Extraction process in Chuantieling (CTL) based on the quality by design (QbD)concept
LI Y,LI Zirui,GUO Zhihu
a. College of Pharmacy,Hunan University of Chinese Medicine,Changsha,Hunan 410208,China
b. College of Traditional Chinese Medicine,Hunan University of Chinese Medicine,Changsha,Hunan 410208,China
ABSTRACT Objective This study aimed to design and optimize the water extraction process for Chuantieling (喘贴灵,CTL) based on the concept of quality by design (QbD).Methods The single-factor experiments were used to select the best experimental points of CTL water extraction. On this basis,the transfer rate of ephedrine hydrochloride and sinapine thiocyanate,and the yield of the extract were evaluated as the evaluation indicators.The liquid-solid ratio,extraction time,and pH value were selected as the main factors to carry out the Box-Behnken design (BBD). A mathematical model of the critical process parameters(CPPs) and critical quality attributes (CQAs) was established,the interaction between CQAs and CPPs was examined,and the CTL extraction process design space was established and optimized,which guaranteed the stability of the process. The high performance liquid chromatography (HPLC) was used to analyze the main active compounds in the CTL extract.Results Through single-factor experiments,the best experimental parameters were found to be a liquid-solid ratio of 8∶1,extraction time of 90 min,pH value of 4,and extraction times of three. The experimental variance analysis results of the BBD showed that the P value of the regression model was less than 0.05,and the lack-of-fit value was greater than 0.01,indicating that the constructed model had good predictive ability. The operating space of the CPPs of the CTL water extraction process was combined with the actual production situation. In the production situation,the best extraction process was nine times of water addition,72 min of extraction time,and 4.5 of pH value. The HPLC results showed that the peak areas of ephedrine hydrochloride,sinapine thiocyanate,tetrahydropalmatine,methyl eugenol,cinnamaldehyde,and 6-gingerol in the CTL extract accounted for 0.94%,14.32%,0.78%,31.23%,0.34%,and 0.44% of the total peak area,respectively.Conclusion The water extraction process design space of CTL based on QbD was conducive to actual production operations,ensuring the stability of the process.
Keywords Chuantieling (CTL) Quality by design (QbD) Box-Behnken design (BBD) Critical quality attributes (CQAs) Critical process parameters (CPPs) Mahuang (Ephedrae Herba) Jiezi (Sinapis Semen) Ephedrine hydrochloride Sinapine thiocyanate
1 Introduction
Bronchial asthma is a chronic inflammatory disease of the airway involving multiple cells (such as eosinophils,mast cells,T lymphocytes,neutrophils,and airway epithelial cells) and cellular components[1]. Its clinical manifestations include wheezing,shortness of breath,tightness of chest,coughing,and other respiratory symptoms accompanied with reversible expiratory airflow limitation. The economic burden caused by the treatment of bronchial asthma exceeds the total economic burden caused by tuberculosis and human immunodeficiency virus (HIV) infection[2,3]. It causes great physical and psychological pain to patients,and affects daily life. Therefore,asthma is considered as a serious health problem and has attracted global attention[4]. Traditional Chinese medicine (TCM) has the unique advantage of eliminating the cause,controlling the symptoms,reducing adverse drug reactions,and also reducing the recurrence of asthma[5]. Acupoint application is a characteristic therapy in TCM. It is a complex intervention method that is affected by factors such as drugs,acupoints,time,and stimulation methods. By stimulating local acupoints,it stimulates the body's internal meridian energy,enhances immune function,and reduces asthma recurrence[6-8].Chuantieling (喘贴灵,CTL) is a hospital preparation of the Second Affiliated Hospital of Hunan University of Chinese Medicine. It is composed of eight herbs:Mahuang (Ephedrae Herba),Jiezi (Sinapis Semen),Xixin(Asari Radix et Rhizoma),Dingxiang (Caryophylli Flos),Rougui (Cinnamomi Cortex),Yanhusuo (Corydalis Rhizoma),Gansui (Kansui Radix),and Shengjiang (Zingiberis Rhizoma Recens). It has been used clinically for many years,and clinical research have shown that CTL plaster exerts a substantial effect on bronchial asthma[9,10].However,there are still problems in the current CTL extraction process such as unclear effective substance content,lack of reasonable extraction process parameters,and weak quality standards. In the prescription,Mahuang (Ephedrae Herba) and Jiezi (Sinapis Semen)are the principal drugs. Ephedrine hydrochloride and sinapine thiocyanate,the main compounds of Mahuang(Ephedrae Herba) and Jiezi (Sinapis Semen),exert definite and strong curative effects on bronchial asthma[9,10].Therefore,in the optimization of the water extraction process,the ephedrine hydrochloride and sinapine thiocyanate content,and extract yield have been proposed to be used as evaluation indicators. To ensure the quality and clinical efficacy of the patch,a single-factor experiment combined with Response Surface Methodology (RSM) was used to optimize the CTL extraction process that can maximize the extraction and reduce the loss of active compounds (ephedrine hydrochloride and sinapine thiocyanate)[11].
Quality by design (QbD) is a scientific,risk-based,comprehensive,and systematic research method for improving product quality by emphasizing the design. The QbD system is composed of a quality target product profile (QTPP),critical material attribute (CMA),critical process parameters (CPPs),and critical quality attributes(CQAs). Through the establishment of mathematical models to characterize the relationships among CMA,CPPs,and CQAs with mutual influence,the corresponding design space and control strategy were adopted to ensure the stability of the QTPP (Figure 1). Recently,QbD has promoted the transformation of the pharmaceutical production model and received widespread attention[12].It is now believed that quality is not only purely dependent on inspection and production processes,but is also conferred by design[13]. QbD has been widely used in the research and development of chemical and biological medicine production technology[14]; it has also been widely utilized in the research of TCM production technology[15,16]. This study focused on QbD by applying experimental design,design space,and other methods to study the extraction process of CTL,reduce the risk of preparation production and post-marketing,and provide an experimental basis for the late-stage research of CTL and development of new drugs.

Figure 1 Application of QbD in products
2 Materials
2.1 Instruments and equipments
A 1200 high-performance liquid chromatography (HPLC)column (Agilent Technologies) with a Hibar C18bonded silica column (150 mm × 4.6 mm,5 μm); an Agilent ChemStation chromatography workstation (Agilent Technologies); a DK-98-11A electric heating constant temperature water bath (Tianjin Test Instrument Co.,Ltd.),a CP114 electronic balance (Ohaus Instrument Co.,Ltd.),and a KQ-500DE CNC ultrasonic cleaner (Kunshan Ultrasonic Instrument Co.,Ltd.) were used in this study.
2.2 Reagents and drugs
Methanol and acetonitrile (chromatographically pure,American Tiandi Company,USA),phosphoric acid (analytically pure,Sinopharm Chemical Reagent Co.,Ltd.,China),and ultrapure water (Option RT ultra AN ultrapure water system,Gemany) were used in this study.Cinnamaldehyde,6-gingerol,tetrahydropalmatine,sinapine thiocyanate,and methyl eugenol (purity ≥ 98.0%)were purchased from Chengdu Aifa Biological Technology Co.,Ltd. (China). Ephedrine hydrochloride (purity ≥98.0%) was purchased from the Chinese Medicine Food and Drug Control Institute (China). The ingredients Mahuang (Ephedrae Herba),Jiezi (Sinapis Semen),Xixin(Asari Radix et Rhizoma),Dingxiang (Caryophylli Flos),Rougui (Cinnamomi Cortex),Yanhusuo (Corydalis Rhizoma),Gansui (Kansui Radix),and Shengjiang (Zingiberis Rhizoma Recens) were all purchased from Hunan Zhenxing Chinese Medicine Co.,Ltd. (China),in compliance with the requirements of an appendix ofChinese Pharmacopoeia(2020 edition).
3 Methods and results
3.1 QTPP and CQAs establishment of CTL extraction process
The appropriate dosage form design,route of administration,and packaging form of preparation were determined by investigating relevant research literature and combining it with previous experiments[17,18]. The QTPP and CQAs of the CTL extract were established,as shown in Table 1 and 2.

Table 1 QTPP of CTL extract

Table 2 CQAs identification of CTL extract
3.2 CTL test solution preparation and composition identification
3.2.1 CTL test solution preparation The prescribed amount of CTL was weighed including 30 g Jiezi (Sinapis Semen),20 g Mahuang (Ephedrae Herba),6 g Xixin (Asari Radix Et Rhizoma),20 g Dingxiang (Caryophylli Flos),20 g Rougui (Cinnamomi Cortex),20 g Yanhusuo (Corydalis Rhizoma),20 g Gansui (Kansui Radix),and 20 g Shengjiang (Zingiberis Rhizoma Recens). The liquidsolid ratio was 10∶1,the pH value was adjusted to 4,and the extraction time was 120 min. The extraction was performed in triplicate,filtered,the filtrate combined,concentrated,filtered through a microporous membrane,and the filtrate was ready for use.
3.2.2 Reference solution preparation In total,10.8 mg ephedrine hydrochloride,10.0 mg sinapine thiocyanate,11.8 mg tetrahydropalmatine,8.8 mg cinnamaldehyde,9.1 mg 6-gingerol,and 10.0 mg methyl eugenol reference substance were precisely weighed and dissolved in an appropriate amount of methanol. The volume was made up to 10 mL in a volumetric flask with methanol,and 1 mL was transferred to 25 mL volumetric flasks and mixed evenly. A mixed reference solution was prepared.The mass concentrations of ephedrine hydrochloride,sinapine thiocyanate,tetrahydropalmatine,cinnamaldehyde,6-gingerol,and methyl eugenol were 43.2,40.0,47.2,35.2,36.4,and 40.0 μg/mL,respectively.
3.2.3 High Perfomance Liquid Chromatography (HPLC)conditions Hypersil BDS C18column (250 mm × 4.6 mm,5 μm); mobile phase: acetonitrile (A) - 0.1% phosphoricacid (B); elution gradient: 0 - 10 min,4% A; 10 - 20 min,4% - 12% A; 20 - 40 min,12% - 25% A; 40 - 60 min,25% - 35% A; 60 - 80 min,35% - 50% A; 80 - 90 min,50% - 90% A; 90 - 100 min,90% - 4% A; flow rate:1.0 mL/min; column temperature: 30 °C; detection wavelength: 210 nm,310 nm; injection volume: 10 μL.
3.2.4 Linear relationship investigation The appropriate amounts of ephedrine hydrochloride and sinapine thiocyanate reference samples were taken and accurately weighed. Methanol was used to prepare mixed solutions containing 0.26 mg/mL ephedrine hydrochloride and 0.38 mg/mL sinapine thiocyanate. 1,1.5,2,3,and 5 mL of the above-mentioned reference solution were placed in a 10 mL measuring flask,diluted to the mark with methanol,shaken well,and analyzed. Linear regression was performed on the mass concentration of ephedrine hydrochloride and sinapine thiocyanate by the peak area. The regression equations of ephedrine hydrochloride and sinapine thiocyanate were Y = 20 895X - 28.348 (R = 0.999 6)and Y = 19 454X - 226.960 (R = 0.999 7),rspectively. The linear relationship between the acid salt concentration was 0.038 - 0.190 mg/mL,and the linear relationship between ephedrine hydrochloride was 0.026 - 0.130 mg/mL.
3.2.5 Precision inspection The same test solution of CTL extract was injected six times for analysis (conditions according to section 3.2.3) and the chromatograms were recorded. Using ephedrine hydrochloride as an example,the Relative Standard Deviation (RSD) value of the relative retention time of the six main chromatographic peaks was 1.02%,the RSD value of the relative peak area was 1.24%,and the similarity value of each chromatogram was 0.998,indicating that the precision of HPLC was high.
3.2.6 Stability investigation The same test solution of the CTL extract was used,and samples were injected at 0,2,4,6,8,12,and 24 h (conditions according to section 3.2.3) for analysis. The chromatograms were recorded at 210 and 310 nm. Using ephedrine hydrochloride as an example,the RSD value of the relative retention time of the six main chromatographic peaks was 0.73%,the RSD value of the relative peak area was 2.51%,and the similarity value of each chromatogram was 0.998,indicating that the test solution remained stable within 24 h,and the stability was good.
3.2.7 Repeatability investigation The precision inspection test (according to section 3.2.5) was repeated to determine repeatability. Using ephedrine hydrochloride as an example,the RSD value of the relative retention time of the six main chromatographic peaks was 0.93%,the RSD value of the relative peak area was 0.63%,and the similarity value of each chromatogram was 0.998,indicating that the repeatability of this method was good. Relative peak area was 2.51%,and the similarity value of each chromatogram was 0.998,indicating that the test solution remained stable within 24 h,and the stability was good.
3.2.8 Transfer rate calculations for ephedrine hydrochloride and sinapine thiocyanate 10 μL each of the reference substance solution was prepared (preparation according to section 3.2.2) and measured under the chromatographic conditions (according to section 3.2.3). The peak areas of ephedrine hydrochloride and sinapine thiocyanate were recorded and the ephedrine hydrochloride content (M0) and sinapine thiocyanate content(N0) in ephedra decoction pieces,ephedrine hydrochloride content (M) and sinapine thiocyanate content (N) in CTL extract were calculated. The transfer rate of ephedrine hydrochloride in CTL extract was calculated using formula (1),and the transfer rate of sinapine thiocyanate in CTL extract was calculated using formula (2).

3.2.9 HPLC analysis of the main compounds of CTL extract The test solution (according to section 3.2.1) and the mixed control solution (according to section 3.2.2)were injected under the chromatographic conditions (according to section 3.2.3),and the chromatogram was recorded. Ephedrine hydrochloride and sinapine thiocyanate were the active compounds in the principle drugs Mahuang (Ephedrae Herba) and Jiezi (Sinapis Semen) in the prescription of CTL; tetrahydropalmatine was the sedative compound in Yanhusuo (Corydalis Rhizoma);methyl eugenol was the active compound in Xixin (Asari Radix et Rhizoma) and Dingxiang (Caryophylli Flos); cinnamaldehyde was the active compound in Rougui (Cinnamomi Cortex),and 6-gingerol was the active compound in Shengjiang (Zingiberis Rhizoma Recens). Gansui (Kansui Radix) was used as an envoy drug in the prescription,and the main active compound had no evident effect on the treatment of bronchial asthma. Therefore,we selected these six active compounds as detection indicators.
As shown in Figure 2,peaks one to six in the chromatogram of the mixed control solution were ephedrine hydrochloride,sinapine thiocyanate,tetrahydropalmatine,methyl eugenol,cinnamaldehyde,and 6-gingerol. Compared with that seen in the reference chromatogram,it could be seen that the CTL extract contained ephedrine hydrochloride,sinapine thiocyanate,tetrahydropalmatine,methyl eugenol,cinnamaldehyde,and 6-gingerol.The peak area of these components accounted for 0.94%,14.32%,0.78%,31.23%,0.34%,and 0.44% of the total peak area of the CTL extract.
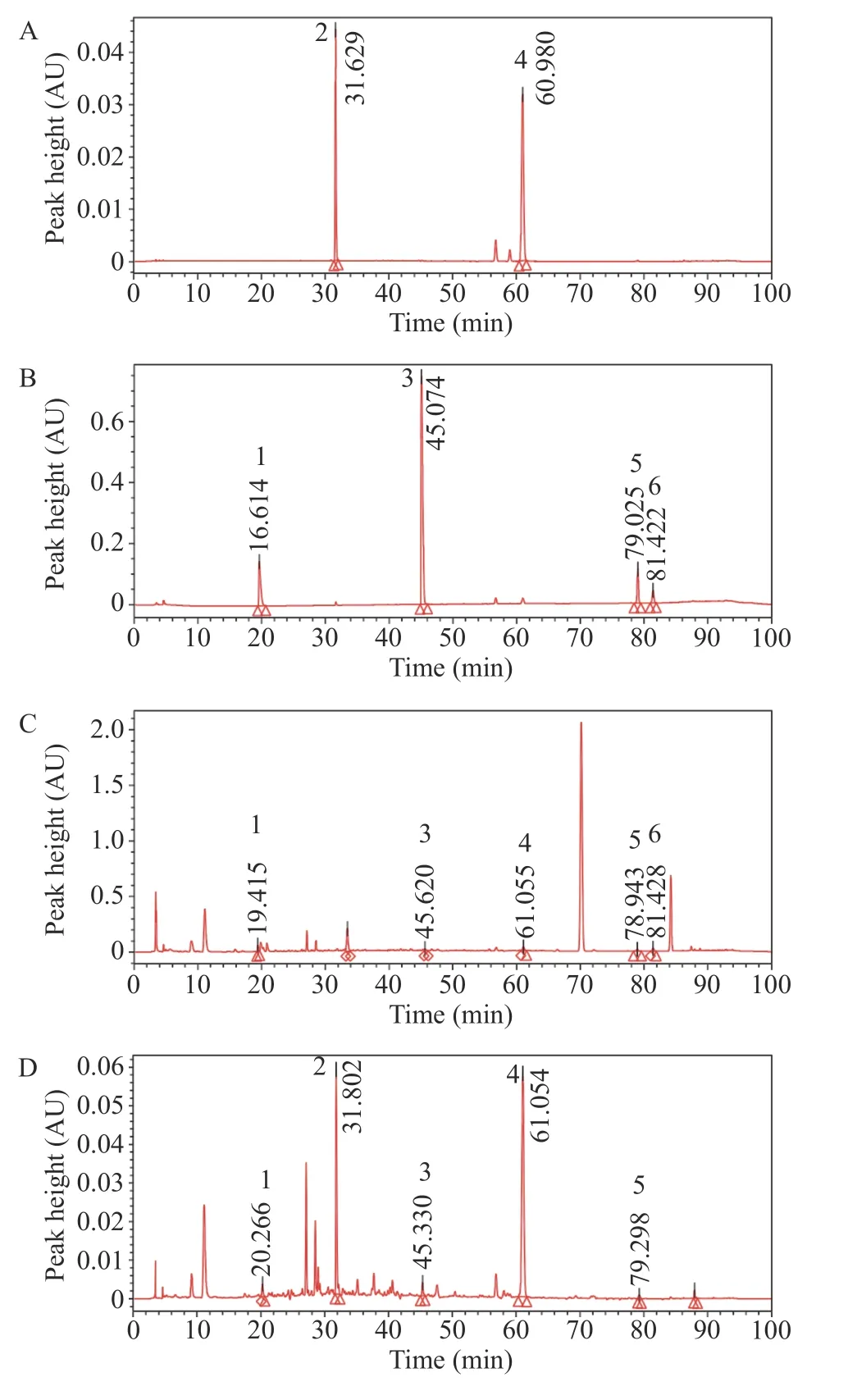
Figure 2 HPLC chromatogram of mixed reference substance and CTL extract
3.3 Determination of extract yield
25.00 mL of the CTL water extract preparation refered to section 3.2.1 was precisely pipetted and placed in an evaporating dish that had dried to a constant weight,evaporated to dryness in a water bath,dried at 105 °C for 3 h,and transferred to a desiccator after cooling for 30 min,weighed,and calculated according to formula (3).

3.4 Single-factor investigation
3.4.1 Liquid-solid ratio Five doses of CTL were weighed for the single-factor experiment of the liquid-solid ratios of 6∶1,8∶1,10∶1,12∶1,and 14∶1,pH value was adjusted to 4,the extraction time was 120 min,extraction was done three times,and then filtered. The filtrate was combined,500 mL of which was concentrated to approximately 100 mL,the content of ephedrine hydrochloride and mustard thiocyanate,and their extraction rates were determined. The comprehensive score was calculated as(ephedrine hydrochloride transfer rate × 0.4)/maximum ephedrine hydrochloride transfer rate + (sinapine thiocyanate transfer rate × 0.4)/maximum sinapine thiocyanate transfer rate + (extract yield × 0.2)/maximum extract transfer rate.
When the liquid-solid ratio was approximately 8∶1,the comprehensive score peaked (Figure 3A). When the liquid-solid ratio was greater than 8∶1,a substantial increase in the liquid-solid ratio could exert a certain impact on the pH value of the extract. This would change the content of ephedrine hydrochloride,sinapine thiocyanate,and other substances in CTL that were pH-sensitive; therefore,8∶1 was selected as the best liquid-solid ratio.
3.4.2 Extraction time Five doses of CTL were weighed for the single-factor experiment of extraction time. The extraction duration was 60,90,120,150,and 180 min,the liquid-solid ratio was 10∶1,pH value was adjusted to 4,extracted three times,and filtered. The filtrate was combined,500 mL of which was concentrated to approximately 100 mL,and the content of ephedrine hydrochloride and mustard thiocyanate and the extraction rate were determined. The comprehensive score was calculated as(ephedrine hydrochloride transfer rate × 0.4)/maximum ephedrine hydrochloride transfer rate + (sinapine thiocyanate transfer rate × 0.4)/maximum sinapine thiocyanate transfer rate + (extract yield × 0.2)/maximum extract transfer rate.
The comprehensive score peaked when the extraction time was approximately 90 min (Figure 3B). Because ephedrine hydrochloride had a small molecular weight and a certain volatility,and sinapine thiocyanate would be degraded with the extension of the extraction time,resulting in a downward trend in the comprehensive score of extraction time greater than 90 min,90 min was selected as the best extraction time.
3.4.3 pH value Five doses of CTL were weighed for the single-factor experiment of pH value. Hydrochloric acid was added to adjust the pH value to 1,2,3,4 and 5,the ratio of liquid-solid was 8∶1,extracted three times and filtered. The filtrate was combined,500 mL of which was concentrated to approximately 100 mL,and the content of ephedrine hydrochloride and mustard thiocyanate and the extraction rate were determined. The comprehensive score was calculated as (ephedrine hydrochloride transfer rate × 0.4)/maximum ephedrine hydrochloride transfer rate + (sinapine thiocyanate transfer rate ×0.4)/maximum sinapine thiocyanate transfer rate + (extract yield × 0.2)/maximum extract transfer rate.
When the pH value was approximately 4,the comprehensive score reached its peak (Figure 3C).
3.4.4 Times of extraction screening Under the conditions of a extraction time of 90 min,a liquid-solid ratio of 8∶1,and a pH value of 4,the effects of different of decoction extraction times (1,2,3,4,and 5) on the comprehensive score were investigated.
The results showed that the extraction rate increased with the increased extraction times,but when the extraction times was greater than three,the comprehensive score decreased (Figure 3D). Excessive extraction times would increase the cost and affect extraction efficiency.Therefore,three times of extration were selected for the follow-up experiments.

Figure 3 Results of single-factor experiments
3.5 Response surface optimization of extraction process
3.5.1 Box-Behnken Design (BBD) and RSM According to the single-factor experiment,the best experimental point for water extraction of CTL ingredients was selected. Taking the ephedrine hydrochloride and sinapine thiocyanate content,and the comprehensive score of the extract yield as the response value,a mathematical model was established to optimize the prescription. The factor levels are presented in Table 3.
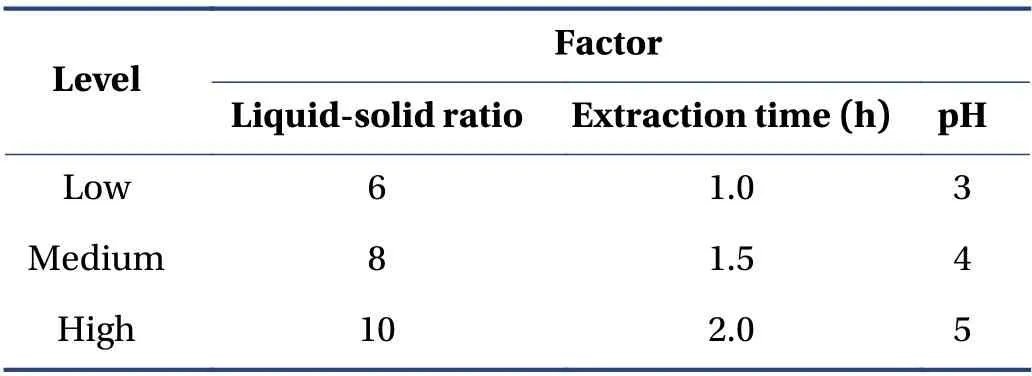
Table 3 Factor levels of BBD
3.5.2 Model fitting and analysis of variance The selected variables were studied at three different levels,and experiments were performed according to BBD (Table 4).

Table 4 Box-Behnken design and results
3.5.3 BBD and RSM The quadratic polynomial regression equation referring to the three independent variables was as follows: Y = - 117.19 + 29.00A + 201.90B -7.66C + 0.33AB - 1.01AC - 7.36BC - 1.10A2- 42.04B2+1.44C2,R = 0.945 1,indicating that the model fitted well.Table 5 shows that the model was statistically significant(P< 0.05),and described the experimental results well.ThePvalue of the model's lack-of-fit was 0.6987,indicating that there was no significant difference in the lack-offit term. The probability that the actual value did not fit the predicted value was low,the model was significant,and the fitting equation had a good predictability for the experimental results. It can be seen that among the three factors investigated in the extraction process,the liquidsolid ratio (A) was a very significant item,the extraction time (B) and pH value (C) were significant,and B2(P =0.006 9) indicated the relationship of the factor and the response surface value was not a simple linear relationship; the BC interaction was significant,indicating that the interaction between the extraction time (B) and pH value (C) was evident. The response surface three dimensions (3D) diagram is shown in Figure 4.
3.6 Establishment and verification of design space
A single-factor experiment was used to select extraction times,and liquid-solid ratio was to be as the main investigation factor. BBD was then used to establish the mathematical models of CQAs and CPPs,and the MODDE software (Umetrics) self-edited program was used for calculations. Based on the literature[19],a comprehensive score of the yield of the CTL extract and the transfer rate of active compounds through water extraction greater than 80 was ideal,and the comprehensive score optimization standard was set to 80.
The design space for the CTL water extraction process was obtained. The operating space range was8.9 -10.0 times the water addition,extraction time was 72.0 - 103.8 min,and the pH value was 4.47 - 5.00(Figure 5).
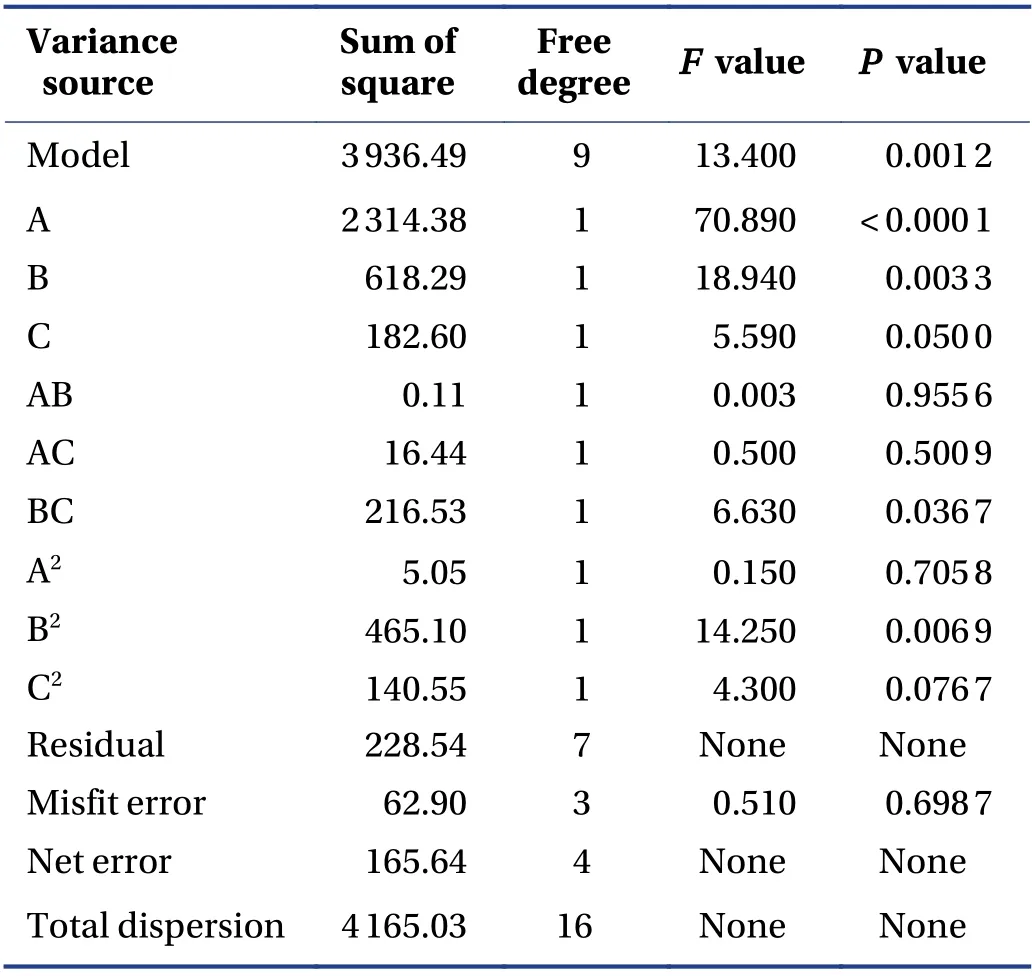
Table 5 Response surface analysis of variance and significance test
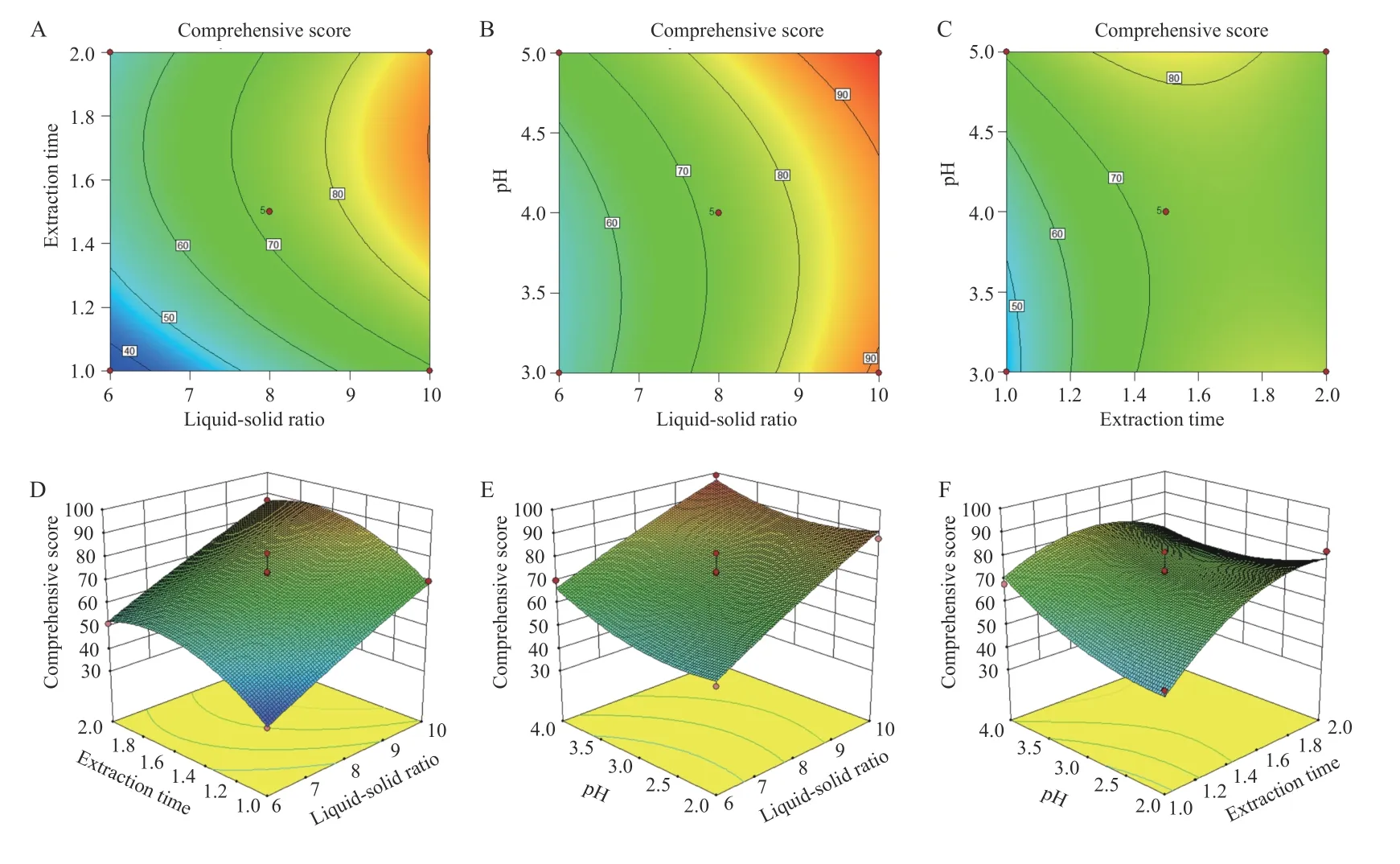
Figure 4 Contour plots and 3D response surface plots of the comprehensive evaluation
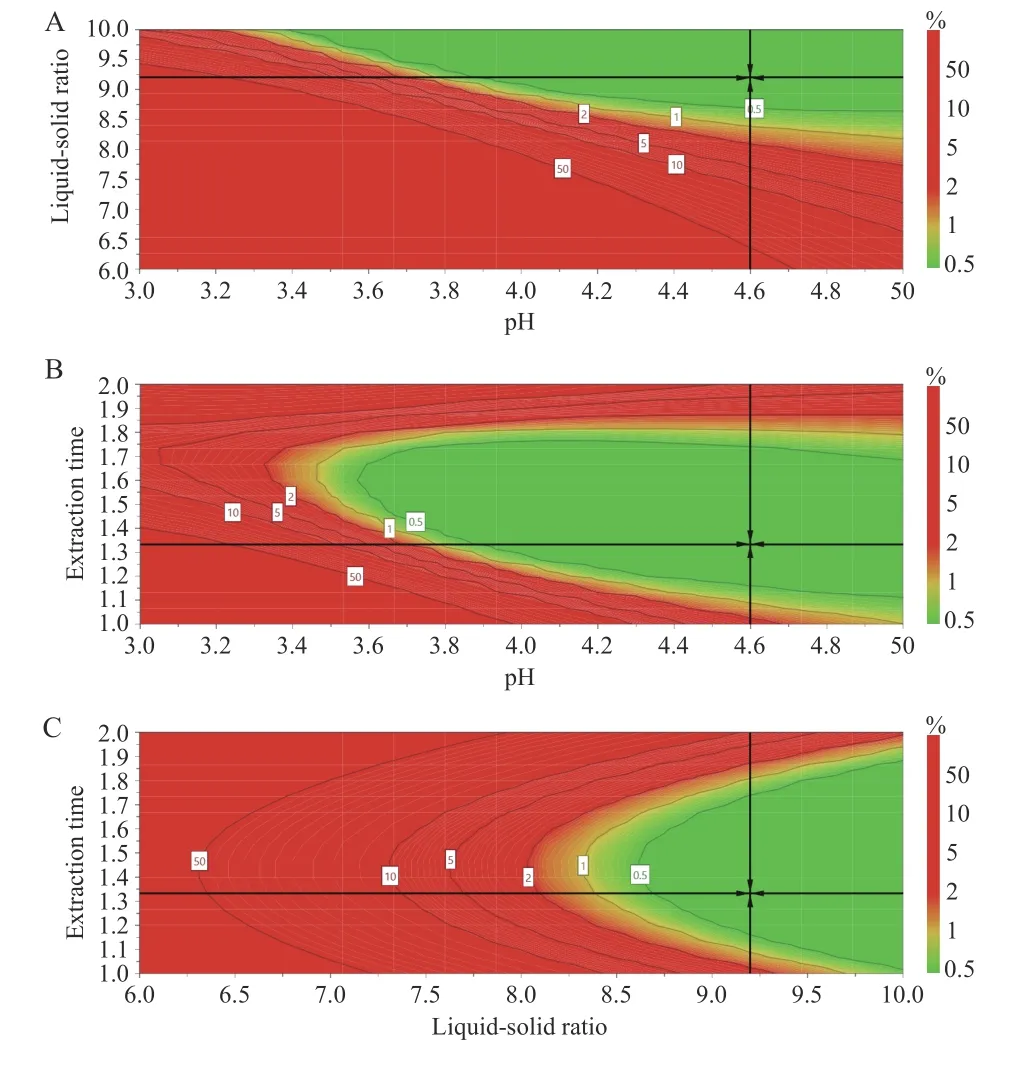
Figure 5 Design spatial result
The verification results (Table 6) showed that the design space operation of the process parameters could ensure the stable quality of the CTL water extraction process and effectively reduce the variability in product quality. The design space was irregular. To facilitate the operation and subsequent industrialization,the recommended operating space range was nine times the water addition,the extraction time was 72 min,and the pH value was 4.5.
Table 6 Verification points and results (±s,n = 3)
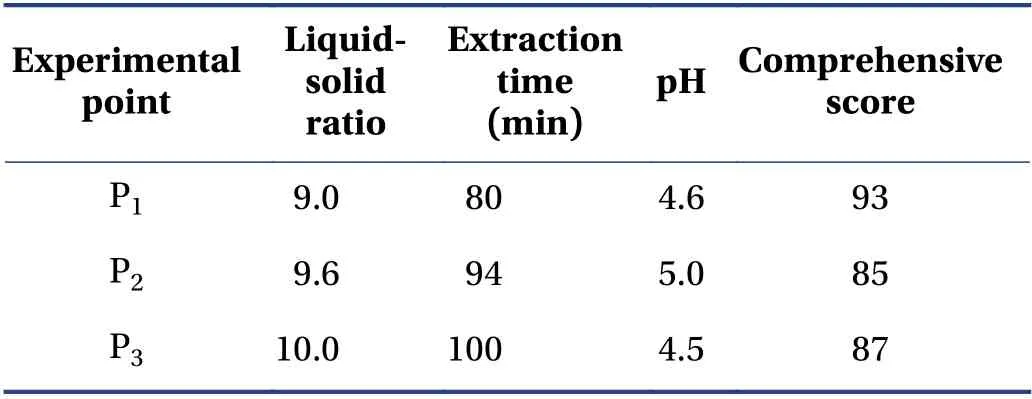
Table 6 Verification points and results (±s,n = 3)
P1,P2,and P3 represent three random experimental points in the design space.
Experimental point Liquidsolid ratio Extraction time(min)pH Comprehensive score P1 9.0 80 4.6 93 P2 9.6 94 5.0 85 P3 10.0 100 4.5 87
4 Discussion
Mahuang (Ephedrae Herba),Jiezi (Sinapis Semen),Xixin(Asari Radix et Rhizoma),Dingxiang (Caryophylli Flos),Rougui (Cinnamomi Cortex),and Shengjiang (Zingiberis Rhizoma Recens) in the prescription of CTL are rich in volatile oil components that can be extracted by water,and they exert certain curative effects on bronchial asthma[20]. The HPLC method established in this study was simple and effective,with accurate results and good precision. It can simultaneously determine the six effective components in CTL. With the improvement in people's awareness regarding drug safety,it is extremely important to strengthen the quality control of the extraction process of TCM ingredients.
Based on the influencing factors,different pH values were selected as the investigating factors. When the pH value was 4.5 - 5.0,the acid promoted alkaloid leaching,improved alkaloid stability,and freed the organic acid to facilitate the removal of acid-insoluble impurities[21]. The results of this experiment showed that if the extraction time was too short or too long,the comprehensive score would be reduced,and both ephedrine hydrochloride and sinapine thiocyanate showed a downward trend,suggesting that high-temperature conditions might cause the destruction of active compounds,thereby affecting the test results. Therefore,in the preparation process of the CTL patch,it is particularly necessary to pay attention to the quality control in the extraction process and select the appropriate pH value,liquid-solid ratio,and extraction time to avoid reducing of the efficacy and the transfer of effective components due to unreasonable extraction processes.
In this study,the extraction process of CTL as an example. By combining a single-factor test and BBD and introducing QbD into the experimental design method of CTL extraction,a quantitative mathematical model and process of CQAs and CPPs were constructed[22]. This method effectively improved the transfer rate and extract yield of ephedrine hydrochloride and sinapine thiocyanate,reduced the loss during the extraction process,and demonstrated a stable process and good reproducibility.The design space provided an experimental basis for future research on CTL and the development of new drugs.Combining the operating space obtained in the experiment with the actual production situation,the best extraction process was nine times of water addition,72 min of extraction time,and 4.5 of pH value.
5 Conclusion
Compared with the results obtained by using fixed process parameters,the establishment of a design space made process operations more flexible and effectively reduced the variability of product quality. The application of QbD in the pharmaceutical industry can effectively improve production efficiency and is of great importance to the development of the pharmaceutical field.
Fundings
Hunan University of Traditional Chinese Medicine Graduate Training Quality Engineering Project(2019CX57),and the First-class Discipline Project on Chinese Pharmacology of Hunan University of Chinese Medicine (201803).
Competing interests
The authors declare no conflict of interest.
 Digital Chinese Medicine2022年2期
Digital Chinese Medicine2022年2期
- Digital Chinese Medicine的其它文章
- Visualization analysis of the international standard ISO/TC 249 for traditional Chinese medicine
- MEDICLOUD: a holistic study on the digital evolution of medical data
- Ancient and modern medication laws of aromatic Chinese medicines in treating angina pectoris based on data mining
- Intra-set correlation analysis of medical records of thyroid cancer treated by traditional Chinese medicine Master ZHOU Zhongying
- Traditional Chinese medicine Master XIONG Jibo’s medication experience in treating arthralgia syndrome through data mining
- Screening influencing factors of blood stasis constitution in traditional Chinese medicine
