Effect of electrical stimulation on red meat Neu5Gc content reduction:a combined experimental and DFT study
Aqi Xu, Rui Chang, Qiujin Zhu*
1 School of Liquor and Food Engineering, Guizhou University, Key Laboratory of Agricultural and Animal Products Store and Processing of Guizhou Province, Guiyang 550025, China
Keywords:
Electrical stimulation
Neu5Gc
In flammation
Electronic structure
Density function theory
A B S T R A C T
The hazardous substance Neu5Gc (N-glycolylneuraminic acid), which is rich in red meat, is related to chronic inflammation but is hard to eliminate. Here, electrical stimulation, as a food-friendly nonthermal processing technology, was applied to red meat samples to reduce the Neu5Gc content. To explore the Neu5Gc structure changes during this process, electronic structure parameters were evaluated, and AIM ( atom in molecules)theory and DFT (density function theory) calculations were further used. The results showed that the content of Nue5Gc in red meat can be reduced by (74.24 ± 0.69)% at 120 V for 50 s, with little impact on the meat texture and color. Theoretical calculations indicated that the Neu5Gc molecule becomes very unstable under electrical stimulation by increasing the O-H bond length, reactive activity, strength of intermolecular dipole forces and total energy through reducing the values of bond dissociation energy and strength of intramolecular hydrogen bonds. Overall, this research provides an economical method to effectively control red meat safety.
1.Introduction
Red meat and its products, such as beef, pork and lamb, are sources of high-quality nutrients, such as proteins, polyunsaturated fatty acids, B vitamins and selenium [1]. However, since October 26, 2015, the International Agency for Research on Cancer (IARC)defined red meat as probably carcinogenic to humans [2]based on continuous updated research revealing the strong relationship between consuming processed meat and colorectal cancer [3]. As far as we know, colorectal cancer is the third most commonly occurring cancer in humans, and there were over 1.8 million new cases in 2018 [4].
Epidemiological research shows that the possible reason for red meat-induced colorectal cancer is nonhumanN-glycolylneuraminic acid (Neu5Gc). Neu5Gc is biosynthesized through cytidine-5′-monophospho-N-acetylneuraminic acid (CMP-Neu5Ac) hydroxylase,which catalyzes Neu5Ac [5,6]and only accumulate in the normal human body by consuming daily food. Approximately 2 to 3 million years ago, the human gene encoding CMP-Neu5Ac hydroxylase mutated. The Neu5Gc content in red meat follows the order beef(231 μg/g) > pork (40 μg/g) > lamb (14 μg/g) [7]. Human intake of Neu5Gc can cause anti-Neu5Gc antibodies in the body and change the intestinal flora [8], thus promoting chronic inflammation and tumors [9,10]. Therefore, Neu5Gc is an important risk factor in red meat products.
The methods used to eliminate Neu5Gc in red meat mainly include thermal and nonthermal processing and synthetic pathway intervention.Typical thermal processing strategies, such as frying and microwaves,can reduce Neu5Gc by almost 70% in red meat, but the byproduct leads to health risks [11]. When the boiling time reached 2 h, the Neu5Gc content dropped by approximately 80%, but this released Neu5Gc into the free state and caused nutrient loss [12]. Varki et al. [13]proposed a method to reduce Neu5Gc content by adding sialic acid precursor Neu5Ac to intervene in the Neu5Gc enzyme synthesis pathway, but it was not economically viable. Recently, we observed that feeding Xiang pigs sialyltransferase inhibitor cytidine 5’-monophosphate after 30 days reduced Neu5Gc levels in muscle by 58.18% [14]. We also found that exoinulinase reduced the Neu5Gc content by (50.52 ± 0.88)% [15]. Above all, an effective and safe elimination method must still be identified.
Electrical stimulation, with the advantages of convenience and efficiency, is widely used in the meat slaughter process and safety control. It can enhance meat quality, including preservation [16],tenderness [17], postmortem glycolysis, and protein hydrolysis [18],reducing cold contraction and changing the protein structure [19].In the red meat industry, electrical stimulation treatment is usually used to improve tenderness after slaughter and includes high-voltage electrical stimulation (> 500 V), low-voltage electrical stimulation(45-100 V) and ultralow-voltage electrical stimulation (< 45 V) [20].In addition, the most used voltage frequencies are 14.5, 50, 60, and 100 Hz [21]. In the poultry meat industry, electrical stimulation stunning treatment is a good auxiliary slaughter tool. The stimulation voltage range is 45-150 V, with low frequencies (50-200 Hz) or high frequencies (633-1 500 Hz) [22]. In addition to the postmortem process synergy, electrical stimulation can also reduce the level of hazardous substances. Liu et al. [23]built a bio film-electrode reactor to degrade azo dye; when the direct electrical stimulation cultivating voltage for activated sludge cells was 2.5 V, the decolorization efficiency was 81.56%, which suggests that electrical stimulation can potentially reduce the hazardous substance content. However, the effect of electrical stimulation on Neu5Gc safety control in red meat is still unknown.
In this study, we first comprehensively evaluated the effect of electrical stimulation on controlling Neu5Gc contents in red meat. The handing time and intensity of electrical stimulation on the Neu5Gc content in red meat samples were both investigated.Further information on the electrical stimulation of Neu5Gc to induce structural property changes was simulated by density function calculations, and the structural properties studied mainly included structural parameters (bonds, charges, intramolecular hydrogen bonds,and the global activity index) [24].
2. Materials and methods
2.1 Chemical reagents
Neu5Gc, 1,2-diamino-4,5-methylene dioxybenzene (DMB),sodium thiosulfate, sodium sul fite, and 2-mercaptoethanol standards were purchased from Sigma-Aldrich, USA. Acetonitrile and methanol(chromatographic grade, AppliChem, Germany) were used. Glacial acetic acid, NaOH, and acetic acid (Analytical grade, Tianjin Komiou Chemical Reagent Co., Ltd., China) were used. Ultrapure water(Purification System, Shanghai Canrex Analytic Instrument Co., Ltd.,China) was used.
2.2 Electrical stimulation treatment performed on red meat
Beef tenderloin samples were purchased from a local supermarket(He Li supermarket, Guiyang, China) on the day of the experiment and stored at 4 °C. Blocks of approximately 400 g with a length,width and height of approximately 12 cm × 7.5 cm × 4.8 cm were prepared and frozen at -20 °C.
200 g of beef tenderloin was homogenized with a meat grinder(QSJ-B02R1, Xiao Xiong Electric Co., Ltd., China). Then, 1 g of processed beef in 10 mL was mixed by an electric glass homogenizer(DY89-II, Ningbo Xin Zhi Co., Ltd., China). Next, electrodes (Direct current system, MP3001D, China Instrument Co., Ltd., China) were inserted into the minced beef center to conduct electrical stimulation,and the system and treatment were regulated for 10, 20, 30, 40, and 50 s at a voltage of 90 V. Another treatment was performed for 50 s at 30, 60, 90, and 120 V, and the voltage frequency was 50 Hz.
2.3 Electrical stimulation treatment performed on Neu5Gc standards
The electrodes were placed directly in a 50 mL tube that contained 3 mL of Neu5Gc standard solution (300 μmol/L) to conduct electrical stimulation. The stimulation times evaluated were 30, 60, 90, 120,150 and 180 s, and the electric field intensities were 50, 100, 150,200, 250 and 300 V.
2.4 The Neu5Gc content detection
Neu5Gc was measured by HPLC fluorescence using an external standard through peak area normalization [25]. An HPLC system(1260, Agilent Technologies Co., Ltd., USA) equipped with a LiChrosorb RP-18 column (Merck KGaA, Germany) was used. The mobile phase was water, acetonitrile and methanol (85 : 8 : 7,V/V),and the flow rate was 0.9 mL/min. The excitation and emission wavelengths were 373 nm and 448 nm, respectively. The column temperature was 30 °C, and the injection volume was 10 μL. The derivatizing agent was a mixture of 8 mmol/L DMB, 1.5 mol/L glacial acetic acid, 0.25 mol/L sodium thiosulfate, 0.25 mol/L sodium sulfite and 0.8 mmol/L 2-mercaptoethanol. The standard curve was built with a series of Neu5Gc standard solutions at concentrations of 50, 100, 200, 300, and 400 μmol/L.
Detection procedure: Meat samples were freeze-dried for 48 h.Then, 10 mL of 30% saturated ammonium sulfate solution was added to precipitate the protein at 25 °C for 1 h. After hydrolysis, the samples were freeze-dried for 48 h again and mixed with 10 mL of 2 mol/L acetic acid solution to release Neu5Gc in a water bath at 80 °C for 3 h. Following centrifugation and filtration, the supernatant was freeze-dried to a powder. Next, the powder was dissolved in 1.0 mL of ultrapure water and 0.2 mL of 0.1 mol/L NaOH at 37 °C for 30 min for deacetylation. After membrane filtration was achieved with a 0.22 μm filter, 900 μL of 100 μL DMB was added to the derivative at 50 °C for 150 min.
2.5 The color and Warner-Bratzler shear force changes
The color and Warner-Bratzler shear force changes of red meat samples after electrical stimulation treatment were measured by a portable colorimeter (High-quality Colorimeter NH350, Beijing Western Yuanda Science and Technology Co., Ltd., China) and digital meat tenderness meter (C-LM3B, Northeast Agricultural University,China), respectively. The CIE color coordinates of lightness (L*),redness (a*), and yellowness (b*) were applied to evaluate the meat samples (1 cm-thick slices) at room temperature (approximately 25 °C). The control groups were not subjected to electrical stimulation.
2.6 Electrical stimulation simulation and DFT calculation
In general, the electrical stimulation process is dominated by the electric field effect [26,27], which can impact the molecular structure(geometry, bond energy, Hamiltonian, and electron density) and active parameters by the redistribution charge and the dipole moment.These electronic structure and activity parameter changes can be studied by DFT calculations. Therefore, electrical stimulation of the external electric field was simulated by DFT calculations to explore the changes in the Neu5Gc structure.
Firstly, electronic structure parameters were calculated.Geometry optimization with frequency vibration analysis and the natural population analysis (NPA) of Neu5Gc were performed at the M062X/6-311G (d, p) theory level under external electric fields of 0.005, 0.01, 0.015, 0.02, 0.025, 0.03 a.u. (1 a.u. = 51.423 V/angstrom),which range from 0.257 V/angstrom to 1.80 V/angstrom. To avoid disordered movement, Neu5Gc was fixed and always oriented in the external electric field direction. The external electric field direction was determined at theXaxis of the Cartesian coordinates because there are more atoms than theYaxis.
Based on the optimized structure with no imaginary frequency,the O-H bond length and bond dissociation energy (BDE) values were obtained. The accurate single point energy was calculated at the M062X/def2TZVP level. For enthalpy values, the vibrational zeropoint energy (ZPE) scale factor was 0.97 [28].

In equation (1), whereH(MO·) represents the enthalpy of the free radical fragment after dehydrogenation,H(MOH) is the maternal molecular enthalpy [29]. The molecular Hamiltonian represents the energy of the system electrons and nuclei. Under an external electric field, the Hamiltonian of the molecular system is shown in equation (2). When taking the dipole approximation, the Hamiltonian interaction between the molecule and the external electric field intensity (E) is shown in equation (3) [30].

H0refers to the Hamiltonian out of the external electric field,andHintrefers to the Hamiltonian of the interaction between the external electric field and molecule.μis the electric dipole moment of the molecule. The Hohenberg-Kohn theorem confirmed that the densityρis the fundamental property that characterizes the ground state of a system. Bader’s atoms in molecules (AIM) theory states that the electron density distribution is related to the chemical bond type. The bond critical point (BCP) occurs between attractive atom pairs. The Laplacian electron density (∇2ρ) plays an important role in the characterization of chemical bonding. Equation (4) shows the relationships between energy topological parameters and ∇2ρ(r) at BCPs [31].

whereG(r),V(r), andρ(r) are the kinetic energy, potential energy,and total electron energy densities, respectively. For a closed shell system, a positive ∇2ρindicates a hydrogen bond. The bond energyEHBcan be described asEHB= 1/2V(rBCP) in kcal/mol.
The electron density changes were characterized by a molecular orbital distribution. According to the frontier molecular orbital theory, the energy of the highest occupied molecular orbital (EHOMO)characterizes the ability to donate electrons. The larger theEHOMOis,the stronger the electron donation ability. The energy of the lowest unoccupied molecular orbital (ELUMO) indicates that the molecule attracts electrons. The lower theELUMOis, the weaker the electron attraction ability. The energy gap was the difference betweenELUMOandEHOMO, which is related to the molecular reactive activity. Here,three different electric field intensities of 0.01, 0.02, and 0.03 a.u were selected to investigate the changes after applying an electric field to Neu5Gc.
Secondly, the concept density functional theory (CDFT)was used to investigate the activity parameters of Neu5Gc under an external electric field, which include local (Fukui functions)and global parameters (electronegativityχ, chemical potentialμ,chemical hardnessη, chemical softnesss, and electrophilic indexω).The molecular chemical potential values are equivalent to the electronegativity of the element when they are calculated by density functional theory. Based on the Pearson HASB (hard and soft acids and bases) concept, the chemical hardness is proportional to the band gap of a chemical system and can be calculated by the ionization potential and the electron affinity. Koopmans’ theorem states that the ionization potential and electron affinity can be approximately replaced by the HOMO and LUMO energies, respectively [32]. Using a finite difference method, the global descriptors can be approximated byEHOMOandELUMObased on Koopmans’ theorem. The calculation formulas (5-9) are as follows [33]:

2.7 Software and data analysis
All experimental data were based on three replicate measurements and are presented as the mean ± SD. One-way analysis of variance(ANOVA) was performed to determine statistically significant differences using SPSS 23.0 (SPSS Inc., Chicago, IL, USA) with a 95% confidence level (P< 0.05). The theoretical calculations were performed on Gaussian 16 (Revision A. 03) software provided by the Guizhou University high-performance computing cluster [34].The AIM analysis of the BCP and CDFT index was performed by Multiwfn 3.7 software on a personal computer [35]. The orbital isosurface maps were generated by the VMD 1.9.1 program. All the data were plotted by Origin Pro 9.0 software.
3. Results and discussion
3.1 Effect of electrical stimulation on the Neu5Gc content
Neu5Gc exists in red meat with conjugated and free states;therefore, the electric stimulation treatment was also imposed on the Neu5Gc standard aqueous solution to examine the direct degradation effect. For parameter optimization, different electrical field intensities and treatment times were both investigated. The built Neu5Gc calibration curve is shown in Fig. S1.
3.1.1 The effect of electrical stimulation intensity
In red meat, there are many matrix compounds, such as proteins,peptides and fats, that lead to different current conduction rates and thermal effect under electric stimulation. Therefore, the optimal voltage on standard aqueous solution was slightly different to the red meat samples. When meat samples were gradually treated with voltages from 30, 60, and 90 V to 120 V for 50 s, the content of Neu5Gc was significantly reduced (P< 0.01). As the voltage increased, the content of Neu5Gc continued to decline (Fig. 1A). The Neu5Gc content in meat samples decreased from 70.19 to 18.08 μg/g at 120 V, which is equivalent to a reduction of (74.24 ± 0.69)%.Consistently, the Neu5Gc standard solution was handled for 50 s under voltages of 50, 100, 150, 200, 250 and 300 V. The result is shown in Fig. 1B. Neu5Gc changed slightly when the voltage reached 50 V but decreased significantly (P< 0.01) at 100 V as the concentration decreased from (279.89 ± 0.09) μmol/L to (221.52 ± 1.22) μmol/L.When the voltage continued to increase, the Neu5Gc content continued to decrease, but this decrease was not significant (P> 0.05)at 200 V or 250 V, and the corresponding content was (171.48 ±4.66) μmol/L and (171.93 ± 3.46) μmol/L. When the voltage reached 300 V, the Neu5Gc content was significantly lower (P< 0.05) than that in all previous treatment groups, and the Neu5Gc content decreased to(114.41 ± 0.59) μmol/L, which is equivalent to a reduction of (59.12 ±0.15)% compared to that in the control group. Therefore, as the electric field strength increases, the reduction effect improves.

Fig. 1 Neu5Gc content changes under different electrical stimulation treatment intensity. (A for red meat sample, treatment for 50s; B for Neu5Gc standard solution, treatment for 50 s; Different uppercase letters indicate P < 0.01.)
3.1.2 The effect of electrical stimulation time
To determine the best processing time for red meat samples, the voltage intensity was set at 90 V based on intensity trial results. With changing the electrical stimulation time from 10, 20, 30, and 40 s to 50 s, the Neu5Gc content in meat samples continued to decrease(Fig. 2A). The content of Neu5Gc in all samples was significantly lower than that in the control group (P< 0.01). As the electrical stimulation duration reached 50 s, the Neu5Gc content decreased from 47.19 μg/g to 31.55 μg/g.
For Neu5Gc standard solutions, according to the field intensity results, a voltage of 300 V was chosen for 30, 60, 90, 120, 150, and 180 s. As time increased, the content of Neu5Gc in all experimental groups was extremely significant (P< 0.01) and lower than that in the blank group (Fig. 2B). This indicated that electrical stimulation could effectively reduce the Neu5Gc content. The Neu5Gc concentration was reduced from (241.79 ± 0.21) μmol/L to (177.20 ± 0.29) μmol/L after treatment for 30 s. When the treatment time was extended to 180 s, the Neu5Gc concentration declined to (99.63 ± 0.33) μmol/L,which is equivalent to a reduction of (58.79 ± 0.10)%. Therefore, an electrical stimulation processing time of 60 s could obtain a better effect on both red meat and Neu5Gc standard solutions.
3.2 The color and shear force changes
To obtain a good balance of processing time, intensity and meat quality, the quality evaluation was conducted. As shown in Fig. 3, when handling the meat samples at 100 V or 300 V for 60 s,the colors remained stable, and the lightness (L*), redness(a*) and yellowness (b*) changed slightly. Tenderness is an important indicator of meat eating quality, which affects consumer preference. From Fig. 3, the sharply decreased shear force reflects a tenderness rise as the voltage intensity increases, which is consistent with reports that electrical stimulation can improve meat tenderness [36].In summary, electrical stimulation treatment of red meat at 300 V for 60 s can not only reduce the content of Nue5Gc but also slightly impact meat eating quality.
3.3 Neu5Gc structural properties under electrical stimulation
3.3.1 The optimized structure of Neu5Gc
Combining the experimental results above, the Neu5Gc content reduction may be related to the electric field that formed during electrical stimulation. Density function theory calculations, which can model and gain insight into the reaction mechanism, were applied to simulate the Neu5Gc molecular electronic structure changes under an external electric field. Based on our previous work, the M062X function with 6-311G (d, p) basis sets can well describe the ground state molecular electronic structure of sialic acid compounds [37].The optimized structure with Cartesian coordinate arrows is shown in Fig. 4. Red represents oxygen, black represents carbon, blue represents nitrogen, and white represents hydrogen.

Fig. 4 Optimized Neu5Gc molecular structure.
3.3.2 The natural population charge changes
The NPA analysis of Neu5Gc under an electric field is listed in Table 1. From the +Xaxis results, when the electric field intensity was within the 0.01-0.03 a.u., the charge values of O3, O5, O6, O7 and O9 atoms were all larger than those at 0 a.u., while those of the O2 atoms gradually decreased. Charge transfer loss is related to bond breaking [38], thus weakening the O-H bond of these O3, O5,O6, O7, and O9 atoms, which was consistent with the bond length change results. As the electric field intensity increased in the -Xaxis direction, the charge values of O2, O7 and O10 atoms gradually decreased, while those of the O3 and O5 positions continued to increase. It was obvious that the electric field impacted most oxygen atoms on the +Xaxis. The electron transfer of Neu5Gc varies in different electric field directions, which is similar to the results of the NBO (natural bond orbital) charge analysis of phenalenyl π-dimers under an external electric field [39].

Table 1The Neu5Gc natural charge distribution under X axis external electric field.
3.3.3 The O-H bond length changes
Neu5Gc is a hexose derivative rich in hydroxyl groups. In the +Xaxis electric field, the O-H bond length at the O2, O5, O10, and O7 positions increased with increasing electric field intensity (Fig. 5A).Among them, the length of the O-H bond at the O2 position increased the most. The O-H bond length at O4 sites possessed maximum values at 0.02 a.u., while that at the O6 position increased after an initial decrease. In the -Xaxis electric field, the bond lengths at the O10, O6, O3, O7, O5, and O4 positions all increased with increasing electric field strength (Fig. 5B). Among them, the bond lengths at the O10 and O6 positions increased obviously, which may be caused by charge redistribution across the electric field direction. However,although the hydroxyl group at the O2 position was oriented closer to the -Xaxis when the electric field was 0-0.03 a.u., the O-H bond length also increased, indicating that the bond strength mainly depended on the electric field strength [40].
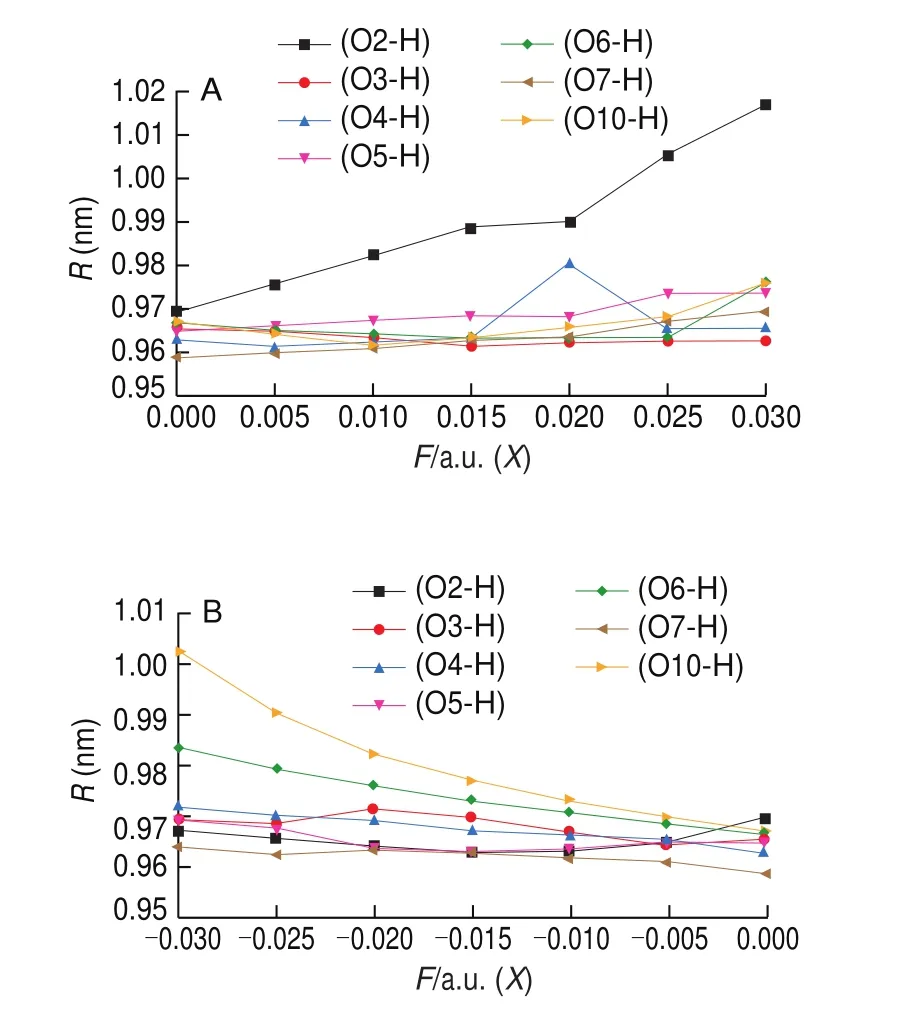
Fig. 5 Effect of electric field on Neu5Gc O-H bond distance (A for the +X axis and B for -X axis).
3.3.4 The bond dissociation energy changes
The BDE values are referred to as the bond disruption energy,which can quantitatively characterize the chemical bond. Here, we evaluated the bond strength changes under a stimulation-induced external electric field. From the calculated results in Table 2, the BDE values of all Neu5Gc O-H bonds under the external electric field were lower than before, indicating that the electric field has a reduction effect on the molecular bond dissociation energy. When the electric field strength was 0.1 a.u., the O-H BDE at the O4 position was the lowest. When the electric field strength was 0.025 a.u., the O-H BDEs at the O2, O3, O5, O6, and O10 positions all reached minimum values. In the range of 0-0.025 a.u., the O7 site has the lowest BDE value of 396.501 6 kcal/mol at 0.05 a.u. However, it changes slightly as the electric field intensity increases further, indicating that the O7 site is easily affected by the electric field. Schirmer et al. [41]also showed that the BDE between two hydrogen atoms can be significantly reduced or even disappear under a certain external electric field.Therefore, the above results infer that electrical stimulation can change the strength of the main Neu5Gc chemical bond.
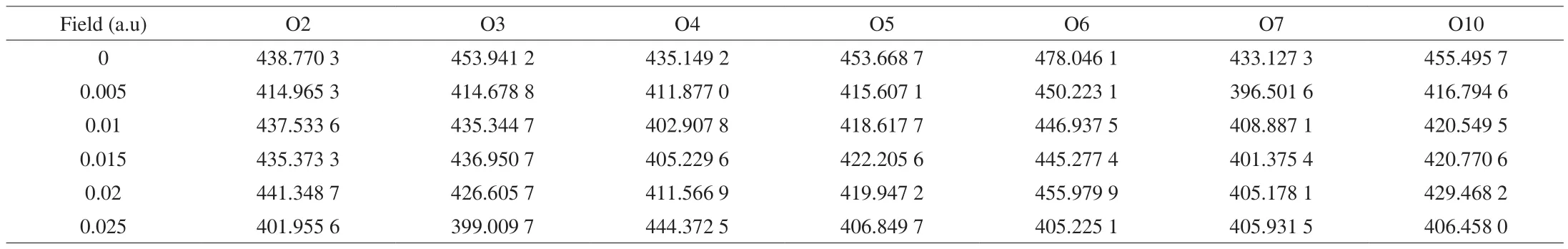
Table 2Effect of external electric field on Neu5Gc O-H bond dissociation energy (kcal/mol).
3.3.5 The total energy changes
Under the external electric field, the total energy of Neu5Gc was the sum of the potential energy and kinetic parameters. In general, the Hamiltonian represents the kinetic and potential energy (dissociation and attraction), and the calculated Neu5Gc results are shown in Fig. 6.From the results, as the electric field strength increased at the +X(0-0.03 a.u.) or -X(-0.03-0 a.u.) axis, the Hamiltonian of Neu5Gc increased, indicating that the total energy also increased, which is similar to the case of CdSe under the external electric field strengths ranging from 0 to 0.2 a.u. [42].

Fig. 6 Neu5Gc total energy changes under X axis external electric field (A for the +X axis and B for -X axis).
3.3.6 The dipole moment changes
The molecular dipole moment can re flect the charge distribution and polarizabilities and is also related to the potential energy parameters [43]. Under an external electric field, the dipole moment changes mainly through the dipole-induced effect and increases with the electric field intensity [44]. The calculated result is shown in Fig. 7.From the picture, as the field intensity increases, the molecular dipole moment tends to increase in both the positive and negative directions of theXaxis. The Neu5Gc dipole moment increased from 4.63 D to 11.94 D in the +Xaxis direction, while it increased from 4.63 D to 15.81 D in the -Xaxis direction. This may be related to the molecular changes under the induced polarization effect.

Fig. 7 Neu5Gc dipole moment changes under X axis external electric field (A for the + X axis and B for -X axis).
3.3.7 The intramolecular hydrogen bonding changes
Intramolecular hydrogen bonds are related to many aspects of molecular stability, such as melting point thermal stability and conformation. Here, the Neu5Gc intramolecular hydrogen bonds were calculated under an electric field, and the results are shown in Table 3.In AIM theory topological analysis, the positive Laplace electron density (∇2ρ) at bond critical points (BCPs) means that hydrogen bonds exist. The hydrogen bonds can be divided into different strengths: weak hydrogen bonds (EHB< 12.0 kcal/mol, λ2(rBCP) > 0,G(rBCP) +V(rBCP) > 0), medium hydrogen bonds (12.0

Table 3The Neu5Gc intra-molecular hydrogen bond under X axis external electric field.
From Table 3, all Laplace density values were positive, and all electron density values were in the range of 0.002-0.04 a.u.,indicating that the hydrogen bonds were not strong. Furthermore,the hydrogen bond -G(rBCP)/V(rBCP) values were larger than 1 under the selected electric field, showing that the hydrogen bonds were noncovalent. The distance and angle of the bonds also met the general criterion of hydrogen bonding. According to theEHBvalues, the strength of the N11-H30... O5 and O3-H34... O1 hydrogen bonds gradually decreased for an electric field of 0.005-0.015 a.u., the same tendency as the hydrogen bond distance, showing that the electric field can reduce the intramolecular hydrogen bond strength.
Compared to the result in the positive electric field direction,Neu5Gc hydrogen bond numbers decreased in the -Xaxis direction at -0.02 a.u and -0.025 a.u. When the field intensity reaches -0.025 or -0.03 a.u., only one hydrogen bond occurs. For the hydrogen bondEHBvalues, the total values at 0, 0.005, 0.01, 0.015, 0.02, 0.025, and 0.03 a.u. were -127.24, -131.81, -79.01, -52.13, -81.92, -50.1, and-24.68 kcal/mol, and the total values at -0.005, -0.01, -0.015, -0.02,-0.025, and -0.03 a.u. were -102.97, -84.74, -51.38, -50.39, -31.83,and -34.37 kcal/mol, respectively. It was obvious that the Neu5Gc intramolecular hydrogen bond strength decreased with increasing electric field strength. Correspondingly, the initial total Laplace electron density of Neu5Gc at 0 a.u. was 0.458 8 a.u., and it changed from 0.457 1 to 0.093 2 a.u. at 0.005-0.03 a.u. and decreased from 0.395 2 to 0.125 1 a.u. at (-0.005)-(-0.03) a.u. From the changes in electron density, the reasons for the strength reduction may be related to the intramolecular charge transfer of Neu5Gc hydrogen bond donor and acceptor atoms under the electric field.
3.3.8 The Neu5Gc HOMO and LUMO distribution
In frontier molecular orbital theory, the HOMO and LUMO represent the ability to donate and attract electrons, respectively.Choosing 0, 0.01, 0.02, and 0.03 a.u. and calculating the corresponding HOMO/LUMO orbital of Neu5Gc, the results are shown in Fig. 8, where light green indicates the positive phase and light blue indicates the negative phase. From Fig. 8, regardless of the electric field, the HOMO and LUMO are distributed near N11-C21=O9 (amide group) and O8=C19-O6 (hydroxyl group),respectively. However, the isosurface sizes were significantly different, indicating that the external electric field can impact the Neu5Gc molecular orbital properties. Furthermore, the HOMO and LUMO isosurface sizes decreased as the electric field intensity increased, providing evidence of charge transfer. This was consistent with the NPA analysis calculation results.

Fig. 8 Neu5Gc HOMO and LUMO diagram under the external electric field(light green for positive phase, light blue for negative phase).
3.3.9 The global activity index changes
The calculated global activity parameters under the external electric field are listed in Table 4. The energy gap (EHOMO-ELUMO)can reflect the molecular reactive activity, and lower Egap values indicate higher reactivity. The electronegativity, χ, corresponds to the ability of an atom to attract shared pair electrons. The higher the electronegativity is, the more attractive the electrons. The chemical potential,μ, can reflect the electron ionization and affinity ability. The electrophilic index,ω, describes the ability of the electrophile to obtain electrons;ω> 1.5 eV corresponds to strong electrophiles, 0.8 <ω< 1.5 eV corresponds to moderate electrophiles, andω< 0.8 eV corresponds to marginal electrophiles [46].

Table 4The global activity index of Neu5Gc under X axis external electric field (unit, eV).
From the Table 4 results, the Neu5Gc Egap values decreased as the external electric field strength increased, which was consistent with the frontier molecular orbital distributions and revealed that the increase in electrons resulted in a loss of ability. This was similar to the results of the conformational analysis of diphenylacetylene under a series of external electric fields [47]. In the +Xaxis direction, as the electric field strength increased, the Neu5Gc Egap decreased from 9.65 eV to 0.71 eV, and the values ranged from 9.65 eV to 5.19 eV along the –Xaxis. This indicated that Neu5Gc becomes more active in the electric field +Xaxis directions.
The Neu5Gc molecular hardness decreased with electric field strength in theXaxis direction. In contrast, softness gradually increased with increasing electric field intensity. When the electric field intensity gradually increased, the electronegativity and chemical potential changed slightly in the +Xaxis direction and reached their minimum at 0.025 a.u. Both increase in the -Xaxis direction,showing that the electric field direction can also impact the electron attraction ability [48]. For the electrophilic index, the empirical rules show that molecules with an electrophilic index ofω> 1.5 eV can act as strong electrophiles and favor polar reactions [49]. From Table 4, theωvalues of Neu5Gc were all larger than 1.5 eV and increased sharply at an electric field intensity of 0.03 a.u., indicating that Neu5Gc was more prone to be electrophiles under an external electric field.
4.Conclusion
In this study, we evaluated the Neu5Gc reduction effect by conducting electrical stimulation with a red meat sample and Neu5Gc standard solutions. To gain deep insight into the mechanisms of Neu5Gc under electrical stimulation, the molecular structure and activity parameters were calculated by theoretical simulations. The electrical stimulation perturbation experiment showed that when treating meat samples at 120 V for 50 s, (74.24 ± 0.69)% of Neu5Gc could be removed, and a certain sensory quality could be maintained.Calculations show that the O-H bond length of Neu5Gc mainly increased in theXaxis direction under the electric field, contributing to the charge distribution changes and the decrease in BDE. The AIM theory revealed that the intramolecular hydrogen bond lengths of Neu5Gc also decreased by electric stimulation. Meanwhile, the total energy and the intermolecular dipole moment increased under the electric field. Finally, the CDFT theory results indicate that the global activity of Neu5Gc increased under an electric field. Above all, electrical stimulation could directly impact the Nue5Gc molecule through charge transfer and the induced polarization effect. With the development of green technologies in food production and processing,safety control tends to be effective, environmentally friendly and convenient. Our study results may help with electrical stimulation applications in the red meat processing industry.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
The author thank to the National Natural Science Foundation of China (No. 31660496), the author thank to the High-level innovative talents training project of Guizhou province-“Hundred” level talents(QKHPTRC[2016]5662).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.03.023.
- 食品科学与人类健康(英文)的其它文章
- Dietary bioactives and essential oils of lemon and lime fruits
- Green tea, epigallocatechin gallate and the prevention of Alzheimer’s disease: clinical evidence
- Simultaneous quantification of 18 bioactive constituents in Ziziphus jujuba fruits by HPLC coupled with a chemometric method
- A systematic study on mycochemical profiles, antioxidant, and anti-inflammatory activities of 30 varieties of Jew’s ear (Auricularia auricula-judae)
- GPP (composition of Ganoderma lucidum polysaccharides and Polyporus umbellatus polysaccharides) protects against DSS-induced murine colitis by enhancing immune function and regulating intestinal flora
- Immunoregulatory polysaccharides from Apocynum venetum L.flowers stimulate phagocytosis and cytokine expression via activating the NF-κB/MAPK signaling pathways in RAW264.7 cells

