Combustion Synthesis of Si3N4-BN-SiC Composites by in-situ Introduction of BN and SiC
ZHANG Ye, YAO Dongxu, ZUO Kaihui, XIA Yongfeng, YIN Jinwei, ZENG Yuping
Combustion Synthesis of Si3N4-BN-SiC Composites byIntroduction of BN and SiC
ZHANG Ye1,2, YAO Dongxu1, ZUO Kaihui1, XIA Yongfeng1, YIN Jinwei1, ZENG Yuping1
(1. State Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, China; 2. Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China)
Si3N4-BN-SiC compositespresent desirable potential for engineering applications because of their improved mechanical properties and oxidation resistance. In present work, Si3N4-BN-SiC composites were successfully fabricated by combustion synthesis using Si, Si3N4diluent, B4C, and Y2O3as initial powders. BN and SiC wereintroduced into Si3N4ceramics by the reaction between Si, B4C, and N2gas. The obtained Si3N4-BN-SiC composites were composed of elongated-Si3N4matrix and hollow spherical composites. The formation mechanism of the hollow spherical microstructure was investigated. The results show that the generated SiC and BN particles and glass phase cover on the raw materials, and hollow spherical microstructure is formed when raw particles are depleted. Furthermore, the impacts of B4C content on the mechanical properties of Si3N4-BN-SiC composites were investigated in detail. Theintroduction of BN and SiC is beneficial to improving mechanical properties of the composites to some extent. Finally, Si3N4-BN-SiC composites with bending strength of 28–144 MPa, fracture toughness of 0.6–2.3 MPa·m1/2, Young's modulus of 17.4–54.5 GPa, and porosity of 37.7%–51.8% were obtained for the samples with 0–20% (in mass) B4C addition.
combustion synthesis; Si3N4-BN-SiC composites;introduction; phase compositions; hollow sphere; formation mechanism
Silicon nitride (Si3N4) ceramics have been extensively used as structural and/or functional components in various engineering fields such as filtration, aerospace, and membrane support. It possesses low thermal expansion coefficient, excellent mechanical properties, good thermal shock resistance, and high chemical stability due to its strongly covalent bonds between atoms[1-3]. Several technologies can be used to fabricate Si3N4ceramics, including pressureless sintering[4-5], reactive sintering[6-7], carbothermal synthesis[8], and combustion synthesis (CS)[9-10]. Among these approaches, combustion synthesis can achieve rapid and low-cost fabrication of Si3N4ceramics by the self-propagating of combustion waves using Si as starting material[9].
Compared with Si3N4ceramics, Si3N4-BN-SiC composites present some improved properties such as lower dielectric constant, lower thermal expansion coefficient, and higher flexural strength, which draws attractive attentions in engineering applications[11-12]. The traditional introduction way of an additional phase is to add the required phase into the initial powders[13]. However, the introduced phase is difficult to disperse homogeneously into the sintered product.introduction can solve this difficulty and achieve good interface bonding between the matrix and introduced phases[14]. But investigations involving theintroduction of BN or SiC into Si3N4matrix are limited. Kusunose[15]reported the preparation of Si3N4/BN nanocomposites by hot-pressing the t-BN coated-Si3N4powders, t-BN wassynthesized by the reducing reaction between boric acid and urea. Zheng[16]used B4C and Si as raw materials to prepare h-BN-SiC compositestheir combustion reaction at high-pressure N2gas (60–120 MPa). Inspired by the above work, present study attempts tointroduce BN/SiC into Si3N4matrix by B4C addition. The microstructural evolution of thefabricated BN/SiC and its effects on the properties of the sintered Si3N4-BN-SiC composites is studied.
1 Experimental
The initial powders were Si powder (Peixian Tiannayuan Silicon Materials Co., Ltd., Jiangsu, China; purity≥99.99%;50=4.1 μm), Si3N4powder (Yantai Tomley Hi-Tech Advanced Materials Co., Ltd., Shandong, China; purity≥99.9%; α-phase content=42.3% (in mass);50= 22 μm), B4C powder (Dalian Jinma Boron Technology Group Co., Ltd, Shandong, China; purity≥99.99%;50= 1.5 μm), and Y2O3powder (Yuelong New Material Co., Ltd, Shanghai, China; purity≥99.999%;50= 5.04 μm). The weight ratio of initial powders was determined as Si: Si3N4: B4C: Y2O3=40 : (60–) :: 2 (=0, 5, 10, 15, 20). The samples were named SBC00, SBC05, SBC10, SBC15, and SBC20 according to the weight ratio of B4C, respectively. To obtain homogeneous mixtures, the ceramic powders were ball-milled for 3 h in ethyl alcohol with a ball/charge weight ratio of 2 : 1. After dried and sieved through a 150 μm (100 mesh) screen, each homogeneous mixture was cold-pressed into a rectangular compact (40 mm×40 mm×10 mm) at 10 MPa. The obtained compact was immersed into a powder bed (homogeneous mixture of 40% Si and 60% Si3N4(in mass)) and ignited under 5 MPa N2atmosphere. Schematic diagram of the reactor and detailed preparation process was mentioned in previous work[17].
The reaction temperature was obtained from the W-Re5/26thermocouple immersed into the powder bed. Rectangular bars with the dimensions of 3.0 mm×4.0 mm×36.0 mm were prepared to measure the bending strength and Young's modulus by three-point bending method (Instron-3443, Instron, USA). Fracture toughness was tested by single-edge notched beam method (SEBN) on pre-notched bars (3.0 mm×6.0 mm×30.0 mm). The microstructure of the sample was observed by scanning electron microscope (SU-1000, Hitachi, Japan) and transmission electron microscope (JEM-2100F, JEOL Company, Japan). The phase composition of the sample was performed by XRD (Diffractor meter D8, Bruker, Germany), and the content of each crystalline phase was calculated based on the XRD results. The open porosity of sintered sample was determined by the Archimedes method in the distilled water. The total porosity () was calculated from the measured bulk density (b), theoretical density (,calculated based on the phase content of each phase) using following equation:=1–b/.
2 Results and disscusion
The possible reactions during the fabrication process are shown in Eq. (1-2). Both the reactions are exothermic, but the adiabatic temperature of reaction (2) is reported to be lower than that of reaction (1)[18-19]. It meets the experimental results as shown in Table 1, the measured reaction temperature decreases from 1850 ℃ to 1765 ℃ with the increase of B4C content. Meanwhile, the reaction time increases with the B4C content increasing, it can be ascribed to the generation of SiC and BN which restrain the propagating of combustion wave as an inert phase.
3Si(s)+2N2(g)=Si3N4(s) (1)
Δθ= –725.615 kJ/mol
Si(s)+2N2(g)+B4C(s)=SiC(s)+4BN(s) (2)
Δθ= –914.459 kJ/mol
Fig.1 displays the phase identification of the obtained composites. The detected crystalline phase of sample SBC00 without B4C is-Si3N4. With the increase of B4C content, the content of BN and SiC increase evidently. Besides, it is worth noting that peak broadening is observed for BN, which reveals that its crystallization is unsatisfactory in such a rapid combustion process. When B4C content is equal to or greater than 10% (in mass), residual Si is detected, which reveals that the nitridation of Si is suppressed by the newly formed BN and SiC. Meanwhile, residual-Si3N4is also detected for samples prepared with high B4C addition. The fabrication mechanism of Si3N4ceramics is primarily controlled by the dissolution of-Si3N4and precipitation of-Si3N4[20]. The decreasing reaction temperature as shown in Table 1 is unfavorable to the phase transition from-Si3N4to-Si3N4, which results in the residual-Si3N4in materials. Besides, the formed BN and SiC disperse in the liquid phase and restrain the mass transport, which is also a significant factor leading to the residual-Si3N4.
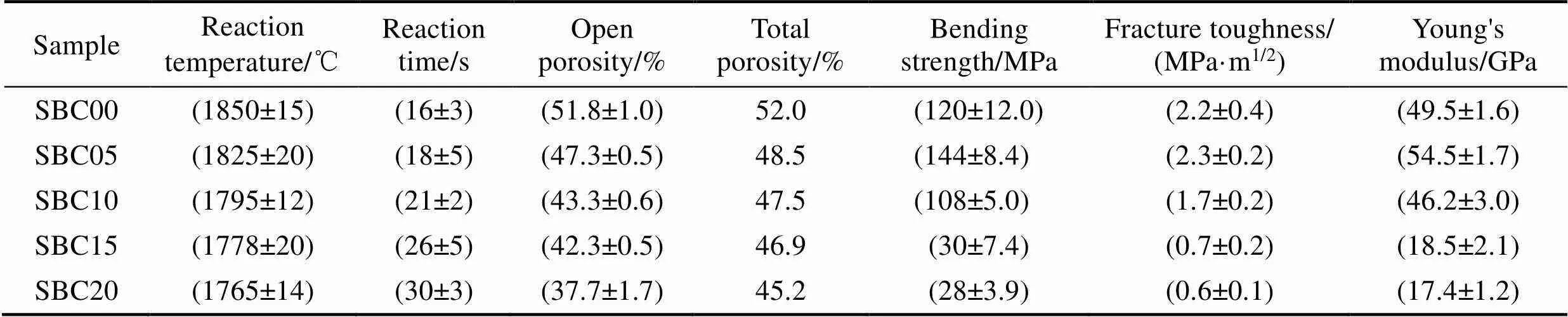
Table 1 Reaction parameters during combustion synthesis and physical properties of the sintered samples with different B4C contents
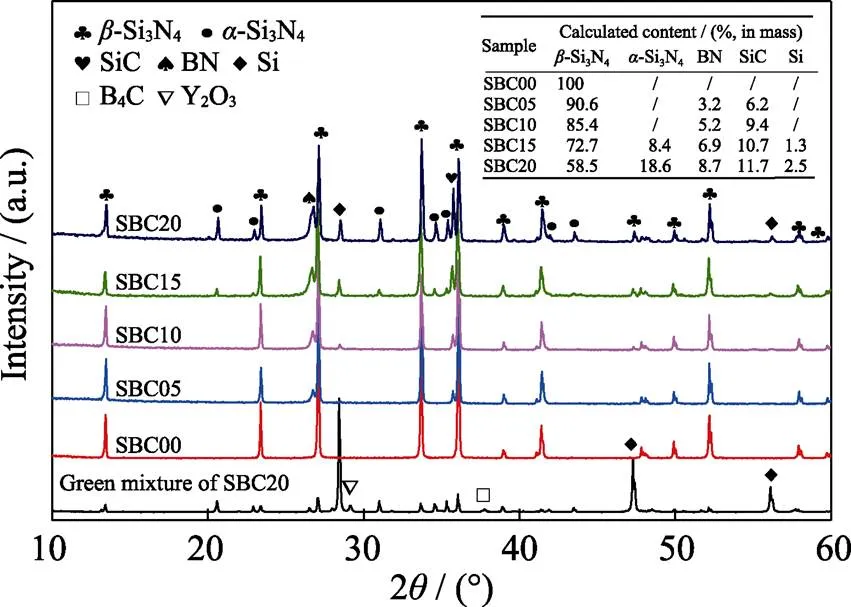
Fig. 1 XRD patterns of the green mixture and sintered samples with varied B4C contents
Fig. 2 shows the microstructure of green mixture of SBC20, and fracture-surface microstructure of the CS- fabricated specimens with varied B4C contents. It could be seen that the microstructure varies evidently after CS process. Before CS process, the green mixture is particulate. After CS process, sample SBC00 without B4C is composed of interlocking-Si3N4grains with high aspect ratio. As B4C is added to the raw materials, the development ofSi3N4grains is evidently inhibited. Average aspect ratio of-Si3N4grains decreases significantly with the increase of B4C content, almost no elongated grains can be observed when B4C content is 20% (in mass). Furthermore, hollow spherical microstructure is observed for the samples prepared with B4C addition. The hollow spheres have very thin wall and evident micropores on their surfaces when B4C content is 5% (in mass). With the increase of B4C content, the thickness of the wall increases and closed hollow sphere is gradually formed. This hollow spherical microstructure is different from that of flaky BN-SiC composites prepared at 60–120 MPa N2gas[16], which illustrates that the microstructure of Si3N4-BN-SiC composites is evidently influenced by the N2gas pressure.

Fig. 2 Microstructure of green mixture of SBC20 (a) and fracture- surface microstructures of the CS-fabricated specimens with B4C contents at (b) 0, (c) 5%, (d) 10%, (e) 15%, and (f) 20% (in mass)
The properties of the sintered samples are shown in Table 1, the open porosity of sample SBC00 is 51.8%. With the increase of B4C content, the open porosity of the sample decreases apparently. When the B4C content is 20% (in mass), the open porosity of the obtained Si3N4-BN-SiC composites is 37.7%. The significant decrease in porosity can be attributed to the higher volume expansion (170%) of reaction (2) than that of nitridation of Si (21.2%)[16], more pores are filled by the generated SiC and BN grains. However, the calculated total porosity based on the XRD results is higher than open porosity, especially for samples prepared with higher B4C content. On one hand, closed pores are formed with increasing addition of B4C as discussed above. On the other hand, B4C might form glass phase with native SiO2film and Y2O3during the high-temperature CS process, the theoretical density calculated based on the XRD results is higher than the actual value of sample, thus leading to the increasing total porosity. This behavior could be proven from the calculated content of each phase by XRD. According to the law of conservation of mass of reaction (2), the content of the generated BN and SiC should be higher than the calculated content. It illustrates that B4C partially forms glass phase instead of BN and SiC after CS process, which could not be detected by XRD.
To investigate the reaction mechanism of the CS process, transmission electron microscope (TEM), high-resolution transmission electron microscope (HRTEM), energy dispersive spectroscope (EDS) analysis, and selected area electron diffraction (SAED) are conduct on sample SBC10 and the results are shown in Fig. 3. The results demonstrate that the hollow sphere is a mixture of polycrystalline phase and amorphous phase. The crystalline phase should be BN and SiC combining the XRD analysis in Fig. 1. The amorphous phase consists of multiple elements including B, C, Si, N, and a little amount of O. On one hand, it has the characteristics of SiBCN ceramics[21]. It is well known that SiBCN ceramics have two compositionsof amorphous SiCN4-x(=1–4) and graphite-like BN(C)[22]. The above-mentioned broadening of BN peak derives from the formation of amorphous BN(C). On the other hand, the amorphous phase contains evident glass phase combining the SEM image. The formation of the hollow spherical microstructure may originate from the comparatively low N2gas pressure and the formation of glass phase. At the initial stage of the combustion reaction, eutectic liquid phase, and small BN and SiC particles are formed and cover the surfaces of raw particles, but the small BN flakes could not grow up because of the low N2gas pressure and restriction of liquid phase. As the reaction proceeding, the newly formed products continue to cover the surfaces thus forming hollow spheres when raw particles are depleted. Ultimately, eutectic liquid phase forms glass phase during the rapid cooling of CS process.
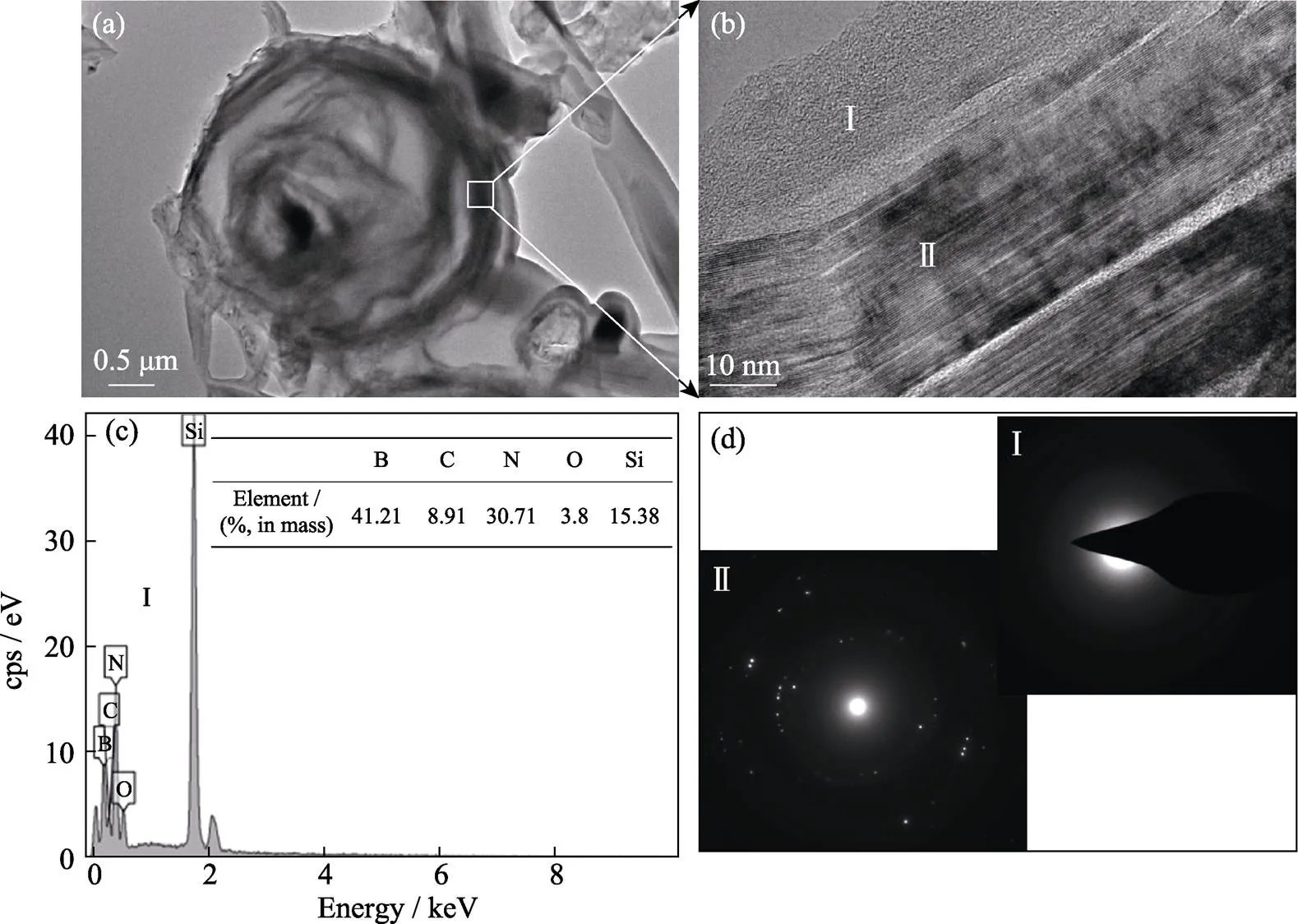
Fig. 3 (a) TEM image, (b) HRTEM image, (c) EDS analysis, and (d) SAED images of sample SBC10
As listed in Table 1, the mechanical properties of the obtained Si3N4-BN-SiC composites fluctuate with different contents of B4C addition. Compared to the monolithic Si3N4ceramics, the composites prepared with 5% (in mass) B4C has higher bending strength of 144 MPa and higher Young's modulus of 54.5 GPa. These improvements mainly result from the decrease of porosity of the sample according to the well-known negative relationship between bending strength and porosity of porous material[23]. Additionally, the generated BN and SiC grains may also benefit the mechanical properties of composites because of their pinning effects within the grain boundary. But the fracture toughness of composites doesnot show apparent increase. It could be ascribed to the introductions of BN and SiC grains, resulting in more lattice defects in Si3N4grains. The elongated Si3N4grains become new crack sources, which is unfavorable to the fracture toughness of composites. With the further increase of B4C content, the porosity of the obtained Si3N4- BN-SiC composites decreases continuously from 47.3% to 37.7%, but their mechanical properties, including bending strength, Young's modulus, and fracture toughness, degrade sharply. These behaviors indicate that the variation of microstructure is the predominated factor degrading the mechanical properties. On the one hand, the introduction of hollow spheres instead of elongated Si3N4grains presents lower mechanical properties than that of Si3N4ceramics with elongated morphology. On the other hand, the growth of Si3N4grain is restrained because of the introduction of B4C and the consequent generation of BN and SiC. Therefore, the degraded average aspect ratio is also a significant factor decreasing the mechanical properties of the Si3N4-BN-SiC composites according to the theory of crack deflection[24].
3 Conclusion
In this research, Si3N4-BN-SiC composites with hollow spherical microstructure were successfully fabricated by combustion synthesis. The microstructural evolution of theintroduced BN/SiC and its impacts on the properties of the obtained Si3N4-BN-SiC composites were studied. As the B4C content increases, the reaction temperature decreases and the porosity of sintered sample decreases evidently. Besides, the nitridation of Si, phase transition from-Si3N4to-Si3N4, and growth of-Si3N4grains are suppressed with the introduction of B4C. Therefore, residual Si and-Si3N4are detected for samples prepared with high B4C content. The bending strength and Young's modulus of the obtained Si3N4-BN- SiC composites increase firstly and then decrease with the B4C content increasing because of the decreasing porosity and degradation of microstructure. Optimal mechanical properties with bending strength of 144 MPa, fracture toughness of 2.3 MPa·m1/2, and Young's modulusof 54.5 GPa are achieved when B4C content is 5% (in mass).
[1] WANG W D, YAO D X, CHEN H B,ZrSi2-MgO as novel additives for high thermal conductivity of-Si3N4ceramics., 2020, 103(3): 2090–2100.
[2] SUN S Y, XIE Z P, CHEN K X. Precisely controlled carbothermal synthesis of spherical-Si3N4granules., 2020, 46(8): 10879–10884.
[3] HAMPSHIRE S. Silicon nitride ceramics–review of structure, processing and properties., 2007, 24(1): 43–50.
[4] LI X Q, YAO D X, ZUO K H,Microstructure and gas permeation performance of porous silicon nitride ceramics with unidirectionally aligned channels., 2020, 103(11): 6565–6574.
[5] KALEMTAS A, TOPATES G, OZCOBAN H,Mechanical characterization of highly porous β-Si3N4ceramics fabricatedpartial sintering & starch addition., 2013, 33(9): 1507–1515.
[6] YANG J G, WU P, ZHANG Y P,Synthesis of Si3N4whiskers by rapid nitridation of silicon droplets., 2020, 17(1): 296–303.
[7] MATSUNAGA C, ZHOU Y, KUSANO D,Nitridation behavior of silicon powder compacts of various thicknesses with Y2O3and MgO as sintering additives., 2017, 14(6): 1157–1163.
[8] ZHI Q, WANG B, ZHAO S,Synthesis and mechanical properties of highly porous ultrafine-grain Si3N4ceramicscarbothermal reduction-nitridation combined with liquid phasesintering., 2019, 45(17): 21359–21364.
[9] ZHANG Y, YU X, GU H,Microstructure evolution and high-temperature mechanical properties of porous Si3N4ceramics prepared by SHS with a small amount of Y2O3addition., 2021, 47(4): 5656–5662.
[10] CANO I G, BOROVINSKAYA I P, RODRIGUEZ M A,Effect of dilution and porosity on self-propagating high-temperature synthesis of silicon nitride., 2002, 85(9): 2209–2211.
[11] LOJANOVÁ S, TATARKO P, CHLUP Z,Rare-earth element doped Si3N4/SiC micro/nano-composites—RT and HT mechanical properties., 2010, 30(9): 1931–1944.
[12] LI X L, ZHANG L, YIN X,Mechanical and dielectric properties of porous Si3N4-SiC(BN) ceramic., 2010, 490(1/2): 40–43.
[13] WANG S J, JIA D C, YANG Z H,Effect of BN content on microstructures, mechanical and dielectric properties of porous BN/Si3N4composite ceramics prepared by gel casting., 2013, 39(4): 4231–4237.
[14] WANG S J, YANG Z H, DUAN X M,Fabrication and characterization ofporous Si3N4-Si2N2O-BN ceramic., 2014, 11(5): 832–838.
[15] KUSUNOSE T, SEKINO T, CHOA Y H,Fabrication and microstructure of silicon nitride/boron nitride nanocomposites., 2002, 85(11): 2678–2688.
[16] ZHENG Y T, LI H B, ZHOU T. Microstructure and mechanical properties of h-BN-SiC ceramic composites prepared by in situ combustion synthesis., 2012, 540(1): 102–106.
[17] ZHANG Y, YAO D, ZUO K,Fabrication and mechanical properties of porous Si3N4ceramics preparedSHS., 2019, 45(12): 14867–14872.
[18] LI H B, ZHENG Y T, HAN J C.-combustion synthesis of h-BN-SiC high-temperature ceramics., 2007, 353–358: 1501–1504.
[19] ZHANG Y, YAO D, ZUO K,Effects of N2pressure and Si particle size on mechanical properties of porous Si3N4ceramics preparedSHS., 2020, 40(13): 4454–4461.
[20] LAI K R, TIEN T Y. Kinetics of-Si3N4grain-growth in Si3N4ceramics sintered under high nitrogen pressure., 1993, 76(1): 91–96.
[21] RIEDEL R, KIENZLE A, DRESSLER W,A silicoboron carbonitride ceramic stable to 2,000 ℃., 1996, 382(6594): 796–798.
[22] THIYAGARAJAN G B, DEVASIA R. Simple and low-cost synthetic route for SiBCN ceramic powder from a boron-modified cyclotrisilazane., 2019, 102(1): 476–489.
[23] YANG J F, ZHANG G J, OHJI T. Fabrication of low-shrinkage, porous silicon nitride ceramics by addition of a small amount of carbon., 2001, 84(7): 1639–1641.
[24] FABER K T, EVANS A G. Crack deflection processes-Ⅰ. Theory., 1983, 31(4): 565–576.
原位引入BN-SiC燃烧合成Si3N4-BN-SiC复合材料
张叶1,2, 姚冬旭1, 左开慧1, 夏咏锋1, 尹金伟1, 曾宇平1
(1. 中国科学院 上海硅酸盐研究所, 高性能陶瓷和超微结构国家重点实验室, 上海 200050; 2. 中国科学院大学, 材料科学与光电工程中心, 北京 100049)
Si3N4-BN-SiC复合材料以其良好的力学性能和抗氧化性能而具有良好的工程应用前景。本研究以Si、Si3N4稀释剂、B4C和Y2O3为原料, 采用燃烧合成法成功制备了Si3N4-BN-SiC复合材料。通过Si、B4C和N2气之间的反应, 在Si3N4陶瓷中原位引入BN和SiC, 制备的Si3N4-BN-SiC复合材料由长棒状的-Si3N4和空心球形复合材料组成。实验研究了空心球微结构的形成机理, 结果表明, 生成的SiC、BN颗粒及玻璃相覆盖在原料颗粒上, 当原料颗粒反应完全时, 形成空心球形微结构。并进一步研究了B4C含量对Si3N4-BN-SiC复合材料力学性能的影响。原位引入SiC和BN在一定程度上可以提高复合材料的力学性能。当B4C添加量为质量分数0~20%时, 获得了抗弯强度为28~144 MPa、断裂韧性为0.6~2.3 MPa·m1/2, 杨氏模量为17.4~54.5 GPa, 孔隙率为37.7%~51.8%的Si3N4-BN-SiC复合材料。
燃烧合成; Si3N4-BN-SiC复合材料; 原位引入; 相组成; 空心球; 形成机理
TQ174
A
2021-07-05;
2021-09-06;
2021-09-27
National Key R&D Program of China (2018YFF01013605); National Natural Science Foundation of China (51902327)
ZHANG Ye (1994–), male, PhD candidate. E-mail: zhangyezn@student.sic.ac.cn
张叶(1994–), 男, 博士研究生. E-mail: zhangyezn@student.sic.ac.cn
ZENG Yuping, male, professor. E-mail: yuping-zeng@mail.sic.ac.cn
曾宇平, 研究员. E-mail: yuping-zeng@mail.sic.ac.cn
1000-324X(2022)05-0574-05
10.15541/jim20210422

