5-Demethylnobiletin and its major metabolites: efficient preparation and mechanism of their anti-proliferation activity in HepG2 cells
Ynping Xin, Ting Zheng, Mn Zhng, Ruiqing Zhng, Siyue Zhu,Dongli Li,b,*, Denggo Zho,b, Ynyn M,b, Chi-Tng Ho, Qingrong Hung,*
a School of Biotechnology and Health Sciences, Wuyi University, Jiangmen 529020, China
b International Healthcare Innovation Institute (Jiangmen), Jiangmen 529020, China
c Department of Food Science, Rutgers University, New Jersey 08901, USA
Keywords:
Hydroxylated polymethoxy flavones
Chemical synthesis
HepG2
Cell cycle
Apoptosis
A B S T R A C T
5-Demethylnobiletin (5-DMN), a hydroxylated polymethoxyflavone (OH-PMF) identified in aged citrus peels, has demonstrated health benefiting effects in previous studies. 5-DMN undergoes biotransformation in vivo, yielding 5,3’-didemethylnobiletin (5,3’-DDMN), 5,4’-didemethylnobiletin (5,4’-DDMN) and 5,3’,4’-tridemethylnobiletin (5,3’,4’-TDMN). However, the anti-cancer effects of 5-DMN and its in vivo metabolites against HepG2 cells remain unclear. In this study, an efficient chemical synthetic method was developed to obtain 5-DMN and its 3 metabolites, and their molecular structures were confirmed by 1H NMR and LC-MS. Cytotoxicity, cell cycle arrestment, apoptosis and caspase-3 expression were investigated to evaluate the anti-liver cancer effects of these OH-PMFs on HepG2 cells. The results showed that all 4 compounds inhibited the proliferation of HepG2 cells in a concentration-dependent manner. Their anti-proliferative activity was exerted through inducing G2/M phase arrestment, cell apoptosis and promoting expression of a key apoptotic protein called cleaved caspase-3. Our results indicated that 5,3’-DDMN and 5,3’,4’-TDMN showed a stronger inhibitory activity on cell proliferation than 5-DMN, followed by 5,4’-DDMN.The expression of cleaved caspase-3 was the highest in cells treated with 5,4’-DDMN, implying that the apoptosis induced by other OH-PMFs might be mediated by other apoptotic execution proteins. Our research reveals the application potential and scientific evidence for the production and functionality of OH-PMFs.
1. Introduction
Hepatocellular carcinoma (HCC), a common primary liver cancer,is one of the main causes of cancer-related deaths [1]. In recent years,the incidence of liver cancer has been on the rise in East Asia and Southeast Asia, seriously threatening human health. Current treatment methods are mainly surgical resection, orthotopic liver transplantation,and local ablation. The most effective one is still the surgery for orthotopic liver transplantation, but due to the lack of organs, this type of treatment is limited to a minor group of patients. In addition,liver transplantation is not suitable for the frail and elderly people [2].Chemotherapy is another promising way to treat liver cancer due to the breakthrough in the development of novel anti-cancer drugs.At present, sorafenib [3], lenvatinib [4]and regorafenib [5]have achieved good results in the treatment of liver cancer. Among them,sorafenib has been approved for marketing as a first-line drug for the treatment of liver cancer.
However, chemotherapeutic drugs often bring negative side effects to patients during the therapy, such as hair loss, diarrhea,vomiting, allergies and other adverse reactions [6]. In order to find safer alternative medicines or functional food for the effective treatment, natural active substances have received increasing attention due to their high efficacy and less side effects.Polymethoxyflavones (PMFs), which areflavonoids rich in citrus peels, contain multiple methoxy groups on C6-C3-C6 skeleton structure. A large number of studies have shown that PMFs possess anti-inflammation, anti-cancer, anti-oxidation, anti-atherosclerosis,anti-obesity, anti-aging and other biological activities [7-15].
Among the health-promoting activities of flavonoids,studies have shown that the anti-cancer activity of hydroxylated PMFs (OH-PMFs) is better than the parent PMFs [16]. OH-PMFs have been identified in natural sources orin vivoas the metabolites of PMFs. For example, 5-demethylnobiletin (5-DMN) was found to accumulate in the aged citrus peels, and has shown various biological activities, such as cancer preventing activity against lung cancer [17],pancreatic cancer [18]and colon cancer [19], anti-obesity,antioxidation [20]and neuroprotection [21]. Fromin vivostudies,the demethylation is a common metabolic pathway of PMFs [22,23].5,3’-didemethylnobiletin (5,3’-DDMN), 5,4’-didemethylnobiletin(5,4’-DDMN), 5,3’,4’-tridemethylnobiletin (5,3’,4’-TDMN)have been identified as the metabolites of 5-DMN [24]. Up to date, investigation of OH-PMFs in the treatment of liver cancer is very limited. And studies have shown thatin vivometabolites of bioactive compounds have shown stronger biological activity in treating/preventing diseases, which help to improve the overall bioefficacy of parent bioactive compounds [25]. Therefore, the comparative study of these OH-PMFs (i.e., 5-DMN, 5,3’-DDMN, 5,4’-DDMN and 5,3’,4’-TDMN) on the inhibitory effect using HepG2 cells will strengthen our understanding of the benefits of 5-DMN against liver cancerin vivo.
In this research, we firstly obtained 5-DMN, 5,3’-DDMN,5,4’-DDMN, 5,3’,4’-TDMN by chemical synthesis according to the 5-step method based on Li et al.’s report [26]with modifications.Our method improved the yield and shortened the reaction time from the second step. In order to investigate the anti-proliferation effect of these compounds on HepG2 cells, we conducted MTT assay,cell cycle arresting and apoptosis assays. To further understand the pro-apoptotic effect of the 4 OH-PMFs, molecular docking and protein expression quantification were performed. This work will provide scientific evidence for the promotion of developing functional/medicinal foods from citrus peels.
2. Materials and methods
2.1 Materials
The orange peel crude extract was purchased from Qiongge Biochemicals Inc. (Wuhan, China). Ethanol, methanol,n-hexane,ethyl acetate, dichloromethane, anhydrous acetonitrile, dimethyl sulfoxide, calcium carbonate, sodium chloride, potassium hydroxide and potassiumtert-butanol were purchased from Saan Chemical Technology Co., Ltd. (Shanghai, China). Human hepatocellular cell line HepG2 (ATCC), DMEM (Gibco, Amarillo, TX, USA), 10% bovine fetal serum (ATCC, Manassas, VA,USA), 1% penicillinstreptomycin (Sigma Aldrich, St. Louis, MO, USA), PI and Annexin-FITC apoptosis kit (Carlsbad, CA), Elisa kit of caspase-3 (Cell Signaling Technology, Danvers, MA, USA), multiplate microplate reader (Biotek, Winooski, VA, USA).
2.2 Chemical synthesis
In our work, a 5-step synthesis method (Fig. 1) was used to obtain 4 kinds of OH-PMFs according to published method with modification. Compared with Li et al. [26]method, in the second step, we used the more effective reagent-potassiumtert-butoxide instead of potassium hydroxide for the Claisen-Smit condensation reaction, reducing the time by 10 h and increasing the yield by 10%.We also combined the third and fourth steps to one quick step, giving over 90% yield in just 2-3 h of reaction. In the final step, we used aluminum trichloride and acetonitrile instead of ethanol hydrochloride as the reaction system for the demethoxylation of the 5 position of A ring, and it took only 2.5 h to obtain a yield higher than 95%. Overall,the optimized method is not only simplified, but also significantly reduces the time and increases the yield.

Fig. 1 Synthesis of 5-DMN and its metabolites. (a) KOH, C2H5OH, 80 °C, 48 h, yield: 46%-52%; (b) potassium tert-butoxide, C2H5OH, 80 °C, 6 h, yield: 85%;(c) I2, DMSO, 100 °C, 3 h, yield: 85%; (d) 10% Pd/C, NH4COOH, CH3OH, 65 °C, 2 h, yield: 95%; (e) AlCl3, CH3CN, 60 °C, 3 h, yield: 97%.
2.2.1 Synthesis of compound 2
The orange peel crude extract (4.02 g) was purified by silica gel column to obtain a mixture of tangeretin and nobiletin 2.8 g, white solid.Then 50% KOH solution (40 mL) was added to this mixture (3.0 g,1.00 equiv) dissolved in ethanol (80 mL) and stirred for 48 h at 80 °C. To end the reaction, the mixture was cooled in an ice bath and the reaction was quenched by slow addition of 20% citric acid solution (20 mL). After that, the mixture was extracted with ethyl acetate trice (20 mL), and the organic layer was washed with saturated aqueous NaCl (25 mL) and dried over Na2SO4, followed by concentratingin vacuo. The obtained crude residue was purified by silica gel column chromatography using hexane/ethyl acetate(6:1) as eluent to afford compound 2 (1.4 g, 47%) as yellow oil.1H NMR (500 MHz, CDCl3):δ13.17 (s, 1H), 4.09 (d,J= 0.7 Hz, 2H),3.96 (d,J= 0.7 Hz, 2H), 3.86 (d,J= 0.7 Hz, 2H), 3.81 (d,J= 0.8 Hz, 2H),2.68 (d,J= 0.8 Hz, 2H).
2.2.2 Synthesis of compound 4c
Compound 2 (1.4 g, 5.46 mmol, 1.00 equiv) in ethanol (40 mL)was added with potassiumtert-butoxide (0.91 g, 8.19 mmol, 1.50 equiv) and 3c (2.09 g, 6.55 mmol, 1.20 equiv) and stirred at 80 °C for 16 h. To end the reaction, the mixture was poured into a separatory funnel and washed by 20% HCl solution until pH value reached 3-4.The mixture was extracted with ethyl acetate (3 × 20 mL), and the organic layer was washed with saturated aqueous NaCl (25 mL),dried over Na2SO4, concentratedin vacuo. The obtained crude residue was purified by silica gel column chromatography using hexane/ethyl acetate (2:1) as eluent to afford compound 4c (2.58 g, 4.46 mmol,85%) as yellow solid.1H NMR (500 MHz, CDCl3)δ7.86 (s, 1H),7.42 (dd,J= 6.9, 1.8, 0.9 Hz, 4H), 7.39–7.34 (m, 1H),7.38–7.30 (m, 4H), 7.33–7.27 (m, 1H), 7.21–7.14 (m, 2H), 7.03 (d,J= 7.5 Hz, 1H),5.17 (t,J= 1.0 Hz, 2H), 5.07 (t,J= 1.0 Hz, 2H), 3.97–3.90 (m, 8H),3.86 (s, 2H).
2.2.3 Synthesis of compound 5c
Compound 4c (2.58 g, 4.46 mmol, 1.00 equiv) in DMSO (30 mL)was added with I2(0.707 g, 2.78 mmol, 0.62 equiv) and stirred for 3 h at 100 °C. To end the reaction, the mixture was poured into a separatory funnel and washed by 10% Na2O3S2solution. The mixture was extracted with CH2Cl2(3 × 20 mL); and the organic layer was washed with saturated NaCl solution (25 mL), dried over MgSO4and concentratedin vacuo. The obtained crude residue was purified by silica gel column chromatography using hexane/ethyl acetate (2:1)as eluent to afford compound 5c (2.10 g, 3.79 mmol, 95%) as yellow solid.1H NMR: (500 MHz, CDCl3)δ7.44 (ddd,J= 7.9, 4.0, 1.4 Hz,4H), 7.41–7.34 (m, 2H), 7.38–7.25 (m, 4H), 7.06 (d,J= 1.3 Hz, 1H),6.95 (d,J= 1.1 Hz, 2H), 5.95 (s, 2H), 5.31 (dd,J= 12.8, 3.0 Hz, 1H),5.18 (s, 3H), 4.05 (s, 3H), 3.89 (s, 2H), 3.84 (s, 3H), 2.96 (dd,J=16.6, 12.8 Hz,1H), 2.78 (dd,J= 16.7, 3.0 Hz, 1H).
2.2.4 Synthesis of compound 6c
Compound 5c (2.35 g, 4.24 mmol, 1.00 equiv) in methanol(25 mL) was added with ammonium formate (0.40 g, 6.36 mmol, 1.50 equiv)and 10% Pd/C (0.10g, 0.51 mmol, 0.3 equiv), and stirred for 2 h at 60 °C. To end the reaction, 10% Pd/C was filtered off and the solvent was removed under vacuum. The crude residue was purified by silica gel column chromatography using hexane/ethyl acetate (1:1) as eluent to afford compound 6c (1.50 g, 4.00 mmol, 95%) as a white solid.1H NMR (500 MHz, DMSO-d6):δ9.84 (s, 1H), 9.51 (s,1H), 7.42 (d,J= 2.2 Hz, 1H), 7.38 (dd,J= 8.4, 2.3 Hz, 1H), 6.89 (d,J= 8.4 Hz, 1H), 6.58 (s,1H), 4.02 (s, 3H), 3.97 (s, 3H), 3.83 (s, 3H), 3.76 (s, 3H).
2.2.5 Synthesis of compound 7c (5,3’,4’-TDMN)
Compound 6c (1.50 g, 4.00 mmol, 1.00 equiv) in acetonitrile (25 mL)was added with AlCl3(2.13 g, 16 mmol, 4.00 equiv) and stirred for 60 °C for 3 h. Then acetonitrile was evaporated, and 3% HCl (15 mL)was added and the mixture was heated under reflux for 30 min. To end the reaction, the mixture was extracted with ethyl acetate (3 × 20 mL), and the organic layer was washed with saturated aqueous NaCl (25 mL), dried over Na2SO4and concentratedin vacuo. The crude residue was purified by silica gel column chromatography using hexane/ethyl acetate (1:1) as eluent to afford compound 7c (1.3 g,97%) as a white solid. LC-MS (AP-ESI)m/z, calcd for C18H16O8[M + H]+m/z361.0918, foundm/z361.091 9.1H NMR (500 MHz,DMSO-d6):δ12.80 (s, 1H), 9.97 (s, 1H), 9.54 (s, 1H), 7.46 (d,J= 8.4 Hz, 2H), 6.91 (d,J= 8.1 Hz, 1H), 6.78 (s, 1H), 4.02 (s, 3H), 3.93 (s, 3H),3.82 (s, 3H).13C NMR (126 MHz, DMSO-d6)δ182.53, 164.43,152.49, 150.11, 148.62, 145.91, 145.29, 135.89, 132.74, 121.46,119.24, 116.22, 113.44, 106.29, 102.72, 62.06, 61.57, 60.64.
2.2.6 Synthesis of compound 7a (5,4’-DDMN)
Compound 7a (0.65 g, total yield 34.62%) was prepared using 6a via the same procedures described for 7c. LC-MS (AP-ESI) calcd for C19H18O8[M + H]+m/z375.1074.1H NMR (500 MHz, DMSO-d6)δ10.34 (s, 1H), 9.00 (s, 1H), 8.67 (s, 1H), 7.94 (s, 1H), 7.85 (d,J= 8.6 Hz,2H), 7.60 (d,J= 8.6 Hz, 2H), 3.25 (s, 2H), 1.93 (s, 2H), 1.51 (s, 4H),1.27 (s, 4H),13C NMR (126 MHz, DMSO-d6):δ169.18, 154.60,154.57, 151.32, 133.96, 129.43, 126.46, 125.77, 124.34, 39.00, 32.32,28.92, 28.4 0, 26.21, 25.18.
2.2.7 Synthesis of compound 7b (5,3’-DDMN)
Compound 7b (0.45 g, total yield 34.17%) was prepared using 6bvia the same procedures described for 7c. LC-MS (AP-ESI)m/zcalcd for C19H18O8[M + H]+m/z375.107 4, foundm/z375.107 4.1H NMR (500 MHz, DMSO-d6):δ10.34 (s, 1H), 8.92 (s, 1H), 8.67(s, 1H), 7.79–7.71 (m, 3H), 7.08 (d,J= 8.9 Hz, 2H), 3.81 (s, 3H), 3.22(s, 2H), 1.93 (s, 2H), 1.53–1.45 (m, 4H), 1.31–1.22 (m, 4H);13C NMR(126 MHz, DMSO-d6):δ169.19, 160.20, 154.76, 153.86, 152.55,126.39, 122.07, 119.49, 114.79, 55.46, 38.95, 32.32, 28.96, 28.41,26.22, 25.19.
2.2.8 Synthesis of compound 8 (5-DMN)
The orange peel crude extract (1.0 g) was separated and purified by silica gel column to obtain nobiletin (0.42 g, 1.04 mmoL).Compound 8 (0.39 g, 1.01 mmoL, 97%) was prepared using the nobiletin via the same procedures described for 6c-7c. LC-MS(AP-ESI)calcd for C20H20O8[M + H]+m/z389.123 1, foundm/z389.123 4.1H NMR (500 MHz, DMSO-d6):δ10.34 (s, 1H), 8.98(s, 1H), 8.67 (s, 1H), 7.90 (s, 1H), 7.83 (d,J= 7.2 Hz, 2H), 7.52 (t,J=7.6 Hz, 2H), 7.44 (t,J= 7.4 Hz, 1H), 3.24 (q,J= 6.7 Hz, 2H), 1.93 (t,J=7.4 Hz, 2H), 1.53–1.47 (m, 4H), 1.30–1.24 (m, 4H);13C NMR (126 MHz,DMSO-d6):δ169.18, 154.68, 154.45, 152.36, 129.51, 129.32, 126.87,124.69, 123.70, 38.98, 32.32, 28.93, 28.40, 26.21, 25.18.
2.3 Cell culture
2.3.1 Cell culture and treatment
Human hepatocellular cell line HepG2 cultured in DMEM supplemented with 10% bovine fetal serum, 1% penicillin-streptomycin in a 5% CO2and humidified atmosphere at 37 °C. Cells between passages 12 to 25 were used for experiments in this study. PI and Annexin-FITC apoptosis kit were purchased from Invitrogen. Cells were handled according to manufacture protocols.
2.3.2 Analysis of cell viability (MTT assay)
HepG2 cells were seeded in a 96-well plate at a density of 1 × 104cells/well and were incubated for 24 h to allow cell attachment before treatment. Four OH-PMFs dissolved in DMSO were diluted to 0.5-50 μmol/L with DMEM, and 1% DMSO in DMEM was used as control. After 24 h treatment, cells were washed with PBS thrice to remove unabsorbed compounds, 100 μL MTT at concentration of 0.5 mg/mL was added to each well followed by 3 h incubation.Subsequently, medium in each well were decanted and 150 μL DMSO was added to each well to dissolve the formed formazan crystals. After shaking for 10 min to allow complete solubilizing of the crystals, the absorbance at 570 nm was measured by multiplate microplate reader. Cell viability was calculated as percentages of the absorbance of treated cells divided by that of control. There were 6 replicates for each treatment.
2.3.3 Cell cycle analysis
HepG2 cells were seeded in 6-well plates at a density of 3 × 105cells/well. After reaching 80% confluency, cells were treated with 4 OH-PMFs for 24 h, respectively. Then cells were washed with PBS 3 times and harvested with trypsin. After that, cells were fixed with 70% ice cold ethanol and stained with PI containing RNAse for 30 min before being subjected to flow cytometric analysis using an Accuri C6 flow cytometer (10 000 events were collected, cell debris excluded by gating). The experiment was repeated three times, data were processed using Flowjo_10 software.
2.3.4 Cell apoptosis
Apoptosis was performed in a similar manner to the cell cycle experiment. Briefly, after treating cells with 4 OH-PMFs for 24 h, cells were stained with Annexin/PI kit without fixing. Staining procedures were performed according to vendor’s protocol.Binding buffer was used instead of PBS to allow attachment of Annexin-FITC to the PS proteins on cell membrane. 5 μL Annexin-FITC and 10 μL PI were diluted in 185 μL cell suspension and incubated at room temperature for 15 min beforeflow cytometry analysis.Cells with minor Annexin-FITC staining and PI staining were considered as unaffected cells; cells with high intensity of Annexin-FITC fluorescence represented early apoptosis; cells with high intensity of PI and Annexin-FITC fluorescence represented late apoptotic cells.
2.4 Quantification of cleaved caspase-3
After treating HepG2 cells with 12.5, 25, 50 μmol/L OH-PMFs for 24 h, cell lysates were collected and centrifuged at 14 000 ×gfor 10 min at 4 °C. Total protein concentration was quantified by Bradford protein assay and all samples were diluted to the same concentration with dilution buffer. Quantification of caspase-3 was performed using ELISA kit following the manufacturer’s instructions.Absorbance at 450 nm was read by multiplate microplate reader.
2.5 Molecular docking
All molecular docking studies were performed with AutoDock Vina [27]. The crystal structure of caspase-3 (PDB code: 3KJF) was downloaded from the RCSB Protein Data Bank (http:// www.rcsb.org/pdb). Molecular docking simulations of ligands and proteins in the caspase-3 complex crystal structure were first performed using the following docking parameters, and the RMSD of the small molecule ligand in the optimal conformation to the real model was 1.469 Å.AutoDock Tools was used to remove ligands and water molecules,redistribute charges, and add hydrogen atoms to the protein structures.The calculation method adopted the Lamarckian genetic algorithm,the maximum number of evaluations for molecular docking is 25 000,and the number of models generated by molecular docking is 20. The ligand molecular coordinated in the original crystal structure as the center of the box, and the default settings were used for the remaining parameters. Subsequently, Autodock Vina was used to calculate and analyze the docking between the four compounds and the modified protein. The best conformation was chosen to analyze the ligand-protein interactions. The image representing the best pose was prepared using PyMOL (https://pymol.org).
2.6 Statistical analysis
All data were presented as mean ± standard deviation (SD) or standard error (SE). Student’st-test was used to test the difference between the mean two groups between the two groups. Analysis of variance (ANOVA) models were used to compare differences between more than two groups.P< 0.05 was considered statistically significant.
3. Results
3.1 Preparation of 5-DMN and its metabolites
Compared with traditional separation of PMFs from the natural products, chemical synthesis is a highly accurate and efficient way to obtain the targeted PMFs. This study used the crude orange peel extract (mainly containing nobiletin and tangeretin) (Fig. 2) as the substrate for chemical synthesis. As nobiletin and tangeretin have the same A-ring structure, the mixture nobiletin and tangeretin can be subjected to a base ring opening reaction to produce 2’-hydroxy-3’,4’,5’,6’-tetramethoxyacetophenone, which is product 2 of the 5-step synthetic reaction. The structure formulas of 4 compounds obtained by chemical synthesis were shown in Fig. 3. The structure and purity of 5-DMN and its metabolites were con firmed by NMR and LC-MS,as shown in support information.
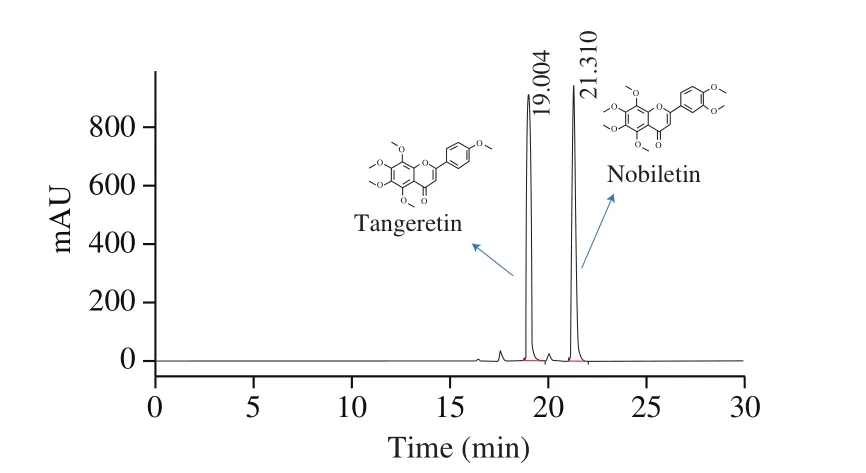
Fig. 2 Pro files of crude extract of orange peel by HPLC.

Fig. 3 Structure of 5-DMN and its metabolites.
3.2 Growth inhibitory effect of 5-DMN and its metabolites on HepG2 cells
In recent years, studies have shown that OH-PMFs have anti-cancer activity against a variety of cancer cells, such as colon,breast and lung cancers, etc. [28]. Compared to several other PMFs found in citrus peels, 5-DMN exhibited stronger anti-cancer activity according to previous cell studies [26]. However, due to the poor water solubility of 5-DMN, its absorption in human body is relatively limited. In contrast, the three metabolites of 5-DMN exhibited better water solubility due to the increased number of hydroxyl groups, and they were found to be effective against colon cancer in anin vitrostudy [19].In order to evaluate the anti-liver cancer potential of 5-DMN and its metabolites, we evaluated the growth inhibitory activity of 5-DMN and its 3 metabolites, 5,3’-DDMN, 5,4’-DDMN, 5,3’,4’-TDMN, on HepG2 cells. HepG2 cell model has been used as anin vitromodel for studying the pathology of human liver cancer, therefore, the anti-cancer activity of the 4 compounds against HepG2 cells can be used as important reference for their efficacy in human body.
In general, all 4 compounds inhibited the growth of HepG2 cells in a concentration-dependent manner (Fig. 4). According to Wang et al. method [29], the IC50values of 5-DMN, 5,3’-DDMN, 5,4’-DDMN,and 5,3’,4’-TDMN treatment of HepG2 cells in this study were 47.31,41.37, 54.46 and 46.18 μmol/L. Compared to other compounds, 5,3’-DDMN and 5,3’,4’-TDMN treatment of HepG2 showed clearer doesresponse relationship. Overall, the inhibitory effects of 5,3’,4’-TDMN and 5,3’-DDMN were better than 5-DMN, while the inhibitory effect of 5,4’-DDMN seemed to be the weakest among them.

Fig. 4 Growth inhibitory effects of 5-DMN and its metabolites on HepG2 cells. Data were shown as mean ± SD (n = 6), and viable cells of controls were used as 100%.
3.3 G2/M cell cycle arrest of HepG2 induced by 5-DMN and its metabolites.
Arresting of cell cycle interferes the normal division and proliferation of HepG2 cells, thereby potentially inhibiting the development of live cancer. To further evaluate the growth inhibitory activity of 5-DMN and its three metabolites, DNA was stained in HepG2 cells to quantify the phase distribution of cells. As shown in Fig. 5, cells treated with 5-DMN, 5,3’-DDMN, 5,4’-DDMN and 5,3’,4’-TDMN for 24 h all showed G2/M phase arrestment.Compared with the control group, 5,3’-DDMN exhibited the strongest cell cycle arresting effect with the percentage of cells arrested in G2/M phase increased to 71%. Cells treated with 5,3’,4’-TDMN showed a 68% G2/M phase arresting, which was slightly lower than 5,3’-DDMN and followed by 5,4’-DDMN (51%). Among them, the cell cycle arresting effect of 5-DMN was the weakest, which is not significantly different from the control group. The results were consistent with the MTT study summarized previously. And a study conducted by Ma et al. [30]also confirmed that nobiletin showed G2/M phase arresting on human SMMC-7721 HCC cells with higher concentration and long incubation time. By which it implies that metabolites of 5-DMN can achieve stronger cell cycle arresting effect than nobiletin.
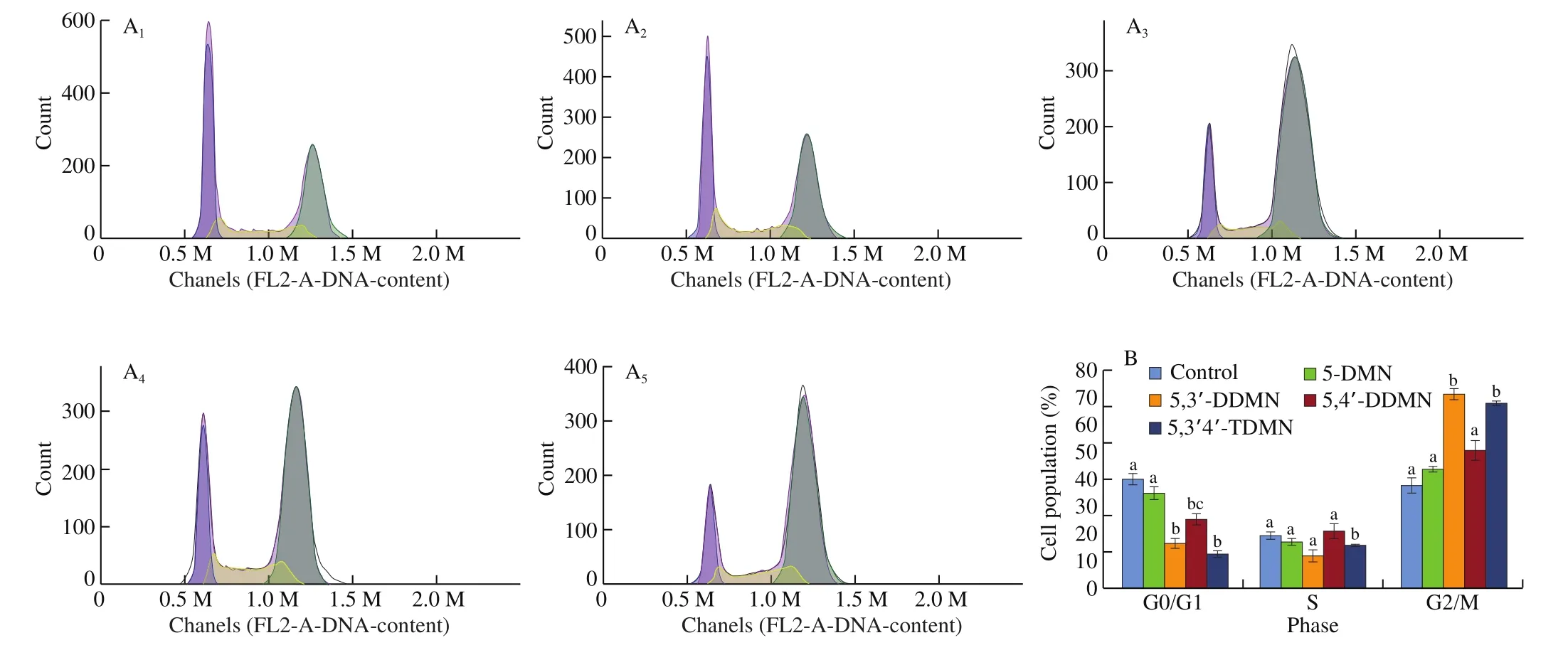
Fig. 5 Effects of 5-DMN and its metabolites (25 μmol/L) on cell cycle progression of HepG2. (A) DNA histogram of cell cycle distribution of HepG2 cells.(A1-A5) Control, 5-DMN, 5,3’-DDMN, 5,4’-DDMN and 5,3’,4’-TDMN, respectively. (B) Percentages of cell population distribution. Different letter symbols in the bar charts indicate statistical significance (P < 0.05, n = 3) by ANOVA.
3.4 Pro-apoptotic activity of 5-DMN and its 3 metabolites on HepG2 cells
Similar to cell cycle abnormalities, apoptosis is another route to evade aberrant growth of cancer cells. Therefore, inducing cancer cell apoptosis is an important way to prevent and treat cancer [31].The effect of 5-DMN and its metabolites on apoptosis were detected by Annexin-V/PI double staining at the concentration of 12.5, 25, and 50 μmol/L on HepG2 cells. As shown in Fig. 6, after 24 h of treatment, the pro-apoptotic effect of the 4 compounds increased with increasing concentration. At concentration of 25 μmol/L,the number of apoptotic cells in treatment group was 2-3 times to that of the control group. At lower concentrations (12.5-25 μmol/L),5,3’,4’-TDMN exhibited the strongest pro-apoptotic effect among the 4 compounds, followed by 5,3’-DDMN and 5-DMN, with 5,4’-DDMN being least effective in apoptosis induction.
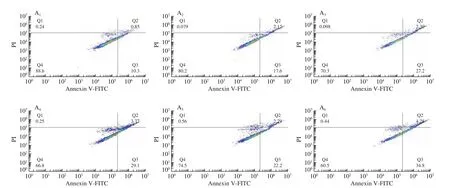
Fig. 6 Dot plots of apoptotic cells of HepG2 cells (A) and effects of 5 OH-PMFs (12.5-50 μmol/L) on apoptosis of HepG2 cells (B). (A1-A13) Control,12.5 μmol/L 5-DMN, 25 μmol/L 5-DMN, 50 μmol/L 5-DMN, 12.5 μmol/L 5,3’-DDMN, 25 μmol/L 5,3’-DDMN, 50 μmol/L 5,3’-DDMN, 12.5 μmol/L 5,4’DDMN, 25 μmol/L 5,4’-DDMN, 50 μmol/L 5,4’-DDMN, 12.5 μmol/L 5,3’,4’-TDMN, 25 μmol/L 5,3’,4’-TDMN, 50 μmol/L 5,3’,4’-TDMN, respectively.Data are shown as mean ± SD. Different letter symbols in the bar charts indicate statistical significance (P < 0.05, n = 3) by ANOVA.
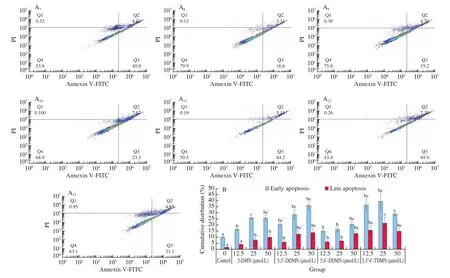
Fig. 6 (Continued)
3.5 Quantitative analysis of cleaved caspase-3 expression induced by 5-DMN and its metabolites on HepG2 cells
Caspase family runs through the whole mechanism of apoptosis,and is the protease system that directly leads to the disintegration of apoptotic cells. When cells are exposed to external or internal stimuli,the expression of downstream proteins are regulated under complex internal gene regulation, which directly affects cell apoptosis [32].In the programmed death pathway, caspase-3, as an apoptosis executioner, is activated/cleaved and then leads to the destruction of cellular structures and contributes to cell death directly. Therefore,the higher the level of cleaved caspase-3, the stronger the proapoptotic effect. HepG2 cells treated with the 4 OH-PMFs showed a significantly higher content of cleaved caspase-3 than the control group (Fig. 7). It indicated that OH-PMFs promoted the expression of cleaved caspase-3 protein to facilitate HepG2 cells apoptosis.It is worth noting that after treating cells with 5,4’-DDMN, the expression of cleaved-caspase-3 protein increased significantly in a concentration-dependent manner, the results demonstrated a good pro-apoptotic effect of 5,4’-DDMN in the programmed death pathway.

Fig. 7 5-DMN and its metabolites (12.5, 25, 50 μmol/L) treatment HepG2 cells induced cleaved caspase-3 protein expression. Data are shown as mean ± SD. Different letter symbols in the bar charts indicate statistical significance (P < 0.05, n = 3) by ANOVA.
3.6 Molecular docking studies
Caspase-3 is a protease that carries out proteolysis events and leads to final cell death in the apoptosis pathway [33]. Therefore,it has been used as an important marker in cancer therapy. To evaluate pro-apoptotic effect of the 4 OH-PMFs from the structural perspective, we calculated the binding affinity of the 4 compounds with caspase-3. Docking simulation results (Fig. 8) showed that 5,3’-DDMN and 5,3’,4’-TDMN were able to bind tightly with caspase-3, and the binding energy was estimated as -7.8 kcal/mol.In comparison, the binding energy of 5-DMN and 5,4’-DDMN with capspase-3 were -6.9, and -6.8 kcal/mol, respectively.
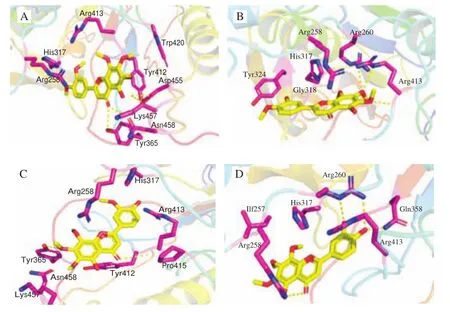
Fig. 8 5-DMN and its metabolites Molecular docking with caspase-3 was performed by AutoDock Vina program. (A) 5-DMN; (B) 5,3’-DDMN;(C) 5,4’-DDMN; (D) 5,3’,4’-TDMN.
Specifically, 5,3’-DDMN mainly interacted with amino acid residues Met61, His121, Arg207, Trp206 and Phe256 through hydrophobic bonds, and formed hydrogen bonds with Tyr204; while 5,3’,4’-TDMN mainly interacted with amino acid residues Phe128,Met61, His121, Trp206, Phe256, Tyr204 and Glu123 by hydrophobic interactions and formed hydrogen bonds with Arg204.
4. Discussion
Aged citrus peels, namely “Chenpi” in Chinese, have been used as the traditional medicine against the gastrointestinal disorders. 4 PMFs are found mainly in fresh citrus peels: nobiletin, tangeretin,sinensetin and 3,5,6,7,8,3’,4’-hepamethoxyflavone [34]. During the aging process of Chenpi, 5-demethylated PMFs, especially 5-DMN, are found to accumulate in the peels. Zheng et al. [35]studied PMFs profile change in the fresh citrus peel under different drying processes, including hot-air drying (HAD), vacuum-freeze drying (VFD), sun drying (SD), and natural drying. The results showed that the citrus peels after natural drying process contained the highest content of 5-demethylated PMFs.
CYP-450s are the main enzymes that metabolize drugs in the body and is found in the liver and intestine [36]. It has been reported that CYP-450s in human liver microsomes can demethoxylate NOB to give OH-PMFs [37,38]. In our previous study, we found that NOB was metabolically converted to 5-DMNin vivo[39]. As mentioned earlier, 5-DMN has good health-promoting activity, and the liver is the important organ for the metabolism of PMFs. Therefore, it is of great interest to study the anti-proliferative effect of 5-DMN and its metabolites on hepatocellular carcinoma.
Compared with nobiletin, 5-DMN and its metabolites contain more hydroxyl groups on the flavone skeleton. Previous studies have shown that the number of methoxy (or hydroxyl) groups is closely related to the biological activity of PMFs [40,41]. Our results showed that 5-DMN and its metabolites interfered with the growth and division of HepG2 cells and induced G2/M phase arrest. On the contrast, nobiletin and its metabolites (3’-demethylnobiletin,4’-demethylnobiletin, 3’,4’-didemethylnobiletin) were less effective in inhibiting HepG2 cell growth (supporting information Fig. 4). From our screening results, the hydroxyl group on the 5-position was very critical for the anti-proliferative activity on HepG2 cells. Furthermore,it suggested that more hydroxyl groups on the skeleton of PMFs were associated with stronger anti-proliferative activity.
There are several key proteins in cell cycle regulation, such as cyclin dependent kinase (CDK), cyclins and casein kinase 1 (CK1),that affect cell apoptosis signal [42]. Proceeding of G2/M phase is mainly regulated by CDK1 and cyclin B. We used molecular docking to study the theoretical fit values of 4 OH-PMFs with CDK1 protein in G2/M phase. The results showed that all 4 compounds were closely bound to CDK1 protein (see supporting information). CDK1 is a cleavage target of caspases and cleaved caspases in cells. It is also a Bcl-2 kinase candidate, which phosphorylates Bcl-2 and enhances pro-apoptotic activity. Basically, in the apoptotic pathway, Bcl-2 is associated with several signaling pathways that directly control apoptosis, such as ROS, caspase-3/7, caspase-6, etc. [43].
Cleaved caspase-3, which is a key apoptotic protein, is commonly used to evaluate the apoptotic effect. From the results of apoptosis and cleaved caspase-3 protein expression study (Figs. 6 and 7), the expression of cleaved caspase-3 was increased by 5,4’-DDMN in a concentration-dependent manner, which indicated that caspase-3 was the primary target of 5,4’-DDMN to exert pro-apoptotic effect.It was striking that 5,4’-DDMN had the strongest activity on cleaved caspase-3 expression among the 4 compounds, while it had weaker apoptosis effect than 5-DMN, 5,3’-DDMN and 5,3’4’-TDMN at gradient concentrations (12.5, 25 and 50 μmol/L), (Fig. 6). The possible reason was that 5,4’-DDMN induced cell apoptosis mainly through facilitating expression of cleaved caspase-3, while the other three compounds may target other effectors, caspase-6 and caspase-7.And according to previous study on the apoptotic mechanisms,active compounds can interact with membrane receptor FADD to activate caspase-2/-8, and further cleave caspase-6 to promote nuclear degradation and DNA damage, resulting in cell death [31]. It was also reported that caspase-3 and caspase-7 exhibited distinct roles in inducing cell apoptosis, when caspase-3 was essential for cell killing,caspase-7 contributed to ROS production and cell detachment, which could also lead to positive results in cells apoptosis studies [44]. It is also possible that the other three compounds induce apoptosis through the interplay with autophagy and necrosis pathway due to sharing of some regulatory proteins such as Necrostatin-1, Bcl-2/Bcl-XL,p53, PI3k, etc. [45]. The 3 pathways could play synergistic or antagonistic roles, for example, when autophagy pathway is activated,apoptosis was inhibited, as reported in previous research [46].Therefore, other apoptotic pathways need to be further investigated as to con firm the molecular mechanism of the anticancer activity of OH-PMFs in hepatocellular carcinoma cells.
Through the above discussion, we, for the first time, investigated the anti-proliferative effect of 5-DMN and its 3in vivometabolites against liver cancer (HepG2). Among the 4 OH-PMFs, 5,3’-DDMN and 5,3’4’-TDMN possess stronger pro-apoptotic effect. It suggests that the PMFs from Chenpi could be developed as functional food with health benefits against liver cancer. Moreover, our cell studies indicated that the overall efficacy of PMFsin vivomight rely on the metabolites. There should be an interplay between the hepatocytes and PMFsin vivo. On the one hand, the liver is regarded as the major site for the metabolism of PMFs in the host. On the other hand,the generated metabolites by hepatocytes would in turn function on the liver cancer cell. In the future study, the dynamics of PMFs and hepatocytes needs further exploration, especially the specific mechanisms/pathways of PMFs on the liver cancer cells.
5. Conclusion
In conclusion, we optimized the synthetic route for obtaining OH-PMFs with half of the time required in previously reported method, the overall yield was also increased by 20%. The evaluation of the anti-proliferative activity of 5-DMN and its metabolites on HepG2,for the first time, provided evidence of their potential in liver cancer prevention. And this protective effect was related to G2/M phase cell cycle arresting and induction of cell apoptosis. Our results also demonstrated that 5,3’-DDMN, 5,3’,4’-TDMN showing stronger pro-apoptotic activity among the 4 compounds, while expression of caspase-3 was highest in 5,4’-DDMN treated cells. This implies that other caspase proteins such as caspase-6/-7 might also participated in the cell death pathway and further investigation is needed to clarify the cell apoptotic mechanisms for all 4 PMFs.
conflict of interest
The authors have declared no conflict of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.04.018.
- 食品科学与人类健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species

