Hyperpolarization-activated cyclic nucleotide-gated 2 contributes to electroacupuncture analgesia on lumbar disc herniation-induced radicular pain through activation of microglia in spinal dorsal horn
QIN Qingguang,CHEN Zujiang,FAN Weimin,LI Junhua,LIAO Liqing,LI Yikai
QIN Qingguang,CHEN Zujiang,FAN Weimin,LI Junhua,LIAO Liqing,LI Yikai,School of Traditional Chinese Medicine,Southern Medical University,Guangzhou 510515,China
Abstract OBJECTIVE:To explore the mechanisms of dorsal root ganglia and spinal microglia cascade cross in electroacupuncture (EA) analgesia in the treatment of lumbar disc herniation.METHODS:A rat model of lumbar disc herniation (LDH)was established,EA was administered at Huantiao(GB30) acupoint 30 min once a day,for 3 d.Before and after modeling,and after EA,mechanical allodynia thresholds were detected.Hyperpolarization-activated cyclic nucleotide-gated 2 (HCN2) in dorsal root ganglia was detected by quantitative polymerase chain reaction(qPCR) and Western blot.C-X3-C motif chemokine ligand 1 (CX3CL1) and activity of microglia in spinal cord was observed separately via qPCR and immunofluorescence staining.RESULTS:The mechanical allodynia threshold of the right planta of model rats was significantly reduced (P <0.01),EA increased the mechanical pain threshold of rats(P < 0.01),and decreased HCN2 mRNA,and protein expression,reduced the expression of CX3CL1 and the activation of microglia.ZD7288 (a blocker of HCN channel) reduced the analgesic effect of EA from 1.83 ±0.84 to 0.74 ± 0.20 (P < 0.05),and the expression of CX3CL1 in the spinal cord decreased from 0.52 ± 0.11 to 0.15 ± 0.05 (P < 0.01).CONCLUSION:EA analgesia on the radicular pain of LDH is definite.EA reduced the expression of HCN2 channel in the dorsal root ganglion,thereby decreasing the noxious stimulation entered to microglia in spinal dorsal horn. Our work supports EA is an effective treatment for radicular pain of LDH.
Keywords:intervertebral disc displacement;radicular pain;electroacupuncture;ganglia,spinal;hyperpolarization-activated cyclic nucleotide-gated channels;microglia
1.INTRODUCTION
lumbar disc herniation (LDH) is one of the most common disorders.The prevalence of symptomatic lumbar disc herniation is 1% to 3%.1LDH is known to be prone to chronic pain and central sensitization.2In LDH,the nucleus pulposus’ mechanical compression and the damage to the dorsal root ganglion (DRG) caused by released inflammatory chemicals are important factors for low back pain and radicular pain.3DRG neurons,as primary neurons of sensory afferents,play an important role in the generation and transmission of pain.4HCN2 in the dorsal root neuron is involved in mechanical allodynia and thermal hyperalgesia.5Microglia in spinal dorsal horn is a type of immune cells within the nervous system.They receive noxious stimuli transmitted from the DRG,and activated to participate in immune responses,produce pro-inflammatory factors and chemokines,and promote central pain sensitization.6In the LDH model,spinal microglia proliferate in a large amount from resting to reactive state,and a variety of pro-inflammatory factors,such as tumor necrosis factorα,interleukin1-β,IL-6 are released.7
In the acute stage of LDH,electroacupuncture (EA) can be used to relieve pain and delay pain progression.The mechanism involved DRG to microglia are ill defined.In the present study,we aimed to explore whether that EA directly reduce HCN2 channel in the DRG and that this could in turn reduced activity of microglia in spinal dorsal horn through an CX3CL1-dependent mechanism.
2.MATERIALS AND METHODS
2.1.Subjects
Adult specified pathogen free-grade Sprague-Dawley male rats weighted 200-250 g were purchased from Lab Animal Center of Southern Medicine University(Guangzhou,China).The animals were kept under standardized conditions:room temperature 25 °C,circadian rhythm 12 h/12 h,and they were given adequate food and water.All rats were allowed to acclimate to laboratory environment for 7 d.The animal experiments were performed in accordance with the guidelines of Guangdong Province on the Review of Welfare and Ethics of Laboratory Animals.The experimental protocol was approved by the Experimental Animal Ethics Committee of Guangzhou University of Chinese Medicine.
2.2.lumbar disc herniation model
Rats were randomly divided into LDH model group and sham model group.
(a) Nucleus pulposus harvested referring to the methods reported by Shoji Yabuki.8Rats were intraperitoneally anesthetized with 40 mg/kg pentobarbital sodium.A 1.5 cm midline incision was made in the back of the tail.The tail nucleus pulposus about 2 mg was scrape.A longitudinal 2 cm incision was made along the dorsal midline,which was at the midpoint of L5 spinous process,to expose the right L5/6 facet joint.Under the microscope,the right L5 DRG was exposed after L5 lower articular process and part of the lamina polished off using an electric drill.The harvested nucleus pulposus was placed to the exposed DRG.There was no nucleus pulposus placed in the sham model group,and the rest of the operations were the same as the model group.The mechanical allodynia threshold of right plantar were measured 3 d after the operation,The model was successful if the mechanical allodynia threshold was reduced by 50%.
2.3.Group and electroacupuncture
The rats were randomly divided into normal group,LDH model group (model group),EA group,EA+ZD7288 group,and sham EA+ZD7288 group (n=7 animals per group).
The EA treatment was performed on rats under isoflurane anesthesia,Bilateral Huantiao (GB30)acupoints were selected,which is situated at the junction of the lateral 1/3 and medial 2/3 of the line connecting the prominence of greater trochanter of femur with the sacral hiatus on the rat.9Disposable sterile acupuncture needle (0.13 mm diameter,25 mm length,HuaTuo acupuncture needle,China Suzhou Acupuncture Goods.Co.,Ltd.) were straightly penetrated depth 6mm,stimulation parameters:continuous wave 2 Hz,2 mA,wave width 4 ms,30 min.EA was performed 3 d after surgery,once a day,for 3 consecutive days.
2.4.Pain behavior
Mechanical allodynia thresholds of right plantar sole were measured by paw mechanical withdrawal threshold,which was performed before,3 and 6 d after the operation.Rats were put in the test box before 30 min,to acclimatize testing environment.Mechanical allodynia was measured with a set of Aesthesio Von Frey filaments(Aesthesio Pain Test Kit,4,6.0,8.0,15,26,60,100,180,300 g).Filament was vertically pressured to the right plantar surface,with a sustaining force for 5 s,with an interval of 30 s between stimulations.The appearance of lifting,avoiding or licking is a positive reaction.The“updown”method was used to determine 50% force withdrawal threshold.10
2.5.Drug administration
(a) Lumbar intrathecal catheterization:according to the method described by Hou Yongheng.11A PE-10 catheter was implanted into the subarachnoid space,the other end of the PE tube was fixed to the back of the neck,exposing the skin about 2 cm.The sterilized red wire (outer diameter 0.28 mm) seals the catheter to prevent the outflow of cerebrospinal fluid.Rats were kept in single cages after operation.
(b) ZD7288 administration:each rat was injected with 50 μg ZD7288 according to reported method.12ZD7288 was diluted with sterile saline to prepare a concentration of 5 μg/μL.A volume of 10 μL physiological saline and 10 μL prepared solution was drawn successively using a micro-syringe.Under isoflurane anesthesia,the volume of 20 μL liquid was injected into the subarachnoid space through PE catheter for 3 consecutive days.
2.6.Immunofluorescence staining
After anesthesia with chloral hydrate,the heart was perfused with saline water and fixed with 4%paraformaldehyde.T13-L1 spinal cord was harvested,13and placed in 4% paraformaldehyde.After embedding,slicing (2 μm thickness),permeabilizing,and sealing operations,the primary antibody was added dropwise and incubated at 4 ℃overnight.The next day,the sections incubated with secondary antibody in the dark.Primary antibodies:rabbit anti-ionized calcium binding adaptor molecule 1 (Iba1) (1∶200,ZEN BIO),goat anti-CX3CL1 polyclonal antibody (1∶100,Immun-oClone),secondary antibody:goat anti-mouse IgG (1 ∶100,Abcam),Fluorescence images were taken with a fluorescence microscope (Olympus,BX51).
2.7.Real-time quantitative polymerase chain reaction(RT-qPCR) analysis
RT-qPCR was performed to detect expression of HCN2 in the right L5 dorsal root nerve and CX3CL1 in the T13-L1 spinal cord.Total RNA was extracted from T13-L1spinal cord or DRG using TRIzol reagent.The total RNA yield was determined by Eppendorf BioPhotometer D30.Using 2 μg total RNA as a template,a reverse transcription reaction system was prepared according to the Bestar qPCR RT Kit instructions,the total system was 10 μL,and the first strand of cDNA was synthesized.RT-PCR amplification reaction system is 20 μL,prepared according to the following table:10 μL Bestar® SybrGreen qPCR masterMix,0.5 μL PCR Forward Primer (10 μLM),0.5 μL PCR Reverse Primer (10 μM),1 μL cDNA template,8 μL ddH2O,The primers had the following sequences (Table 1).

Table 1 Primers used in this study
The PCR reaction was as follows:95 ℃ for 2 min,94 ℃for 20 s,58 ℃ for 20 s,72 ℃ for 20 s,and 40 cycles,GAPDH was used as an internal reference gene,the relative expression of gene mRNA was calculated according to the 2-△△Ctmethod.
2.8.Western blotting
The harvested L5 dorsal root ganglion was subjected to protein extraction in alysis buffer,and the protein concentration was determined by BCA protein assay.Western blotting analysis was executed in the normal series of steps.After SDS-PAGE electrophoresis and transfer,sections were block with 5% skim milk for 2 h,add primary antibody HCN2 (1 ∶200,Santa Cruz Biotech.,Santa Cruz,CA,USA) and incubate overnight at 4 ℃,add followed by goat anti-rabbit secondary antibody (1∶200,Abcam) and incubate for 1 h.The protein bands were visualized on the film with Immobilon Western chemiluminescent HRP substrate(WBKLS0500,MilliporeSigma,Billerica,MA,USA),taken by X-ray photo,β-actin served as an internal reference protein.
2.9.Statistical analysis
Statistical analysis was performed with SPSS 25.0 software (IBM SPSS Statistics for Windows,Version 25.0.IBM Corp.,Armonk,NY,USA).All data were presented as the mean ± standard deviation.One-way analysis of variance was performed for comparison between groups,Pairedt-test was used for statistical analysis within a group.APvalue < 0.05 was considered statistically significant.
3.RESULTS
3.1.EA alleviating pain and microglia activation in rat model of LDH
The mechanical pain threshold of the right foot of the model rats was significantly reduced,on postoperative 3 day (t=-18.580,P=0.01),which in sham model group did not change markedly (Figure 1A).We selected Huantiao (GB30) acupoint to start EA on the 3rd day of the model,for 3 consecutive days.The mechanical pain threshold of rats in the EA group was increased (t=6.693,P=0.01) (Figure 1A).
In this experiment,on the 6th day of autologous nucleus pulposus transplantation,we harvested T13-L1 spinal cord for immunofluorescence,staining,found that Iba1,a specific marker of microglia,was highly expressed(Figure 1D),while only a small amount of Iba1was expressed in sham model group (Figure 1C) and normal group(Figure 1B).EA significantly reduces the expression of Iba1 (Figure 1E).

Figure 1 Electroacupuncture (EA) reduces mechanical allodynia thresholds and microglia activation in rats with lumbar disc herniation (LDH)EA was given on the 3rd day after modeling.A:the line graph shows the effect of EA on the mechanical allodynia threshold of the right plantar in model rats.The mechanical allodynia threshold before the intervention was identified as 1.Fluorescence image shows the expression of Iba1+microglia of rat in the ipsilateral lumbar spinal dorsal horn at 6th day.B:normal group;C:the sham model group;D:model group;E:EA group.compared with normal group,bP < 0.05,compared with EA group,aP < 0.05 (n=7).
3.2.EA decrease HCN2 in dorsal root ganglion and CX3CL1 in spinal dorsal horn
We observed the expression of HCN2 in the DRG after EA treatment.The level of HCN2 mRNA in rat DRG increased significantly (P=0.004) (Figure 2A),and the protein expression of HCN2 increased (Figure 2C).Sixth days after autologous nucleus pulposus transplantation,there was no significant change in the sham model group(Figure 2A,2C).The level of HCN2 mRNA in the EA intervention group was lower than that in the model group (P=0.001) (Figure 2A),and the protein expression was also reduced (Figure 2C).CX3CL1 is the only member of the CX3C subfamily of chemokines,which is involved in neuropathic pain.14We found that CX3CL1 mRNA in the spinal dorsal horn increased (P=0.002) (Figure 2B),and the protein expression increased(Figure 2C),6th day after modeling,which is consistent with the phenomenon reported.15CX3CL1 mRNA and protein expression were prominently decreased in EA group compared with that in model group (P=0.038)(Figure 2B,2D).
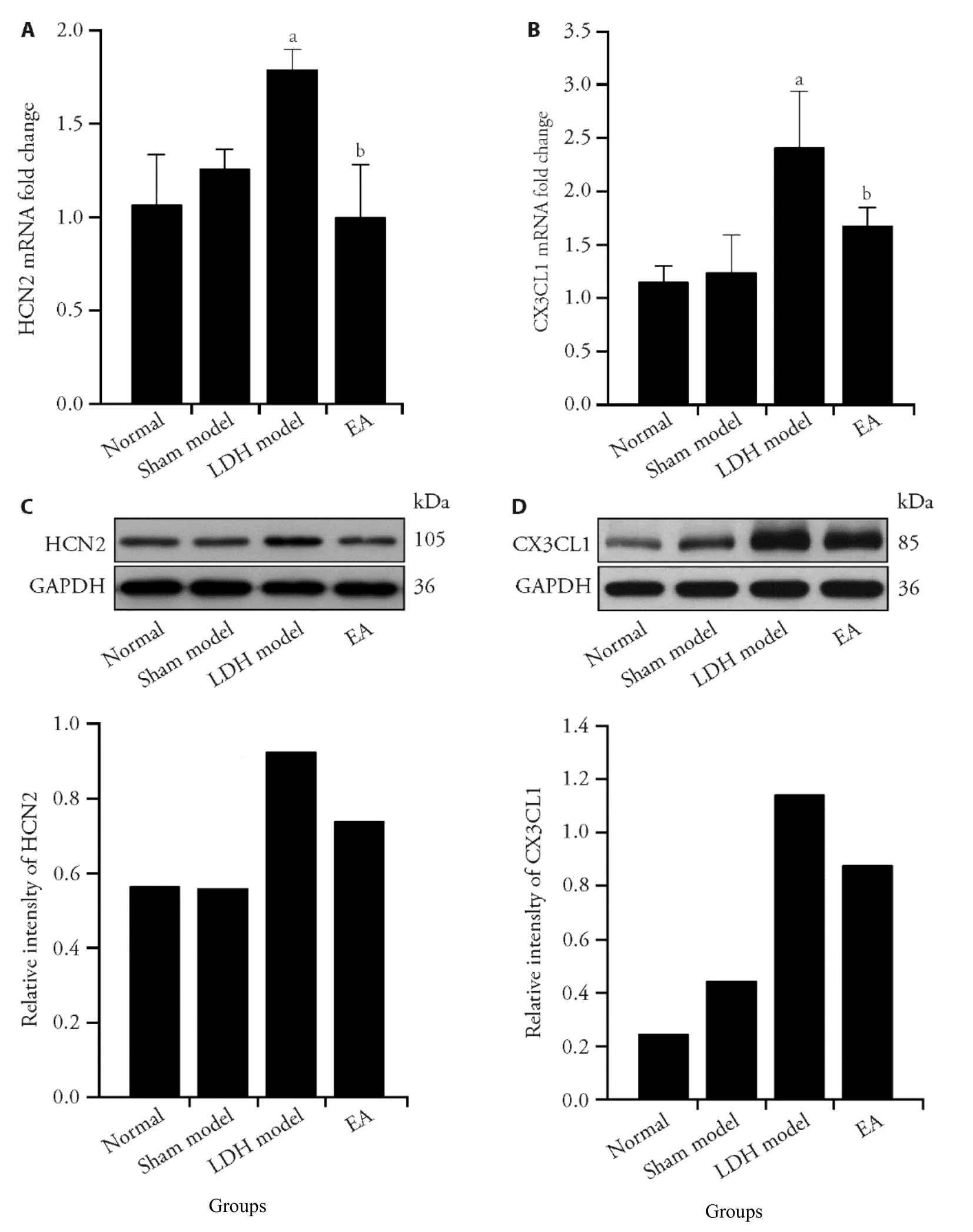
Figure 2 Electroacupuncture (EA) reduces the expression of HCN2 in ipsilateral dorsal root ganglia and CX3CL1 in ipsilateral spinal dorsal horn
3.3.EA’s analgesic effects on lumbar disc hernia are reduced by ZD7288
In order to further confirm the analgesic effects of EA by reducing HCN2,we tested the HNC2 inhibitor ZD7288 on the analgesic effect of EA.We set up ZD7288+EA group and ZD7388+sham EA group,to distinguish the analgesic effects of EA,No current stimulation was given to sham EA group.The analgesic effect of EA decreased from 1.83 ± 0.84 to 0.74 ± 0.20 (t=3.074,P=0.012),after HCN2 blocker ZD7288 (Figure 3A),Western blot results showed that the strip density of HCN2 in ZD7288+Sham EA group is lower than that in ZD7288+EA group (Figure 3C).
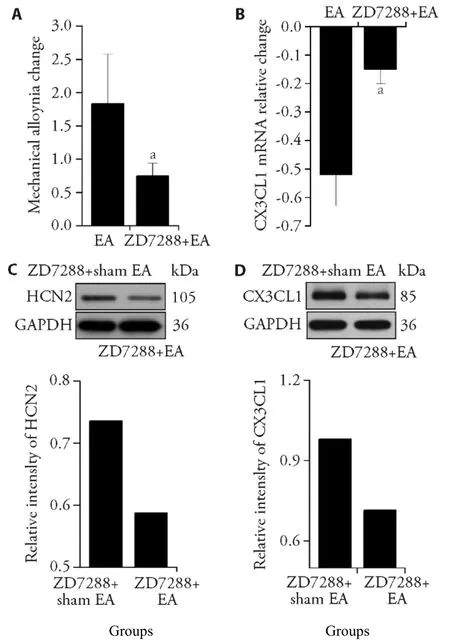
Figure 3 HCN2 antagonist reduces the analgesic effect of electroacupuncture (EA) on rats with lumbar disc herniation (LDH)HCN2 antagonist (50 μg of a rat) were injected into subarachnoid space of LDH model rats,per day for 3 d.A:the analgesic effect of EA on mechanical pain was significantly reduced;B:the expression of CX3CL1 in the spinal cord decreased;C,D:Western blot showed that HCN2 protein and CX3CL1 protein expression of the ZD7288 +sham EA group was higher than that of the ZD7288+EA group,respectively (n= 3).Compared with EA group,aP < 0.05 (n=7).
EA reduced the expression of CX3CL1 mRNA in the spinal cord from 0.52 ± 0.11 to 0.15 ± 0.05 (t=-7.483,P=0.000) after HCN2 blocker ZD7288 (Figure 3B).CX3CL1 protein expression in D7288+Sham EA group is lower than ZD7288+EA group (Figure 3D).These results support that the analgesic effect of EA on LDH is mediated by HCN2.
4.DISCUSSION
In this study,we established a LDH model with autologous nucleus pulposus transplantation.We found that the mechanical pain threshold of the right foot was significantly reduced on the third day,which is the same as reported by Yabuki.8On the 6th day after surgery,both expression of HCN2 in ipsilateral L5 DRG and CX3CL1 in ipsilateral dorsal horn were increased significantly,and microglia in ipsilateral dorsal horn was activated.EA treatment significantly attenuated the expression of HCN2 channels and CX3CL1,reduced activation of microglia.Pharmacological blocking HCN2 channel with selective HCN inhibitor ZD7288.It was found that the analgesic effect of EA and expression of CX3CL1 was significantly reduced.Our research results showed that EA at Huantiao (GB30) acupoint can significantly alleviate the pain caused by nucleus pulposus transplantation,which is the same as the clinical study reported previously.16
The distal ends of the DRG neurons are in the skin,muscle and other surrounding tissues,and the central ends in the dorsal horn of the spinal cord.12These neurons transmit nociceptive stimuli to the spinal dorsal horn through primary afferent Aδ and C fibers.17,18In the DRG,HCN2 channel exists in small-diameter neurons and large-diameter neurons.19HCN2 plays an important role in both inflammatory pain and neuropathic pain.20ZD7288 as selective HCN inhibitor,alleviated pain sensitivity caused by inflammation or nerve injury.21The results of this study showed that the expression of HCN2 in the L5 ganglion was significantly higher in model group than that in sham model group,normal group,and in the EA group.In the EA group,HCN2 decreased with the increase of mechanical pain threshold.It means that HCN2 in the dorsal root nerve is related to the pain sensitivity of model rats.We used ZD7288 to block HCN2,the analgesic effect of EA decreases significantly,indicating that HCN2 participates in the analgesic effect of EA.
Microglia,as the resident macrophage in central nerves system,play an important role in immune responses.22On the 6th day of nucleus pulposus transplantation,we observed that the expression level of microglia marker Iba1 was significantly higher than that of the normal group and the sham model group,which is consistent with previous studies.23Activated microglia release cytokines,such as TNF-α,IL-1β,and IL-2,which drive central sensitization.24The proliferation of microglia caused by nerve injury is accompanied by the development of pain hypersensitivity.Therefore,blocking microglial cell proliferation can reduce painful behaviors.25Microglia are activated rapidly in the early stage of pain development,and astrocytes are activated in the middle and late stages of pain.The early and middle stages of pain development are dominated by the microglia-astrocyte cascade,so early inhibition of microglia activity is also a better choice to control chronic pain.26In this study,EA at Huantiao (GB30)acupoint reduced the expression of Iba1 and increased the mechanical pain threshold.
The chemokine CX3CL1 is only expressed in primary afferent neurons and spinal neurons.CX3CR1,the only receptor of CX3CL1,is only expressed on the microglia membrane.The combination of CX3CL1 and CX3CR1 induces microglia activation.27Although CX3CL1 is synthesized by activated astrocytes,however,astrocytes are involved in chronic pain and to be activated 14 d after surgery.28We found that the expression of CX3CL1 in spinal dorsal horn increased on the 6th day after nucleus pulposus transplantation,which is the same as related research.15A question for further study is that whether CX3CL1 produced by dorsal root neurons was retrogradely transmitted to the spinal dorsal horn,or CX3CL1 produced by spinal dorsal horn neurons.
After blocking the HCN2 channel with ZD7288,the expression of CX3CL1 decreased significantly.It suggests that the blockade of HCN2 reduces the transmission of noxious signals to the spinal dorsal horn.EA reduces the expression of HCN2,it is suggested that EA reduces the expression of HCN2,thereby reducing the activation of CX3CL1 on microglia,and has an analgesic effect.
In conclusion,reducing the transmission of nociceptive information to microglia is an important way to relieve pain.29Our research confirms that EA at Huantiao (GB30)acupoint has analgesic effect on radicular pain caused by LDH.The results of this study have shown that EA reduces transmission of noxious stimuli to the spinal cord,though descending HCN2 in the dorsal root ganglia neurons,leading decrease in CX3CL1 expression and a decrease in microglia activity.Our work supports EA at Huantiao (GB30) acupoint as a potential therapeutic option for radicular pain with LDH.
5.ACKNOWLEDGMENTS
The authors would like to thank Prof.Zhu Bing (Institute of Acupuncture and Moxibustion,China Academy of Chinese Medical Sciences) and Prof.Chen Yong Jun(Guangzhou University of Chinese Medicine) for guidance.The authors would like to acknowledge the facilities supported by South China Acupuncture and Moxibustion Center.
6.REFERENCES
1.Kim YK,Kang D,Lee I,Kim SY.Differences in the incidence of symptomatic cervical and lumbar disc herniation according to age,sex and national health insurance eligibility:a pilot study on the disease's association with work.Int J Environ Res Public Health 2018;15:2094.
2.Huang Y,Li Y,Zhong X,et al.Src-family kinases activation in spinal microglia contributes to central sensitization and chronic pain after lumbar disc herniation.Mol Pain 2017;13:1-13.
3.Sanzarello I,Merlini L,Rosa MA,et al.Central sensitization in chronic low back pain:a narrative review.J Back Musculoskelet Rehabil 2016;29:625-33.
4.Gore M,Sadosky A,Stacey BR,Tai KS,Leslie D.The burden of chronic low back pain:clinical comorbidities,treatment patterns,and health care costs in usual care settings.Spine (Phila Pa 1976)2012;37:E668-77.
5.Emery EC,Young GT,Berrocoso EM,Chen L,McNaughton PA.HCN2 ion channels play a central role in inflammatory and neuropathic pain.Science 2011;333:1462-66.
6.Ji RR,Nackley A,Huh Y,Terrando N,Maixner W.Neuroinflammation and central sensitization in chronic and widespread pain.Anesthesiology 2018;129:343-66.
7.Gu N,Peng JY,Murugan M,et al.Spinal microgliosis due to resident microglial proliferation is required for pain hypersensitivity after peripheral nerve injury.Cell Rep 2016;16:605-14.
8.Yabuki S,Igarashi T,Kikuchi S.Application of nucleus pulposus to the nerve root simultaneously reduces blood flow in dorsal root ganglion and corresponding hindpaw in the rat.Spine (Phila Pa 1976) 2000;25:1471-76.
9.Yang YQ,Yan C,Branford-White CJ,Hou XY.Biological values of acupuncture and chinese herbal medicine:impact on the life science.Evid Based Complement Alternat Med 2014;2014:593921.
10.Chaplan SR,Bach FW,Pogrel JW,Chung JM,Yaksh TL.Quantitative assessment of tactile allodynia in the rat paw.J Neurosci Methods 1994;53:55-63.
11.Hou Y,Wang L,Gao J,Jin X,Ji F,Yang J.A modified procedure for lumbar intrathecal catheterization in rats.Neurol Res 2016;38:725-32.
12.Wan Y.Involvement of hyperpolarization-activated,cyclic nucleotide-gated cation channels in dorsal root ganglion in neuropathic pain.Sheng Li Xue Bao 2008;60:579-80.
13.Padmanabhan R,Singh S.Observations on the topographical relations of spinal nerve roots in the rat.Acta Anat (Basel) 1979;105:378-80.
14.Zhang ZJ,Jiang BC,Gao YJ.Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain.Cell Mol Life Sci Sep 2017;74:3275-91.
15.Park HW,Ahn SH,Kim SJ,et al.Changes in spinal cord expression of fractalkine and its receptor in a rat model of disc herniation by autologous nucleus pulposus.Spine (Phila Pa 1976)2011;36:E753-60.
16.Liu Y,Zhao J,Tian Y.Efficacy and safety of electroacupuncture in treatment of lumbar disc herniation:a protocol for a cohort study.J Tradit Chin Med 2019;39:127-32.
17.Fernandes EC,Luz LL,Mytakhir O,Lukoyanov NV,Szucs P,Safronov BV.Diverse firing properties and Abeta-,Adelta-,and C-afferent inputs of small local circuit neurons in spinal lamina I.Pain 2016;157:475-87.
18.Braz J,Solorzano C,Wang X,Basbaum AI.Transmitting pain and itch messages:a contemporary view of the spinal cord circuits that generate gate control.Neuron 2014;82:522-36.
19.Kouranova EV,Strassle BW,Ring RH,Bowlby MR,Vasilyev DV.Hyperpolarization-activated cyclic nucleotide-gated channel mRNA and protein expression in large versus small diameter dorsal root ganglion neurons:correlation with hyperpolarizationactivated current gating.Neuroscience 2008;153:1008-19.
20.Song XJ,Hu SJ,Greenquist KW,Zhang JM,LaMotte RH.Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia.J Neurophysiol 1999;82:3347-58.
21.He JT,Li XY,Zhao X,Liu X.Hyperpolarization-activated and cyclic nucleotide-gated channel proteins as emerging new targets in neuropathic pain.Rev Neurosci 2019;30:639-49.
22.Du L,Zhang Y,Chen Y,Zhu J,Yang Y,Zhang HL.Role of microglia in neurological disorders and their potentials as a therapeutic target.Mol Neurobiol 2017;54:7567-84.
23.Zhong Y,Huang YL,Hu YM,Zhu LR,Zhao YS.Puerarin alleviate radicular pain from lumbar disc herniation by inhibiting ERK-dependent spinal microglia activation.Neuropeptides 2018;72:30-37.
24.Hanisch UK.Microglia as a source and target of cytokines.Glia 2002;40:140-55.
25.Chen G,Zhang YQ,Qadri YJ,Serhan CN,Ji RR.Microglia in pain:detrimental and protective roles in pathogenesis and resolution of pain.Neuron 2018;100:1292-1311.
26.Blaszczyk L,Maitre M,Leste-Lasserre T,et al.Sequential alteration of microglia and astrocytes in the rat thalamus following spinal nerve ligation.J Neuroinflammation 2018;15:349.
27.Hughes PM,Botham MS,Frentzel S,Mir A,Perry VH.Expression of fractalkine (CX3CL1) and its receptor,CX3CR1,during acute and chronic inflammation in the rodent CNS.Glia 2002;37:314-27.
28.Li T,Liu T,Chen X,et al.Microglia induce the transformation of A1/A2 reactive astrocytesviathe CXCR7/PI3K/Akt pathway in chronic post-surgical pain.J Neuroinflammation 2020;17:211.
29.Inoue K,Tsuda M.Microglia in neuropathic pain:cellular and molecular mechanisms and therapeutic potential.Nat Rev Neurosci 2018;19:138-52.
 Journal of Traditional Chinese Medicine2022年3期
Journal of Traditional Chinese Medicine2022年3期
- Journal of Traditional Chinese Medicine的其它文章
- Efficacy of meridian massage for motor function after a stroke:a systematic review and Meta-analysis
- Antiviral Activity of Medicinal Plants against Human Coronavirus:a systematic scoping review of in vitro and in vivo experimentations
- Fuzheng Kang' ai decoction (扶正抗癌方) inhibits cell proliferation,migration and invasion by modulating mir-21-5p/human phosphatase and tensin homology deleted on chromosome ten in lung cancer cells
- Correlation between slow transit constipation and spleen Qi deficiency,and gut microbiota:a pilot study
- Efficacy of Kushen decoction (苦参汤) on high-fat-diet-induced hyperlipidemia in rats
- Electroacupuncture preconditioning alleviates myocardial ischemiareperfusion injury through the hypothalamic paraventricular nucleusinterposed nucleus nerve pathway
