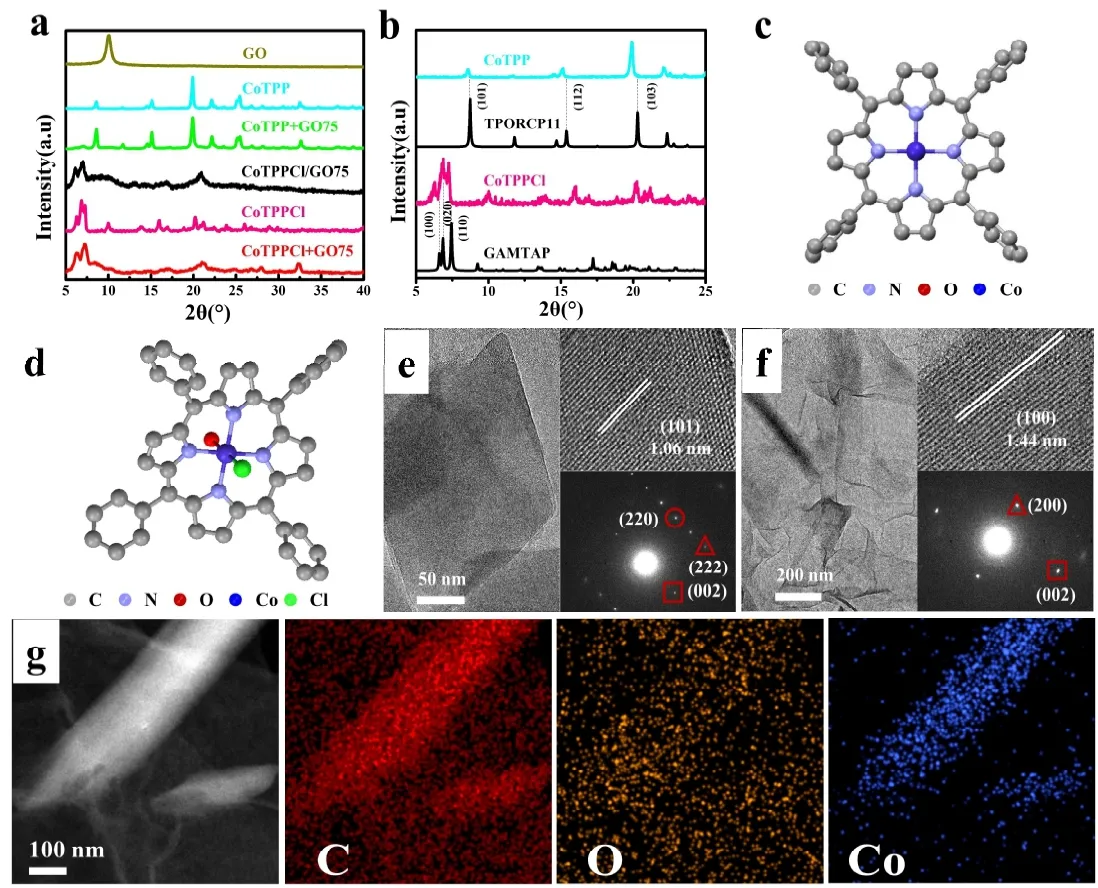
Fig. 2. (a-b) XRD patterns of GO, CoTPP, CoTPPCl, CoTPP+GO75, CoTPPCl/GO75 and CoTPPCl+GO75, (c-d) molecule structure of CoTPP and CoTPPCl, (e-f) HRTEM images and SAED pattern of CoTPPCl/GO75 and CoTPPCl+GO75,and (g) EDS mappings of C, O and Co atoms in CoTPPCl/GO75
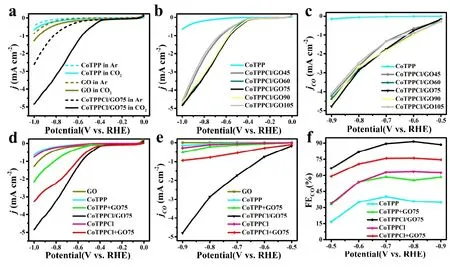
Fig. 3. CO2RR electrocatalytic activity in CO2 or Ar saturated 0.1 M KHCO3 solution in H-type cell. (a) LSV curves of CoTPP, GO and CoTPPCl/GO75. (b-c) Change of j and jCO with potential in CoTPPCl/GO composites. (d-h) j, jCO and FECO of GO CoTPP,CoTPP+GO75, CoTPPCl/GO75, CoTPPCl and CoTPPCl+GO75
3. 2 Performance of CoTPP and GO composites for CO2RR
The LSV curves of GO, CoTPP and CoTPPCl/GO75 in Fig. 3a obtained in the CO2-saturated solution exhibit an increase in the current density (j) and a bigger onset potential than those in Ar-saturated solution, suggesting that both CoTPP and GO should have the activity for electrocatalytic CO2RR[16]. Higherjin CoTPPCl/GO75 than CoTPP and/or GO means the synergistic effect between CoTPP and GO[28].To study the effect of the graphene solid content and the molecular structure on the electrocatalytic CO2RR, LSV curves of different samples were collected. In Fig. 3b and Fig. 3c,the effect of GO solid content in CoTPPCl/GO on the catalytic reduction of CO2RR to CO is shown. With the increase of graphene from GO45 to GO60, thejenhances. However,the increase degree decreases, as compared to the change from pure CoTPP NWs to CoTPPCl/GO45. It means that the catalytic sites have been more exposed in CoTPPCl/GO45 than CoTPP NWs. Interestingly, the catalytic activities of CoTPPCl/GO60, CoTPPCl/GO75 and CoTPPCl/GO90 were almost the same, strongly suggesting the real active sites should result from CoTPPCl on GO rather than CoTPPCl NWs. However, with the GO increase to G105, the catalytic activity reduces. According to the structure evolution of CoTPPCl/GO nanocomposites, a conclusion can be made that lower activity of CoTPPCl/GO45 may result from the more coverage of 1D NWs on GO with active sites, while that of CoTPPCl/GO105 should result from the reduction of active sites on GO[22,27]. Fig. 3c reveals the change of partial current density during the process of CO2RR to CO (jCO) with potential[40], in which the CoTPPCl/GO75 sample exhibits the highest electrocatalytic activity.
In addition, the influences of the ratio of CoTPP to GO, the synthesis method and the molecular structure of cobalt porphyrin in the catalytic performance were also studied. As seen in Fig. 3d and Fig. 3e, as the potential changes, the trends ofjandjCOof the compared samples are almost the same. CoTPPCl exhibits better catalytic activity than CoTPP raw material, suggesting that the axially coordinated CoTPP may be more efficient for catalytic CO2RR. More obvious phenomenon can be observed that the activity of CoTPPCl+GO75 is better than that of CoTPP+GO75 after the CoTPP was mixed with GO. Meanwhile, the synergic catalysis between GO and CoTPP may be clearly identified, as compared with the individual component. More interestingly, CoTPPCl/GO75 composite displays higher activity than CoTPPCl+GO75, implying more enough dispersion of CoTPP molecules on GO through chemical method than thephysical one.It is consistent with their structures (see Fig. 1). In Fig. 3f, the Faradic efficiency (FE) of CO can be found for the different samples. With the decrease of potential, the FE of CO also gradually increased, and then decreased. During the whole process, the competitive hydrogen evolution reaction (HER)may be found. As usual, the phenomenon should be associated with the reduction of active site Co atom from Co2+to Co+,finally to Co0in theCo porphyrin and composites[41,42]. At a potential of -0.8 V, the FECOof different samples was CoPPCl/GO75 > CoTPPCl+GO75 > CoTPP+GO75 >CoPPCl > CoTPP. The highest FECOof > 90% with the current density of -1.7 and -2.9 mA·cm-2can be achieved in CoTPPCl/GO75 at the potential between -0.7 V and -0.8 V(see Fig. 3e). Compared with other cobalt-based nanomaterials, the performance of the axially coordinated cobalt porphyrin graphene nanomaterials CoTPPCl/GO75 is excellent in Table 1.
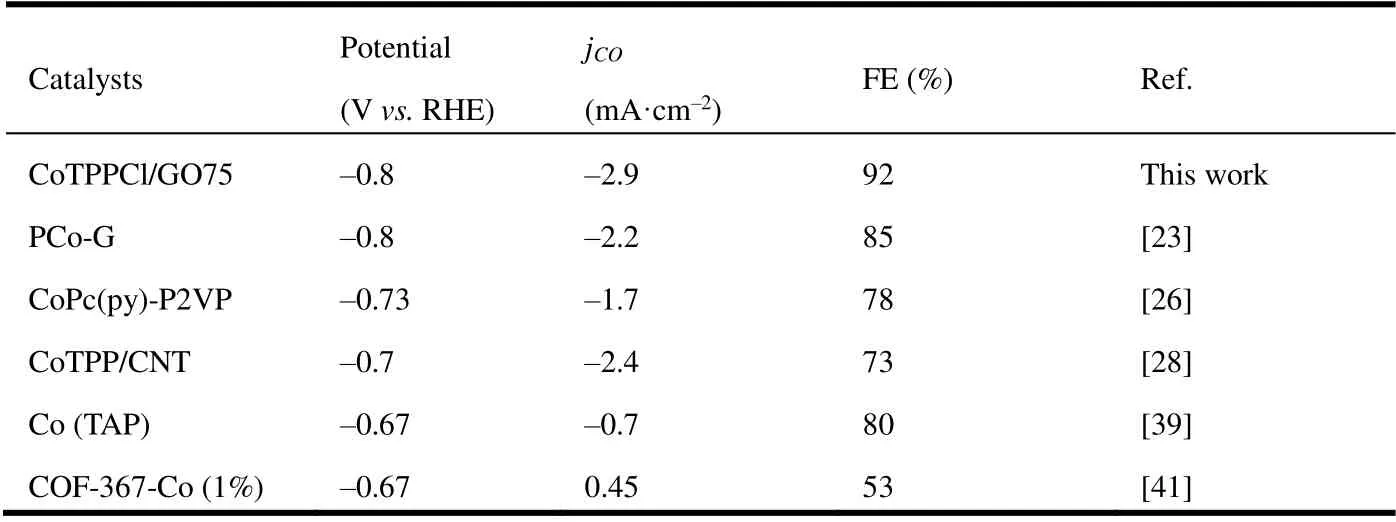
Table 1. Electrocatalytic Performance of the Cobalt-based Nanomaterials
The change ofjCOand FECOwith potential, the CV curve of CoTPPCl/GO75 sample was measured to deeply investigate the oxidation/reduction states of cobalt porphyrin, as shown in Fig. 4a. At potential of 0.21 and -0.49 V[23,43], the reduction peaks should be from the reduction of surface cobalt porphyrin from Co(III)TPPCl to [Co(II)TPPCl]-and from [Co(II)TPPCl]-to [Co(I)TPPCl]2-. It also further proves that Co(I) is a real active site for CO2RR. At about -0.75 V, a little peak exists, meaning the further reduction of[Co(I)TPPCl]2-to [Co(0)TPPCl]2-and the decay of catalytic activity. The most surface component should be Cl-Co(III)-N in CoTPPCl/GO75 and Co(II)-N in CoTPP+GO75. At about-0.49 V potential, the reduction peak of CoTPPCl/GO75 has a more positive potential than that of CoTPP+GO75[41],strongly indicating that the axially coordinated molecules may be beneficial to the formation of Co(I) under low overpotential, and to the higher selectivity of CO in CO2RR[20,44].In the case of a small potential, the Tafel slopes of CoTPPCl/GO75, CoTPPCl+GO75, CoTPP+GO75, Co(Ⅲ)TPPCl and Co(Ⅱ)TPP are 129, 144, 160, 168 and 188 mV·dec-1(see Fig. 4b), suggesting that the electron transfer is the easiest in CoTPPCl/GO75, and the CO2adsorption should be the ratedetermining step[45]. The result is accordant to the results of electrochemical impedance spectroscopy (EIS) in Fig. 4c,revealing the lowest interfacial charge transfer resistance in CoTPPCl/GO75. During the reduction process for at least ten hours, the catalytic performance of CoTPPCl/GO75 remains stable. As seen in Fig. 4d,jand FECOonly changed slightly. It can be concluded that the dispersion of porphyrin on the substrate and axial coordi- nation of central Co atom in CoTPP should significantly affect the electrocatalytic activity of CO2RR to CO.
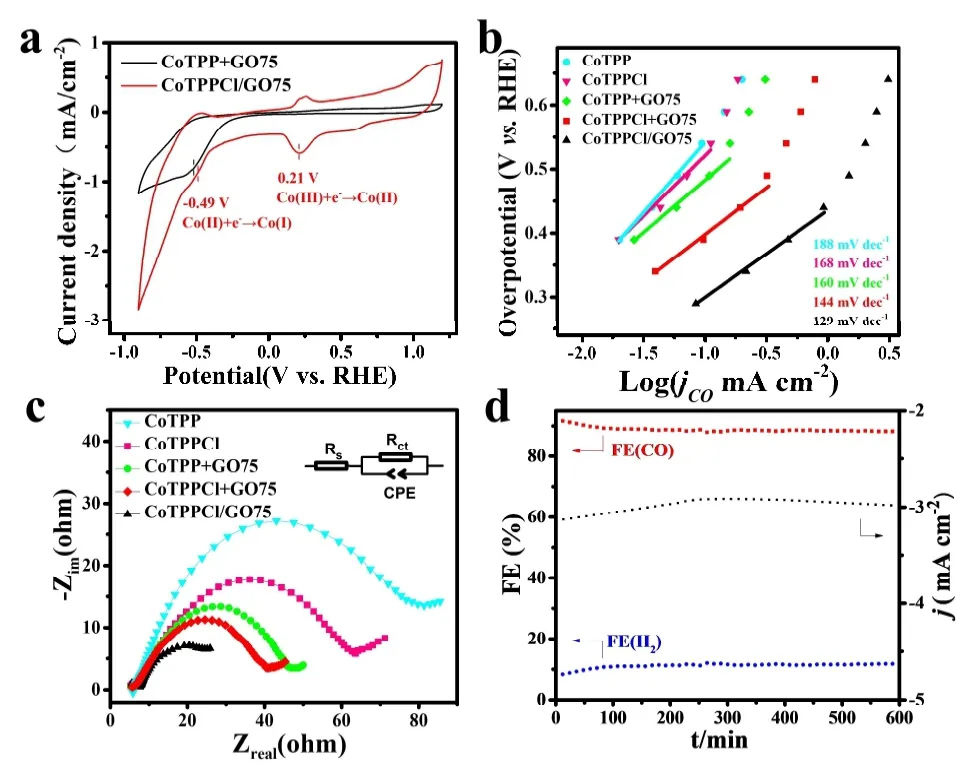
Fig. 4. (a) CV of CoTPP+GO75 and CoTPPCl/GO75. (b-c) Tafel slopes and EIS of CoTPP, CoTPPCl, CoTPP+GO75, CoTPPCl+GO75 and CoTPPCl/GO75. (d) Chronoamperometry curve and FE of CO and H2 at -0.8 V for 600 min in CoTPPCl/GO75
3. 3 Analysis of cobalt porphyrin and graphene composites
The structure, composition and Co valence state of the complex were analyzed to further understand the different catalysis of as-obtained samples in CO2RR. As Fig. 5a shows,the intensity ratio of D/G peak (ID/IG) in Raman spectroscopy was used to measure the structural defects of graphene[44].The values are 1.00 and 0.97 in CoTPPCl/GO75 and CoTPPCl+GO75. They are higher than 0.94 in GO, meaning the decrease ofsp2carbon in graphene. Moreover, the G bands of CoTPPCl/GO75 and CoTPP + GO75 are 1583 and 1585 cm-1,respectively, and blue-shifted compared to 1594 cm-1in GO.It may be caused by the extended delocalization ofπelectrons in GO[46]. The appearance of FT-IR peak at 3745 cm-1in Fig. 5b shows that the representative peaks of CoTPPCl/GO75 at 1003, 1348, 1439 and 1597 cm-1are slightly blueshifted, as compared to those of individual CoTPPCl and GO.It reveals the existence of strong interaction between CoTPPCl and GO[47], resulting in the enough dispersion of CoTPPCl on GO and the improvement of electrocatalytic activity for CO2RR.
The valence states change of Co, O and N atoms in composites are further analyzed through XPS spectra in Fig. 5c-f to study their electrocatalytic environment. The weak peak of O 1sin CoTPP sample can be attributed to the partial oxidization of the raw material in air (Fig. 5b). Obviously, the intensity of O 1speak in CoTPP + GO75 and CoTPPCl +GO75 composites increases. With the change of CoTPP molecular structure, the valence state of Co 2p, the environment of N 1sand the peak number of O 1sshould be theoretically changed. Therefore, the high-resolution XPS spectra of N, O and Co atoms are analyzed. Here, the N 1sspectra in Fig. 5c result from the pyrrolic nitrogen in Co(Ⅱ)TPP molecule[48].After the formation of Co(Ⅲ)TPPCl, the XPS peak of pyrrolic nitrogen shows a slight red-shift of 0.2 eV, indicating the increase of Co-N bond length because the Cl atom combines with the Co atom[49]. Here, the O 1s spectra in Fig. 5d may be divided into the groups of O-C=O (531.7 eV),C-O-C (532.6 eV), C-OH (533.4 eV) and adsorbed water(533.9 eV), respectively[48,50,51]. The first three peaks were attributed to GO, and the last one corresponded to the coordinated water of Co porphyrin. The Co 2pspectrum of CoTPP, CoTPP+GO75, CoTPPCl/GO75, CoTPPCl and CoTPPCl+GO75 in Fig. 5e could be fitted into four subpeaks at 777.9 eV (the 2p3/2orbital of Co3+), 781.4 eV (the 2p3/2orbital of Co2+), 794.7 eV (2p1/2orbital of Co3+) and 796.4 eV (2p1/2orbital of Co3+)[32,52]. The XPS peaks of Co2+atom are from the electron transition of 2p3/2and 2p1/2orbitals in CoTPP and CoTPP+GO75 samples. The weak peak of Co3+may derive from the partial oxidization of Co atom in air. In contrast, the peak intensity of Co3+in CoTPPCl/GO75,CoTPPCl and CoTPPCl+GO75 is stronger[53-55].
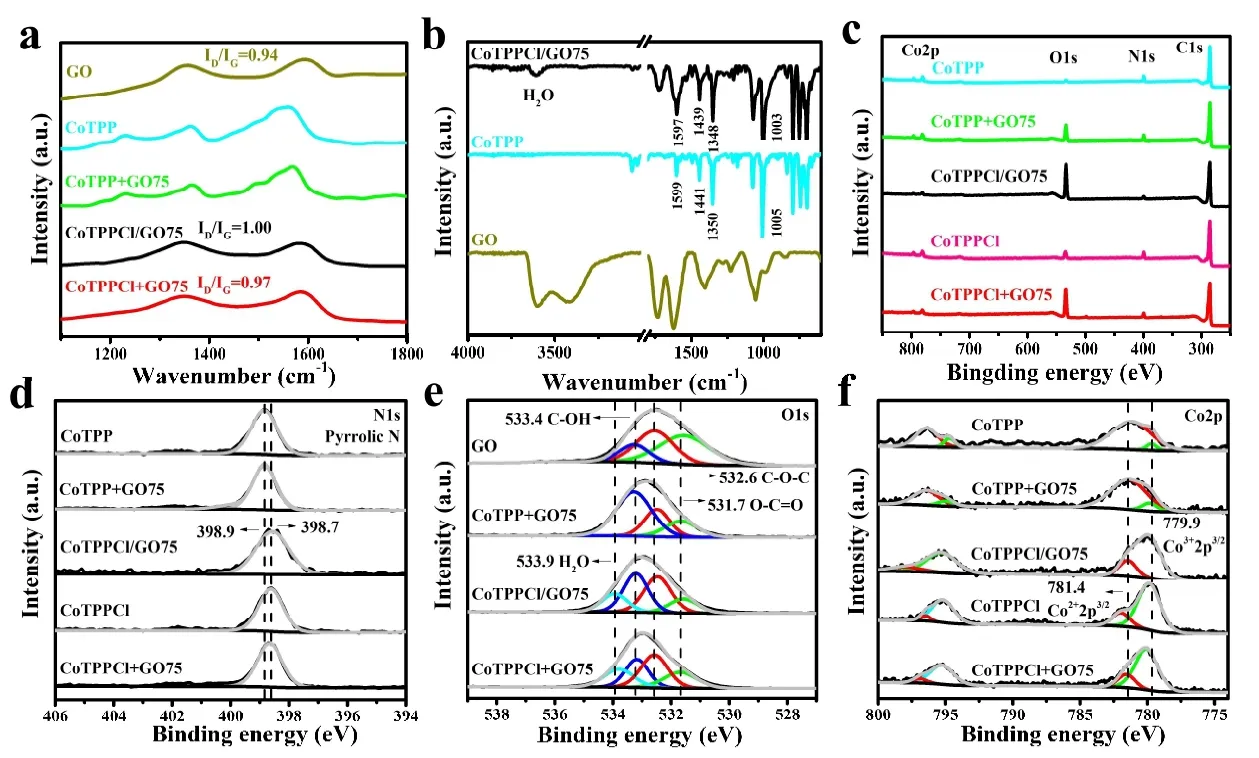
Fig. 5. (a) Raman spectra of GO, CoTPP, CoTPP+GO75, CoTPPCl/GO75 and CoTPPCl+GO75. (b) FTIR spectra of GO, CoTPPCl and CoTPPCl/GO75. (c-f) XPS spectra of N 1s, O 1s and Co 2p in GO, CoTPP, CoTPP+GO75, CoTPPCl/GO75, CoTPPCl and CoTPPCl+GO75
On the basis of all characterizations in this work, we know that the best catalytic activity of CoTPPCl/GO75 for electrocatalytic CO2RR should mainly originate from four sides, as compared with those of Co(II)TPP+GO, Co(III)TPPCl+GO and other CoTPPCl/GO nanocomposites. 1) The production of axially coordinated Co(III)TPPCl is helpful to produce real active site Co(I) at higher reduction potential, as shown in Fig. 4a. 2) The active Co-N-C moiety in Co(III)TPPCl is efficiently dispersed on GO. In fact, Co(III)TPPCl molecules aggregate more easily than the Co(III)TPP ones along the axial direction due to the existence of hydrogen bond of Cl-H(see Fig. 1). It means that the active sites are usually sealed in form of 1D NWs without template like GO, which is beneficial to more efficient dispersion Co(III)TPPCl molecules when solid content is 60~90 mg in 360 mL water. 3) In the wet chemical reaction, the strong electrostatic andπ-πinteractions between CoTPP+ion in precursor and GO in aqueous dispersion can efficiently suppress the face-to-face stacking of CoTPP molecules. Therefore, the CoTPP molecules are more easily dispersed on GO via a chemical synthesis method than physical mixing. 4) GO plays a positive role in the electron transfer and adsorption of CO2, especially the ratedetermining step is the formation of intermediate *COOH during the CO2RR to CO[56-59].
4 CONCLUSION
In conclusion, the graphene-based cobalt porphyrin composites with axial coordination and non-axial coordination have been successfully synthesized via GO-assisted liquidphase physical and chemical methods. The electrocatalytic performance of GO, CoTPP raw material, CoTPPCl, CoTPP+GO, CoTPPCl+GO and CoTPP/GO were systematically studied for CO2RR to CO. After optimizing the mass ratio of CoTPP to GO, CoTPPCl/GO75 composite obtained by a chemical method exhibited the best electrocatalytic activity for CO2RR. The FECOof > 90% can be achieved between-0.7 V and -0.8 Vvs. RHE for > 10 h. Characterizations of SEM, TEM, XRD, Raman, FT-IR, XPS and CV revealed that the successful synthesis of axially-coordinated Co(III)TPPCl on GO should via strong interaction play a key role in its high electrocatalytic performance. This work proves the effect of axially coordinated porphyrin on CO2RR, and will be helpful for the application of metal-pyrrolic N4-C molecules in the electrocatalytic field.
- 结构化学的其它文章
- Structural and Electronic Properties of Lutetium Doped Germanium Clusters LuGen(+/0/-) (n = 6~19):A Density Functional Theory Investigation①
- Discovery of Benzimidazole Derivatives as Novel Aldosterone Synthase Inhibitors: QSAR, Docking Studies, and Molecular Dynamics Simulation①
- QSAR Models for Predicting Additive and Synergistic Toxicities of Binary Pesticide Mixtures on Scenedesmus Obliquus①
- Preparation, Crystal Structure and Fungicidal Activity of N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)amide Compounds①
- Antibiotic Silver Particles Coated Graphene Oxide/polyurethane Nanocomposites Foams and Its Mechanical Properties①
- Planar Tetracoordinate Carbon in 6σ + 2π Double Aromatic CBe42- Derivatives①

