Positive effect of ethanol-induced Lactococcus lactis on alcohol metabolism in mice
Sisi Chn, Shimin Ji, Kk Suo, Qiozhn Kng, Limin Ho*,Lizhng Lu, Xin Liu, Jinyong Hung, Jik Lu,*
a School of Life Sciences, Zhengzhou University, Zhengzhou 450001, China
b Physical Education College, Zhengzhou University, Zhengzhou 450001, China
c The Quartermaster Research Institute of Engineering and Technology, Academy of Military Sciences PLA China, Beijing 100010, China
d Zhengzhou Mindtek Biotechnology Co., Ltd., Zhengzhou 450001, China
e School of Agricultural Sciences, Zhengzhou University 450001, China
Keywords:
Ethanol stress
Intracellular extracts
Alcohol dehydrogenase
Alcohol metabolism
A B S T R A C T
It is well known that exposure to environmental stresses could enhance the adaptability of bacteria and up-regulate the expression of a variety of oxidative stress-related genes and antioxidant enzymes. It is unclear whether the adaptability of microorganisms formed naturally in special environments could transfer to other organisms. The study aimed to evaluate the effects of untreated and ethanol-induced Lactococcus lactis intracellular extracts (U-IE and E-IE) on alcohol metabolism in mice. The positive effects of E-IE on alcohol metabolism in mice were revealed by the enhanced latency of loss of righting reflex (LORR), the reduced duration of LORR, the decrease of blood alcohol concentration, as well as the elevation of alcohol dehydrogenase (ADH) activities in the stomach and liver tissues. Furthermore, the potential benefits of E-IE on the liver were evaluated by biochemical parameters including the activities of serum transaminase, the levels of antioxidant enzymes, and the pathological changes of liver tissue. The present work put forward a new point that appropriate ethanol stress could enhance the intracellular ADH activity of L. lactis, and its intracellular extracts could continue to enhance alcohol metabolism in mice.
1. Introduction
Lactic acid bacteria (LAB) have a variety of biological activities and play an essential role in the fermentation process of numerous foods and beverages. More and more studies have proven that LAB can alleviate alcoholic liver damage by improving gastrointestinal function in mice [1-3], reducing oxidative stress, and regulating inflammation level [4]. The heat-killedLactobacillus brevisstrain SBC8803 could alleviate alcoholic liver damage via inhibiting the accumulation of liver fat [5]. Nowadays, researches on LAB are mainly about the applications of intact cell and fermentation supernatantin vitroorin vivo[6], while few studies are reported on the intracellular extracts. Some studies have shown that intracellular extracts of LAB have more anti-pathogenic effects, antioxidant activity, and stability during storage time [7]. During the industrial processes, LAB could be exposed to some environmental stresses, such as low or high temperatures, oxidative stress, high osmotic pressure,and the presence of ethanol [8,9]. Previous research in our lab showed that the intracellular extracts ofL. lactisunder gradient freezing stress had a protective effect onL. lactisin the frozen environment [10].Some studies also indicated that appropriate osmotic pressure could improve the antioxidant activity ofPediococcus pentosaceusR1 [11]and induce the expression of oxidative stress genes and antioxidant enzymes [12]. From this point of view, ethanol-inducedL. lactisintracellular extracts (E-IE) might have positive effects to improve alcohol metabolism compared with untreated intracellular extracts (U-IE) (Fig. 1).
Fig. 1 Schematic representation of E-IE promoting alcohol metabolism in mice.
Acute heavy drinking does much harm to human health, which may cause gastrointestinal damage, alcoholic hepatitis, liver steatosis,cirrhosis, and hepatocarcinoma [13]. Alcohol-induced oxidative stress is linked to the metabolism of alcoholin vivo. It is known that about 90% of alcohol metabolism occurs in the liver, and alcohol is eliminated and degraded via both multiple enzymatic pathways and non-enzymatic pathways. Alcohol is firstly metabolized into acetaldehyde mainly through alcohol dehydrogenase (ADH), and then acetaldehyde is converted to acetic acid with the catalysis of the aldehyde dehydrogenase (ALDH) [14]. Furthermore, researchers had suggested that ADH rather than ALDH was the major rate-limiting factor in alcohol metabolism, and the activity of ADHin vitro, at least in part, was an indication of the ADH alcohol metabolismin vivo[15].
This study aimed to investigate whether E-IE had a positive effect on ADH activityin vitroand alcohol metabolismin vivocompared with U-IE. Then the underlying mechanism was assessed by bioinformatics tools.
2. Materials and methods
2.1 Preparation of intracellular extracts
L. lactiswas supplied by Zhengzhou Mindtek Biological Technology Co., Ltd. (Zhengzhou, China) and grown at 33 °C for 16–24 h. Gradient ethanol treatment was carried out by adding absolute ethanol, and the final ethanol concentration in the medium was 0%–2.0%. The growth phase was determined by monitoring the optical density at 600 nm (OD600nm), using a spectrophotometer(UV 3600, Shimadzu, Japan). Then the original growth medium was removed by centrifugation at 4 000 r/min for 10 min. The cell pellets were washed in 75 mmol/L PBS buffers and the two previous steps were repeated three times. The cell pellets were resuspended in the PBS buffer again and disrupted using ultrasonication for 40 min,and then the suspension was centrifuged at 12 000 r/min (4 °C) for 10 min. The intracellular extracts were sterilized by filtration through a 0.22 μm microporous membrane and freeze-dried. The samples of U-IE and E-IE were stored at –20 °C for the following experiments.
2.2 Determination of ADH activity
ADH activity was determined by the modified method of Zhao et al. [16].In brief, 1.5 mL sodium pyrophosphate solution (0.1 mol/L,pH 8.8), 0.1 mL of 0.25 U/mL ADH, 0.5 mL ethanol (0.67 mol/L),and 0.1 mL samples were mixed at 37 °C, and then 1.0 mL NAD+(27 mmol/L) was added to initiate the reaction. The absorbance was immediately measured at 340 nm for 5 min continuously. The absorbance in the presence of H2O was measured as the control. One milliunit (mU) of the enzyme activity of ADH corresponds to 1 nmol NADH produced per minute, based on the extinction coefficient of 6.22 mmol/L·cm for NADH at 340 nm. The activity was expressed as a percentage compared to the control.
2.3 Animal experiments
Animal experiments were carried out according to the regulations of the Experimental Animal Welfare and Ethics Committee of the Chinese Association for Laboratory Animal Sciences. Male specific-pathogen-free (SPF) KM mice (8 weeks old, 18–22 g)were obtained from the Henan Experimental Animal Center (Permit number: SCKK (Yu) 2017-0001). All mice were grown in a clean room with no specific pathogens. The room temperature was 21–25 °C, and the dark/light cycle was 12 h. The mice had free access to sterile feed pellets and distilled water.
As showed in Fig. 2, after acclimation for 7 days, mice were randomly divided into 5 groups with 10 mice in each group [17].The control group was given saline, while the model group was given alcohol (Red Star Er Guo Tou White Spirit, 56%,12 mL/kg bw, Beijing Hongxing Limited by Share Ltd., China).The positive group was given bifendate at 150 mg/kg bw [18]. At the same time, the U-IE and E-IE groups were given corresponding intracellular extracts at doses of 200 mg/kg bw. These groups were intragastrically administered 60 min before each alcohol ingestion,once a day, for 7 days. The body weight of mice was monitored daily until the end of the experiments. The time of loss of righting reflex(LORR) was recorded on the first day. The blood samples were collected into sodium citrate anticoagulant tube from the eyeballs for the alcohol concentration determination, and the blood samples were centrifuged at 6 000 r/min at 4 °C for 5 min to collect serum on the last day. The liver and stomach tissues were dissected immediately after mice were sacrificed. These tissues were washed instantly with ice-cold PBS (pH 7.4) and stored at -80 °C until future assays after the weights were recorded (body weight and liver index). Meanwhile,a part of liver tissue was rapidly fixed in 10% neutral formalin buffer for pathological examination.
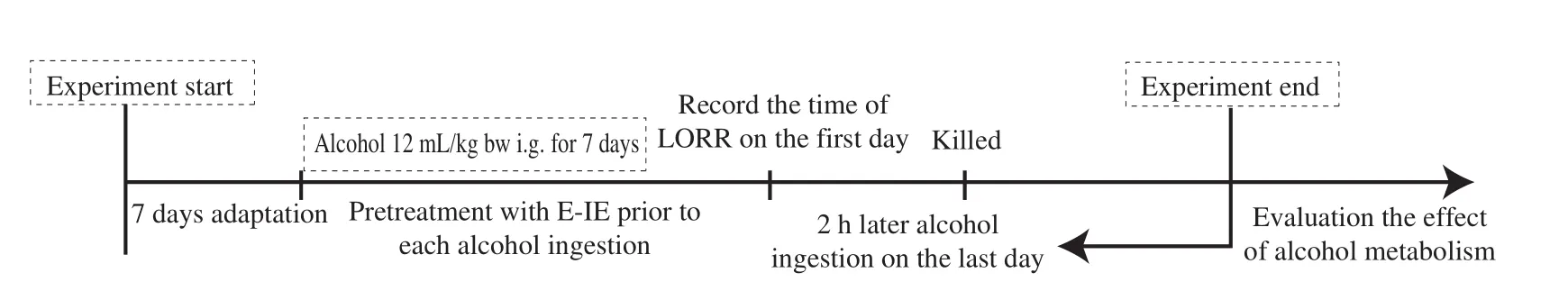
Fig. 2 Experimental design on alcohol metabolism in mice. After acclimatization for 7 days, all mice were randomly divided into 5 groups with 10 mice in each group. These groups were administered intragastrically 60 min before each alcohol ingestion, and alcohol was given for 7 days continuously except for the control group. The time of LORR was recorded on the first day. The liver and stomach tissues were dissected out immediately after mice were sacrificed on the last day.
2.4 Determination of blood alcohol concentration
The blood alcohol concentration was determined using the gas chromatography (GC) method [19]. Blood samples (0.5 mL), 0.1 mL 10.0 mol/L tertiary butanol, 1.0 mL DMSO and 2.0 g sodium sulfate were added to the tube. Then, the suspension was centrifuged at 12 000 r/min (4 °C) for 10 min before GC analysis. The blood alcohol concentration was analyzed using a GC-2010 plus system (Shimadzu,Japan) equipped with an SH-Rtx-Wax capillary column (30 m × 0.25 mm,0.25 μm, Shimadzu, Japan) and a flame ionizing detector (FID).The column temperature was set at 70 °C and maintained 5 min, then heated to 200 °C at 60 °C/min and maintained 6 min. The temperatures of the injector and detector were set at 250 °C.
2.5 Liver index assays
The mice liver index was calculated based on the following formula:

2.6 Liver and stomach biochemical assays
Liver and stomach tissues were mixed with nine volumes of saline (m/V), and homogenized in an ice-water bath. The prepared 10% homogenate was centrifuged at 3 500 r/min and 4 °C for 10 min, and the supernatant was collected. The activities of gastric and hepatic ADH, superoxide dismutase (SOD) and malondialdehyde (MDA)in mice were determined respectively with commercial kits (Jiancheng Bioengineering Institute, Nanjing, China).
2.7 Serum biochemical assays
The activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined using commercial kits (Jiancheng Bioengineering Institute, Nanjing, China).
2.8 Histopathology analysis
For histopathological analysis, the liver tissues were fixed in 10% neutral formalin buffer for over 24 h and embedded in paraffin wax. The paraffin sections were cut and stained with hematoxylin and eosin, and a subsequent examination was conducted under the light microscope for histological analysis (Digital Sight DS-Fi2, Nikon, Japan).
2.9 Interactions network of ADHs-related proteins
The STRING database was used to find the protein-protein interactions (PPIs) networks between ADHs and other proteins(http://string-db.org/). Here, it provided a summary of all the evident channels used to create the link between the proteins. A brief description of the color codes and the representative nodes for each protein was used to draw the network. The lines were set to represent the confidence in the association occurrence, and the results from the 7 evident channels were combined and represented as one single line. In STRING, PPIs that were annotated with one or more “scores”could judge an interaction as true or wrong, given the available evidence. All scores rank from 0 to 1, with 1 being the highest possible con fidence.
2.10 Statistical analysis
The data were expressed as mean ± SD and examined for their statistical significance of difference with variance (ANOVA) andttests (and nonparametric tests) by GraphPad Prism 8.P≤ 0.05 was considered to be statistically significant.
3. Results
3.1 Growth curves of L. lactis in MRS with ethanol
To determine the effect of ethanol on the growth of bacteria,untreated and ethanol-inducedL. lactiswere cultured, and their intracellular extracts were collected during the stable growth phase.As shown in Fig. 3a, with the increased ethanol concentration, the duration of the lag phase was elongated, indicating that the presence of ethanol resulted in growth inhibition. The bacterial biomass of other groups (0%, 0.5%, 1.0% and 1.5%) was the same during the stable growth phase (20–24 h), which excluded the 2.0% group

Fig. 3 (a) Growth curves of Lactococcus lactis in MRS broth with ethanol, and (b) the effects of U-IE and E-IE on ADH activity in vitro. Data were expressed as mean ± SD (n = 3). Significant differences from 0% group were indicated by *P < 0.05, **P < 0.01.
3.2 Effects of U-IE and E-IE on ADH activity
Intracellular extracts of LAB could remarkably increase ADH activityin vitro, and the ADH activity depends on the concentration of bacteria [20]. The effect of U-IE and E-IE on ADH activity was evaluatedin vitro. The result of U-IE (0%) on ADH activity was (20.00 ± 0.66)%, while the effects of E-IE (0.5%, 1.0%, 1.5% and 2.0%) on ADH activity were(33.30 ± 0.95)%, (23.3 ± 0.2)%, (21.70 ± 0.15)% and (16.70 ± 0.35)%,respectively. The results showed that U-IE and E-IE could remarkably increase ADH activity (P< 0.01), and the best stress concentration of ethanol was 0.5% (V/V) (Fig. 3b). Based on the above findings, it was necessary to investigate whether E-IE had similar positive effects on alcohol metabolism by animal experiments.
3.3 Loss of righting reflex and alcohol concentration
The alcohol concentration was determined 2 h after the last alcohol ingestion on the seventh day. Alcohol could act on the central nervous system and cause many behavioral problems including LORR [21]. Meanwhile, anin vivoexperiment indicated that the pre-administration intragastrically of E-IE could significantly promote alcohol metabolism by the enhanced latency of LORR, the reduced duration of LORR, and the decrease of blood alcohol concentration (Table 1). Results of alcohol metabolism further con firmed our conjecture that E-IE could play a positive effectin vitroandin vivo.
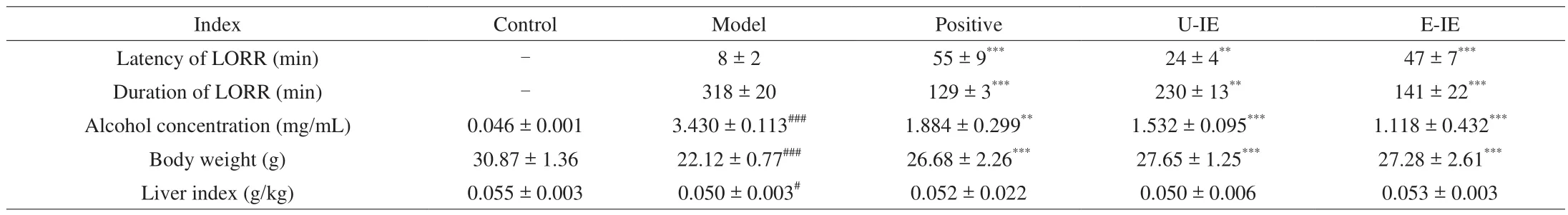
Table 1Effects of U-IE and E-IE on the LORR, alcohol concentration, body weight and liver index in mice.
3.4 Effects of U-IE and E-IE on body weight and liver index in mice
The liver index could also partly reflect the degree of liver injury in mice. Herein, the liver index was calculated as the ratio of the liver mass to the body weight. As shown in Table 1, the body weight of mice treated with alcohol was significantly decreased in comparison with the control group. However, the alcoholic-induced decrease in body weight was reversed by U-IE and E-IE at 200 mg/kg bw. Yet as for the body weight and liver index, there was no significant statistically between U-IE and E-IE groups at the end of the seventh day.
3.5 Effects of U-IE and E-IE on alcohol metabolizing enzymes
LAB could decrease the blood alcohol concentration by increasing the first-pass metabolism (FPM) in the stomach to effectively protect against alcoholic-induced gastric and liver injury [20]. Gastric FPM of alcohol primarily depends on gastric ADH activity. Consequently,the elimination rate of blood alcohol usually depended on the ADH activity [22], and the influences of alcohol exposure on the gastric and hepatic ADH activities were further examined. As for the changes of alcohol metabolism enzymes, the results showed that the alcohol significantly reduced gastric and hepatic ADH activities (Table 2), while administration with E-IE could rescue these decreases compared with the model group. The results showed that E-IE participated in protective actions by increasing remarkably gastric and hepatic ADH activities.
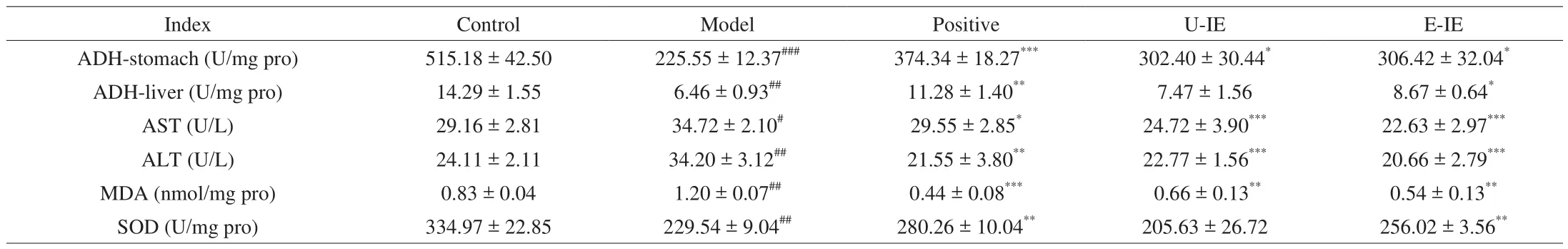
Table 2Effects of CFE-L and CFE-AL on biochemical indices in mice.
3.6 Effects of U-IE and E-IE on biochemical parameters in liver
Alcohol intake led to a noticeable increase in AST activity and ALT level in the serum, which were well-known liver injury biomarkers reflecting liver damage [23]. The elevated ALT and AST activities might be related to hepatocellular plasma membrane damage because these enzymes were normally localized to the cytoplasm and wouldn’t be released into the blood [24]. Administration of E-IE at doses of 200 mg/kg bw revealed a significant protective effect on the liver of mice by attenuating the elevation of the activities of AST and ALT compared with the model group (Table 2).
3.7 Effects of U-IE and E-IE on oxidative stress
There was increasing evidence that oxidative stress was a primary pathological mechanism, and in particular, ROS-induced oxidative stress could lead to liver damage under pathological conditions [25].Hepatic MDA and SOD levels were indicative of oxidative stress [26].To evaluate the protective effect of E-IE against oxidative damage caused by alcohol ingestion, the levels of MDA and SOD in liver tissues were determined. The results showed that the alcohol challenge weakened the activity of SOD and increased the MDA level in mice treated with alcohol only. Whereas E-IE treatment had the opposite effect, as it significantly increased the activity of SOD and reduced the MDA level (Table 2). These results indicated that E-IE had the potential to protect the liver against alcohol-induced oxidative stress.
3.8 Effects of U-IE and E-IE on hepatic pathological changes
Histopathological changes in liver tissue sections were used to evaluate the severity of liver damage in mice. The cellular morphology of liver cells in the control group and positive group were clear and polygonal. Simultaneously, the structure of the hepatic lobules was clear as well, with the hepatic cords arranged in order by radiating from the central vein (Figs. 4a, c). In contrast, typical morphological changes of liver damage were observed in the sections in the model group, including that the liver cord lost the ability to arrange orderly. In addition, the boundary between cells was obscure,and hepatocyte plasma membranes were incomplete (Fig. 4b). In the groups pretreated with U-IE and E-IE, the pathological abnormalities caused by alcoholic damage were significantly improved (Figs. 4d, e).
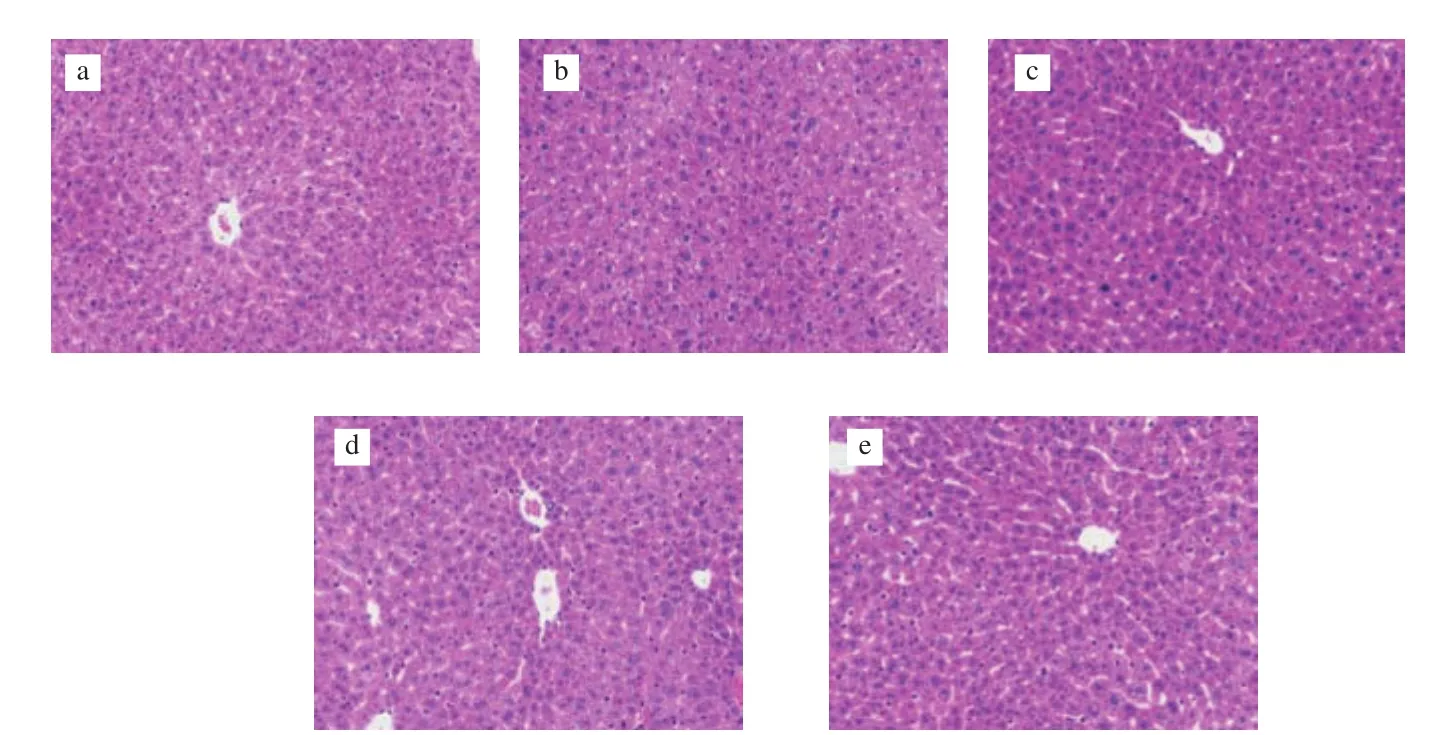
Fig. 4 Effects of U-IE and E-IE on hepatic pathological changes (magnification 200 ×). (a) Control group; (b) model group; (c) positive group; (d) U-IE group;(e) E-IE group.
3.9 ADHs-related proteins interactions analysis
The ADH isozymes amounts for alcohol oxidation were important determinants of alcohol elimination rate [27]. ADHE, a bifunctional alcohol/acetaldehyde dehydrogenase with both ADH and ALDH domains, played a central regulatory role during the adaptation process of bacteria to environmental stresses in the host [28]. Besides,researchers have indicated that dose and time changes of hepatic ADH activity during alcohol intoxication mainly involved ADH I and ADH III which could govern the elimination rate of blood ethanol [22]. In this study,L. lactiscells might initiate the expression of some genes and regulate proteins synthesis involved in stress responses to adapt to the changing environment [29]. PPIs networks diagrams of ADHE(Figs. 5a, c) and ADH1 (Figs. 5b, d) were obtained by a joint search of protein ADHE inL. lactisand protein ADH1 in mice. Alcohol metabolism was primarily governed by these proteins in mice, such as ADH1, CYP2E1, ALDH2, ALDH1, and so on. The results proved the existence of protein interactions with high possible con fidence. Based on current findings, it was inferred that there were protein interactions betweenL. lactisand mice, and the PPIs between different species could increase the activity of ADH, accelerate alcohol metabolism.

Fig. 5 PPIs networks of ADHE (a) and ADH1 (b) based on STRING database. Legends representing color code used to differentiate evidence channels used to establish the link between proteins represented in the ADHE (c) and ADH1 (d) network.

Fig. 5 (Continued)
4. Discussion
To survive and thrive in harsh or ever-changing environments,bacterial cells have evolved intricate mechanisms to mount an appropriate response in time [30].Oenococcus oeniadjusted the expression ofggppsgenes to stable bacterial cell membranes and prevent cell death induced by ethanol[31]. A previous study had shown that the genes of ADH and ALDH co-expressed in the vehicle ofL. lactisNZ3900, exhibited a dose-dependent protective effect to ameliorate alcoholic liver damage in mice [32].L. lactisNZ9700 grown in the presence of 2.0% ethanol could up-regulate genes expression of the ADH pathway to adapt to the stress conditions [33].According to our previous investigation, intracellular extracts ofL. lactisunder gradient freezing stress had a protective effect onL. lactisin the frozen environment [34]. It was inferred that the ethanol stress could up-regulate the expression of ADH-related genes and antioxidant enzymes in the host to expel toxic ethanol and adapt to the changes in the environment. Our results also showed that 0.5% ethanol concentration had no significant effect on the growth (Fig. 3a), while the E-IE (0.5%) could markedly enhance ADH activity (Fig. 3b).
Intriguingly, the bio-adaptability of LAB formed naturally in ethanol stress could transfer to the mice and improve the ability of alcohol metabolism. The protective effect of E-IE was further studied by animal experiments. The disappearance of the righting reflex meant that mice have been intoxicated. The recovery of the righting reflex showed the soberness of the mice [35]. In animal experiments,the intragastric administration of E-IE could enhance the latency of LORR, reduce the duration of LORR, decrease blood alcohol concentration and inhibit the decrease of body weight induced by alcohol in mice (Table 1). Alcohol undergoes an FPM in the stomach tissues. The hepatic ADH activity could govern the elimination rate of blood alcohol during alcohol intoxication, and the activity changes exist dose-dependently [22]. The results strongly indicated that E-IE could promote alcohol metabolism by enhancing gastric and hepatic ADH activities (Table 2). Components about the intracellular extracts ofL. lactismight include some antioxidant enzymes [36],non-enzymatic antioxidants, and alcohol metabolism enzymes [20].While the activities of intracellular alcohol metabolizing enzymes and antioxidant enzymes [37]might be induced significantly under appropriate ethanol stress. Based on current findings, it was inferred that there were protein interactions betweenL. lactisand mice, and the interactions between different species could increase ADH activity and accelerate alcohol metabolism.
A series of evidence has indicated that alcohol can decrease antioxidant levels. Especially, SOD is necessary for the endogenous antioxidant defense system to scavenge reactive oxygen species and maintain the cellular redox balance [26]. MDA is one of the main end products of liver lipid peroxidation, and it has the characteristics of cross-linking cellular macro-molecules of DNA or protein, which can cause extensive cellular damage [25]. Generally, hepatic MDA and SOD levels are major parameters for the status of oxidative stress.Consistent with previous reports, alcohol intake induced significantly oxidative stress as proven by the elevated MDA level and the reduced SOD activity in the mice of the model group. In addition, the levels of AST and ALT in mice serum also reflected liver damage caused by alcohol (Table 2). However, the pretreatment with E-IE for 7 consecutive days could preserve the activities of these enzymes,suggesting that E-IE played a hepatoprotective effect (Table 2).
PPIs are a common physiological mechanism for the protection and actions of proteins in an organism. The identification and characterization of PPIs in different organisms are necessary to better understand their physiology and determine their efficacy [38].Proteins usually interact with other proteins in different waysin vivo, such as affecting the function of one or more proteins;activating/inhibiting different signaling pathways to modulate the functions of the whole organism [39]. In this study, we demonstrated the existence of protein interaction betweenL. lactisIL1403 and mice by the STRING database (Fig. 5). We speculated that the PPIs in different organisms could increase the activity of ADH and accelerate alcohol metabolism, thereby having a positive effect on hangover mice. The bioinformatics results were consistent with our animal experiments. Further studies are still needed to further understand the molecular mechanisms.
5. Conclusions
In summary, our results showed that 0.5% ethanol concentration did not affect the growth ofL. lactis, while E-IE could markedly increase the ADH activityin vitro. Animal experiments also indicated that E-IE could enhance the ability of alcohol metabolism. The positive effects of E-IE on alcohol metabolism were related to the enhanced latency of LORR, the reduced duration of LORR, and the decrease of blood alcohol concentration. Furthermore, the activities of ALT and AST, ADH activities of stomach and liver tissues, the levels of MDA and SOD, together with the pathological changes of liver reflected that E-IE played a hepatoprotective effect. Based on the results of PPIs, it was concluded that there was an interaction between ADHs-related proteins ofL. lactisand mice, which may improve the ability of alcohol metabolism of mice.
conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81870093), the Research Project of People’s Liberation Army (BX115C007, BFP20C006), the Special Subject Funding of Zhengzhou University, and the Natural Science Foundation of Henan Province for Outstanding Youth(202300410365).
- 食品科学与人类健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species

