Pre-clinical study of human umbilical cord mesenchymal stem cell transplantation for the treatment of traumatic brain injury: safety evaluation from immunogenic and oncogenic perspectives
Gang Wang,Hua-Ling Wu,Yue-Ping Liu,De-Qi Yan,Zi-Lin YuanLi ChenQian YangYu-Song Gao,Bo Diao
Abstract Stem cell therapy is a promising strategy for the treatment of traumatic brain injury (TBI). However,animal experiments are needed to evaluate safety; in particular,to examine the immunogenicity and tumorigenicity of human umbilical cord mesenchymal stem cells (huMSCs) before clinical application. In this study,huMSCs were harvested from human amniotic membrane and umbilical cord vascular tissue. A rat model of TBI was established using the controlled cortical impact method. Starting from the third day after injury,the rats were injected with 10 μL of 5 × 106/mL huMSCs by cerebral stereotaxis or with 500 μL of 1 × 106/mL huMSCs via the tail vein for 3 successive days. huMSC transplantation decreased the serum levels of proinflammatory cytokines in rats with TBI and increased the serum levels of anti-inflammatory cytokines,thereby exhibiting good immunoregulatory function. The transplanted huMSCs were distributed in the liver,lung and brain injury sites. No abnormal proliferation or tumorigenesis was found in these organs up to 12 months after transplantation. The transplanted huMSCs negligibly proliferated in vivo,and apoptosis was gradually observed at later stages. These findings suggest that huMSC transplantation for the treatment of traumatic brain injury displays good safety. In addition,huMSCs exhibit good immunoregulatory function,which can help prevent and reduce secondary brain injury caused by the rapid release of inflammatory factors after TBI. This study was approved by the Ethics Committee of Wuhan General Hospital of PLA (approval No. 20160054) on November 1,2016.
Key Words: cell transplantation; immune regulation; inflammation; mesenchymal stem cells; safety evaluation; immunogenicity; traumatic brain injury; tumorigenesis
Introduction
Although much has been learned about the cellular and molecular mechanisms of traumatic brain injury (TBI) in the past two decades,these advances have failed to translate into a successful clinical trial; thus,no therapies are currently available to effectively treat TBI (Saatman et al.,2008; Schepici et al.,2020). Because of their capacity to differentiate into neuronal cells and release neurotrophic factors,stem cell therapy is a promising therapeutic strategy for TBI (Schepici et al.,2020).
Recent stem cell-based therapies,which use sources such as bone marrow mesenchymal stem cells (MSCs) (Lam et al.,2017; Yuan et al.,2021),human umbilical cord MSCs (huMSCs) (Shi et al.,2012),oligodendrocyte progenitor cells (Xu et al.,2015),neural stem cells (Gao et al.,2016; Liu et al.,2021) and neural progenitor cells (Blaya et al.,2015),have been reported to be beneficial in treating TBI. However,because of their allogenicity and their proliferative similarity to tumor cells,questions remain regarding immunological rejection reactions,tumorigenesis and uncontrolled proliferation of stem cells,which could cause sudden onset of glioblastoma after TBI (Tyagi et al.,2016),especially when MSCs are used in glioma patients (Nakamura et al.,2004). These challenges have delayed the application of stem cells in the clinical treatment of TBI. Furthermore,a previous study identified two distinct mesenchymal stromal cell populations in human malignant glioma (Svensson et al.,2017). Therefore,animal experiments are necessary to evaluate the safety of huMSC transplantation prior to clinical application.
Here,we first isolated and immunophenotypically characterized huMSCs. The huMSCs were then transplanted into experimental animalsin situor via the tail vein. A safety evaluation study was conducted of the immunogenicity,immunomodulatory effects,tumorigenesis and main organ distribution of the transplanted huMSCs in the rat model of TBI. The immunogenic and immunomodulatory effects of huMSCs were evaluated by detection of human leukocyte antigen II (HLAII) and serum pro-inflammatory and antiinflammatory cytokines.
Materials and Methods
HuMSC isolation and identification
All procedures for umbilical cord collection and manipulation were performed in accordance with the regulations of the Ethics Committee of Wuhan General Hospital of PLA,and this study was approved by the review board (approval No. 20160054) on November 1,2016. After obtaining informed consent from the donor,fresh amniotic membrane and umbilical cord vascular tissue were cut into 1-mm3pieces with ophthalmic scissors and placed into sterile dishes. StemPro MSC SFM XenoFree (Gibco,Grand Island,NY,USA) culture medium was added into the dish,which was gently shaken to promote even mixing,and transferred into a 5% CO2/37°C incubator (Thermo Scientific,Waltham,MA,USA). Half of the culture medium was replaced every 5 days. On day 10,long spindle-like cells were observed growing from the edge of the tissue block. On day 14,when the cells covered 50% of the surface area,the tissue blocks were removed,and the cells were collected for continuous culture. The culture medium was replaced every 3 days. When cells reached 90% confluence,0.25% trypsin was used to detach the cells. The suspended cells were incubated for 1 hour at room temperature in the dark with fluorescent antibodies (anti-CD29-APC,anti-CD44-PE,anti-CD73-PE and anti-CD105-APC; BD Bioscience,San Jose,CA,USA). Cells were then washed 3 times and analyzed using a FACSCalibur cytometer (Becton,Dickinson and Company,Franklin Lakes,NJ,USA). The huMSCs from the third passage were used in subsequent experiments.
Detection of huMSC HLAII expression by western blot and quantitative real-time polymerase chain reaction
HLAII expression was detected by western blot. First,10 μg huMSC lysate was resolved on SDS-PAGE gels and transferred onto polyvinylidene difluoride membranes (Millipore,Bedford,MA,USA). After blocking in 5% skim milk for 30 minutes,the membranes were incubated with anti-major histocompatibility complex (MHC) Class II (HLAII) mouse monoclonal antibody (Cat# ab55152,1:2000,Abcam,Cambridge,UK) or antiglyceraldehyde 3-phosphate dehydrogenase (GAPDH) mouse monoclonal antibody (Cat# 60004-1-Ig,1:5000,Proteintech,Wuhan,China) at 4°C overnight. After washing,the membranes were incubated with horseradish peroxidaseconjugated goat anti-mouse IgG (Cat# SA00001-1,1:3000,Proteintech) at room temperature for 1 hour. After washing,the blots were developed using Pierce ECL western blotting substrate (Thermo Scientific) and then imaged and analyzed with the Tanon 5200 automatic chemiluminescence image analysis system (Tanon Company,Shanghai,China).
HLA-DPA1,HLA-DQA1andHLA-DRA1were selected from among the 16 humanHLAIIgenes to assess their mRNA expression in huMSCs. Peripheral blood mononuclear cells were separated from human peripheral blood by density gradient centrifugation with Ficoll as a positive control. The GenBank mRNA sequences ofHLA-DPA1(accession: NM_001242524.1),HLA-DQA1(accession: NM_002122.3),HLA-DRA1(accession: NM_019111.4) andGAPDH(accession: NM_001256799.2) were used to design primers for qRT-PCR. The following primers were used:HLA-DPA1forward: 5′-CTG GAC AAG AAG GAG ACC GT-3′;HLA-DPA1reverse: 5′-TCA ATG TGG CAG ATG AGG GT-3′ (product length 224 kb);HLA-DQA1forward: 5′-AAC GCT ACA ACT CTA CCG CT-3′;HLA-DQA1reverse: 5′-TCT GTG ACT GAC TGC CCA TT-3′ (product length 166 kb);HLA-DRA1forward: 5′-AAT GGC CAT AAG TGG AGT CC-3′;HLA-DRA1reverse: 5′-GGA GGT ACA TTG GTG ATC GG-3′ (product length 336 kb);GAPDHforward: 5′-CCA GAA CAT CAT CCC TGC CT-3′,GAPDHreverse: 5′-CCT GCT TCA CCA CCT TCT TG-3′ (product length 185 kb). Primers were synthesized by Tsingke Biological Technology (Wuhan,China). Total mRNA from huMSCs was extracted using TRIzol (Invitrogen,Carlsbad,CA,USA) and then reverse-transcribed into cDNA with a QuantiTect Reverse Transcription Kit (Qiagen,Hilden,Germany). The qRT-PCR mix was prepared with a Taq PCR MasterMix kit (TIANGEN,Beijing,China) according to the user guide and run on a LightCycler 96 (Roche,Basel,Switzerland).
HuMSCs labeled by carboxyfluorescein succinimidyl ester
HuMSCs were labeled using the CellTrace carboxyfluorescein succinimidyl ester (CFSE) Cell Proliferation Kit (Thermo Scientific) following the manufacturer’s instructions. Briefly,huMSCs were suspended in prewarmed 0.1% bovine serum albumin-phosphate buffer saline (PBS) and CFSE. The cell concentration was 1 × 106/mL,and the CFSE concentration was 2.0 μM. The suspension was incubated for 15 minutes at 37°C in the dark. The staining was quenched by adding 5 volumes of ice-cold culture medium followed by incubation on ice for 5 minutes. Labeled cells were then washed with PBS three times and resuspended in 1 × 106/mL for tail vein injection and 5 × 106/mL forin situbrain injection.
Animal modeling,grouping and intervention
All Sprague-Dawley rats (Experimental Animal Center of Hubei Province,Wuhan,China,license No. SCXK-2015-0018) were housed according to the Guide for the Care and Use of Laboratory Animals of the National Academy of Sciences. All animal experiments were performed according to the animal study protocol approved by the Ethics Committee of Wuhan General Hospital of PLA (approval No. 20160054) on November 1,2016. A total of 130 adult healthy Sprague-Dawley male rats (280-300 g,8 weeks old) were fed a standard laboratory diet and water and maintained under temperature-controlled conditions (22 ± 2°C) and a 12-hour light/dark cycle. The rats were randomly distributed into the following four groups: sham,TBI model (TBI),in situinjected (In Situ),and tail vein injected (Tail Vein) groups.
Sham group (n= 25): The skin at the surgical site was cut open and then sutured. For detection,five rats were sacrificed at each time point in synchrony with theIn Situand Tail Vein groups.TBI group (n= 25): The rat model of TBI was prepared using the Feeney method (Feeney et al.,1981). Briefly,animals were anesthetized with sodium pentobarbital (30 mg/kg,intraperitoneal injection; Sigma-Aldrich,St. Louis,MO,USA) and fixed on a cerebral stereotactic device (RuiWoDe,Shenzhen,China),and a craniotomy (4 mm × 4 mm) was performed above the right parietal cortex between the sagittal,lambdoid and coronal sutures. After removing the bone fragments from the dural surface,a 3 mm diameter impactor was employed to impact the dura at a speed of 2 m/s to cause traumatic brain injury. The impact depth was set at 2 mm to avoid dural breakdown. For detection,five rats were sacrificed at each time point in synchrony with theIn Situand the Tail Vein groups.
In Situgroup (n= 40): On the third day after TBI,a single dose of 10 μL 5 × 106/mL CFSE-labeled cells was injected through the cerebral stereotactic device (RuiWoDe) with an aseptic microsyringe (Hamilton,Reno,NV,USA) to transplant the huMSCs into the brain injury impact site; the day after transplantation was considered day 1. For detection,five rats were sacrificed on days 1,3,7,14 and 28. The remaining 15 rats were kept for an additional 12 months for long-term observation.
Tail Vein group (n= 40): After TBI,500 μL of 1 × 106/mL CFSElabeled cells was injected through the tail vein once per day for 3 days; the day after the last transplantation was considered day 1. For detection,five rats were sacrificed on days 1,3,7,14 and 28. The remaining 15 rats were kept an additional 12 months for long-term observation.
Detection of serum cytokines by enzyme-linked immunosorbent assay
Serum was separated from the jugular blood collected on days 1,3,7,14 and 28 from each group. The concentrations of the serum cytokines interleukin 6 (IL-6),interleukin 10 (IL-10) and interleukin 12 (IL-12),transforming growth factor beta (TGF-β) and tumor necrosis factor alpha (TNF-α) were measured using enzyme-linked immunosorbent assay (ELISA) kits. IL-6,IL-10,TGF-β and TNFα ELISA kits were purchased from Boster (Wuhan,China). The IL-12 ELISA Kit was purchased from Biorbyt Company (Cambridge,UK). All tests were conducted according to the manufacturers’ instructions.
Localization of huMSCs by in vivo imaging
We examined the colonization pattern and metabolism of the CFSE-labeled transplanted huMSCs. The rats were sacrificed by decapitation after inhalation anesthesia with diethyl ether (Sinopharm Chemical Reagent Co.,Ltd.,Shanghai,China). After dissection,the brain,liver,lung,kidney and heart were collected and observed on anIn VivoImaging System Lumina II (PerkinElmer,Waltham,MA,USA) in the fluorescent imaging mode (excitation peak = 490 nm,emission peak = 518 nm). After subtracting the background fluorescence with Living Image 4.2 (PerkinElmer),the average radiant efficiency values of these organs were measured and analyzed.
Immunofluorescence staining
After observation with theIn VivoImaging System Lumina II,the brains were fixed in 4% paraformaldehyde overnight at 4°C,and then dehydrated,cleared and embedded in paraffin. The tissue blocks were sliced into 5-μm sections using a Slicer microtome (Leica Biosystems,Buffalo Grove,IL,USA). The brain sections were dewaxed,rehydrated,and placed into microwave-heated citrate buffer (pH 6.0) to retrieve the antigens at high heat for 3 minutes and medium heat for 2 minutes,and then cooled to room temperature. Sections were then rinsed three times in PBS for 10 minutes and incubated for 1 hour at room temperature in blocking solution (5% bovine serum albumin in PBS). The sections were thereafter incubated overnight at 4°C with primary antibodies,including rabbit anti-human CD29 polyclonal antibody (Cat# PB9086,1:100,Boster),proliferating cell nuclear antigen (PCNA) mouse monoclonal antibody (Cat# 2586S,1:4000,CST,Danvers,MA,USA),Caspase 3 (CASP3) rabbit polyclonal antibody (Cat# 19677-1-AP,1:200,Proteintech) and F4/80 rabbit polyclonal antibody (Cat# 28463-1-AP,1:200,Proteintech),diluted in 1% bovine serum albumin containing PBS. Then,after three rinses in PBS,the corresponding secondary antibody,CoraLite594-conjugated goat anti-rabbit IgG (Cat# SA00013-4,1:200,Proteintech) or CoraLite594-conjugated goat anti-mouse IgG (Cat# SA00013-3,1:200,Proteintech) was added to the sections and incubated at 37°C for 30 minutes. The sections were rinsed in PBS before incubation in 4′,6-diamidine-2′-phenylindole dihydrochloride (Cat# 10236276001,Roche) at room temperature for 5 minutes,and then mounted with 50% glycerol/PBS and imaged under a fluorescence microscope (BX51,Olympus,Shinjuku,Japan).
Immunohistochemistry
The paraffin tissue blocks were sliced into 5-μm sections and then dewaxed and rehydrated. After immersion in 3% hydrogen peroxide for 10 minutes,sections were placed into microwave-heated citrate buffer (pH 6.0) to retrieve the antigens at high heat for 3 minutes and medium heat for 2 minutes,and then cooled to room temperature. Sections were then rinsed three times in PBS for 10 minutes and incubated for 1 hour at room temperature in blocking solution (5% bovine serum albumin in PBS). The sections were subsequently incubated with epidermal growth factor receptor variant III (EGFRvIII) rabbit monoclonal antibody (Cat# 64952S,1:100,CST),diluted in PBS containing 1% bovine serum albumin,at 4°C overnight. The next morning,the sections were rinsed three times in PBS and incubated with biotin-labeled goat anti-rabbit IgG (Cat# BA1003,1:200,Boster),diluted in PBS,at 37°C for 30 minutes. The sections were rinsed three times in PBS,incubated with streptavidinbiotin complex (Cat# SA2002,Boster) at 37°C for 30 minutes,rinsed four times in PBS,developed with diaminobenzidine,stained with hematoxylin,mounted with neutral balsam,and imaged under a fluorescence microscope.
Hematoxylin and eosin staining
After observation under theIn VivoImaging System Lumina II,the liver and lung tissues were processed into paraffin tissue blocks and sliced into 5-μm sections for hematoxylin and eosin staining. Briefly,sections were dewaxed,rehydrated and incubated with hematoxylin for 5 minutes,then rinsed with water before incubation with eosin for 30 seconds. Finally,sections were mounted with neutral balsam and photographed under a fluorescence microscope.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 22.0 (IBM,Armonk,NY,USA). The concentration of inflammatory factors and average radiant efficiency values of brains/livers/lungs were compared by one-way analysis of variance followed by the least significant difference test. Repeated measures analysis of variance was used to explore the changes of concentrations of inflammatory factors at different points.P< 0.05 was considered statistically significant.
Results
Morphologic and immunophenotypic characteristics of huMSCs
The third passage huMSCs had a morphology resembling long whirling spindles (Figure 1A). Flow cytometry revealed that cells expressing CD29,CD44,CD73 and CD105 accounted for more than 95% of the total cell population (Figure 1B),indicating that these were indeed huMSCs,and therefore suitable for use in the following experiments.Western blot analysis showed that no HLAII protein was expressed in the huMSCs (Figure 1CandD). qRT-PCR showed no expression ofHLA-DRA1. WhileHLA-DPA1andHLA-DQA1were detected in the huMSCs (Figure 1EandF),the cycle thresholds were ~32 for these cells,compared with 24 for peripheral blood mononuclear cells. These results suggest that huMSC therapy might be immunologically tolerated.
Immunomodulatory effects of huMSCs in TBI model rats
We examined the levels of serum proinflammatory cytokines (IL-6,IL-12 and TNF-α) and anti-inflammatory cytokines (IL-10 and TGFβ) in experimental animals with ELISA. The levels of serum pro- and anti-inflammatory cytokines in the TBI model group were higher than those in the Sham group (P< 0.001). In theIn Situ(huMSCs deliveredin situat the lesion site) and Tail Vein groups (huMSCs delivered through the tail vein),we found that the levels of proinflammatory cytokines were decreased,and the levels of anti-inflammatory cytokines were increased compared with the TBI group (P< 0.001;Figure 1G). Repeated measures analysis of variance was used to examine the changes in serum inflammatory factor concentrations in each group. Compared with the Sham group,the levels of TNF-α and IL-6 in the TBI group were significantly higher during the entire experimental observation period (P< 0.05). The concentrations of IL-12 and IL-10 were significantly higher in the first 7 days (P< 0.05),but there were no significant differences after day 14. In the Tail Vein group,the levels of pro-inflammatory cytokines were decreased significantly (P< 0.05) a short time after huMSC transplantation (IL-6: 3 days later,IL-12: 1 day later,and TNF-α: 3 days later). The immunomodulatory effects of the injected huMSCs persisted during the whole experimental period,such that there were no significant differences in pro-inflammatory cytokine levels between the Tail Vein and Sham groups. However,it took a longer time (IL-6: 28 days; IL-12: 3 days and TNF-α: 3 days) for injected huMSCs in theIn Situgroup to exert immunomodulatory effects. There were no statistically significant differences in pro-inflammatory cytokine levels between theIn Situand Tail Vein groups (P> 0.05).
Organ distribution of injected huMSCs in TBI model rats
Compared with the TBI group,the fluorescence intensity of the brain observed using the small animal live imager was significantly higher on days 1 and 3 in theIn Situgroup (P< 0.001;Figure 2AandB). However,there was no significant difference in the fluorescence intensity of the other organs (liver,lung,heart and kidney) between the TBI andIn Situgroups (Figure 2B). These results indicate that the huMSCs injected into the brain injury site were primarily localized within the brain,and almost no huMSCs entered the blood from the injured brain tissue. Although the fluorescence gradually faded,it could still be seen on day 7,which might indicate that some huMSCs persist for up to a week and have adequate time to secrete regulatory factors before they undergo apoptosis or differentiate.
Compared with the TBI group,live images of the tail vein group showed fluorescence in the liver,but only weak green fluorescence in the brain,lung,heart and kidney (Figure 2A),indicating that the huMSCs injected into the tail vein primarily accumulated in the liver,with only a few cells making their way to the brain,lung and other organs. In addition,the fluorescence intensity of the lungs on days 1 (P< 0.001) and 3 (P< 0.001) and the fluorescence intensity of the liver on days 1 (P< 0.001),3 (P< 0.001) and 7 (P< 0.05) were significantly higher in the tail vein group compared with the TBI group (Figure 2C). Over time,fluorescence values diminished to nearly normal levels by day 14.
Brain sections from theIn Situand Tail Vein groups were used to observe the location of huMSCs and the aggregation of cells under a fluorescence microscope. Considering that the fluorescence intensity of CFSE labeled huMSCs was halved once per generation,we chosed CD29,a surface molecule makers of huMSC,for immunofluorescence staining to track the transplanted huMSCs. The CFSE and CD29 labeled huMSCs were identified in theIn Situgroup and CD29 on days 1,3 and 7 (Figure 2D) in the brain lesions. A few CFSE-labeled huMSCs in the Tail Vein group were also observed in the brain lesions on days 1 and 3 (Figure 2E).
Evaluation of tumorigenesis following huMSC transplantation in TBI model rats
The experimental rats from both theIn Situand Tail Vein groups exhibited no cell degeneration,necrosis,hyperplasia or tumorigenesis in the primary organs,including the liver and lungs,on days 1,3,7,14 and 28,or even 12 months posttransplantation (Figures 3Aand4A). Immunohistochemistry for EGFRvIII in the brain showed no positive signal in huMSCtreated rats from the day of injection to 12 months after injection in either group (Figures 3Band4B). As EGFRvIII is one of the most common carcinogenic mutations in glioma (Kim et al.,2021),these results provide additional assurance to their clinical application.
Furthermore,immunofluorescence staining of PCNA showed that TBI triggered the proliferative activity of adjacent neurocytes,but the fluorescently labeled huMSCs had no proliferative activity in the brain lesion sites in either theIn Situgroup (Figure 3C) or Tail Vein group (Figure 4C). These results demonstrate that uncontrolled proliferation of transplanted huMSCs did not occur,even 12 months after transplantation.
Fate analysis of injected huMSCs in the brains of TBI rats
We also performed immunofluorescence staining for CASP3 and F4/80 in the brains of rats from both theIn Situand Tail Vein groups. In CFSE-labeled huMSCs,CASP3 was observed from days 3 to 7 in both groups (Figure 5AandC). Although no CFSE-labeled huMSCs were found after day 14,CASP3 immunoreactivity persisted until day 28,which may have resulted from apoptosis of damaged neurons. Immunostaining for F4/80,a unique marker of murine macrophages (Dos Anjos Cassado,2017),showed that macrophages aggregated near the lesion site after huMSC injection (Figure 5BandD). Notably,the number of macrophages in theIn Situgroup (Figure 5B) was greater than that in the Tail Vein group (Figure 5D),especially on day 14.
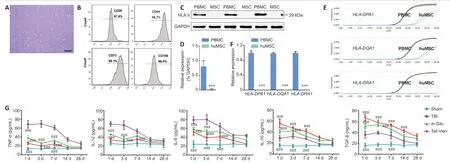
Figure 1|Identification,immunogenicity and immunomodulation of huMSCs.

Figure 2|Sites of accumulation of huMSCs in vivo in TBI rats.
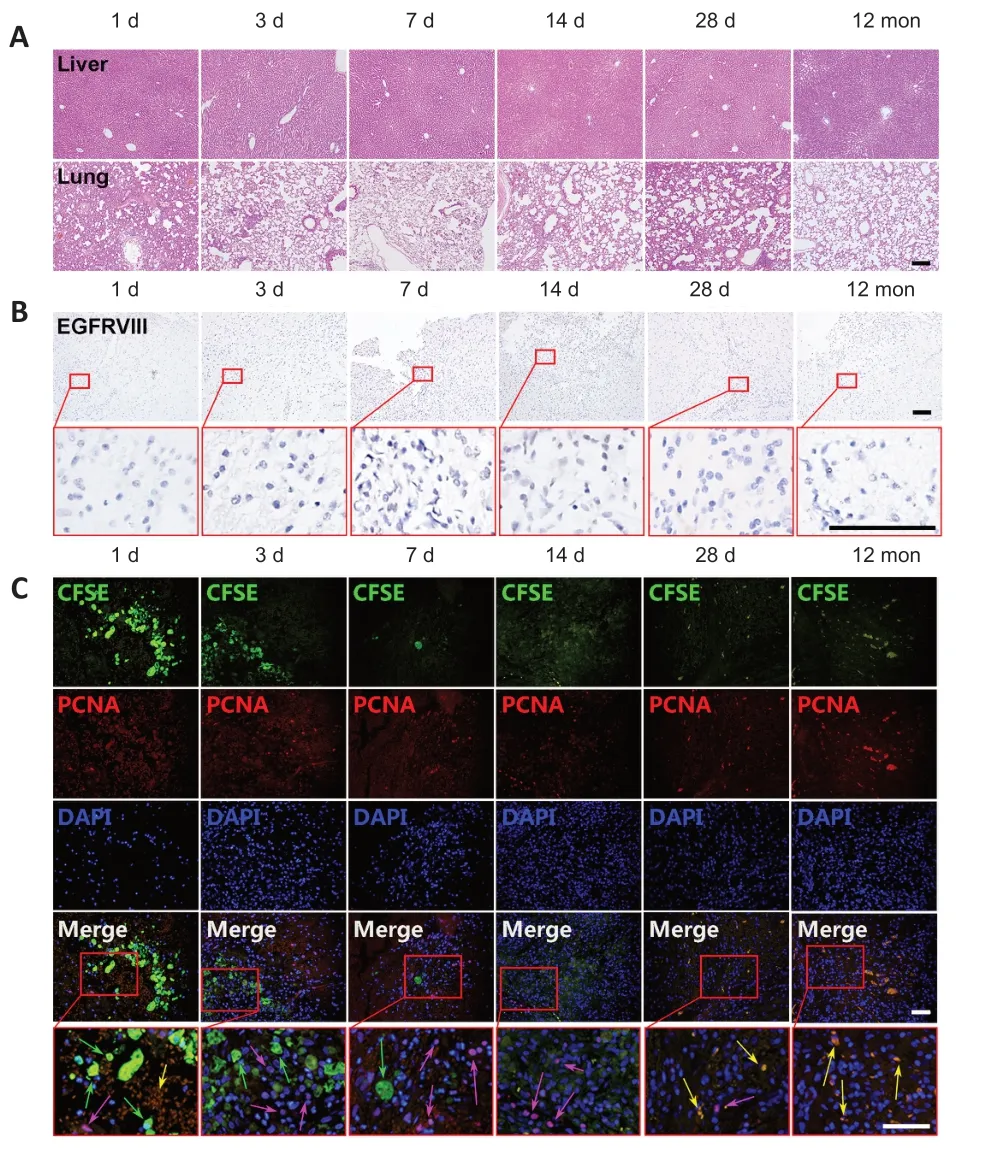
Figure 3|Evaluation of tumorigenicity in TBI rats given in situ huMSC transplantation.

Figure 4|Evaluation of tumorigenicity in TBI rats given huMSC transplantation via tail vein injection.

Figure 5|The fate of huMSCs in TBI rats given in situ or tail vein transplantation.
Discussion
The current management of TBI is mainly supportive,with much more attention given to intensive care and prevention of secondary neopathy. Numerous recent studies have found that MSCs,with their multipotency and low immunogenicity,have potential in treating TBI (Cox et al.,2019; Das et al.,2019; Zhou et al.,2019; Bonsack et al.,2020; Dehghanian et al.,2020; Schepici et al.,2020; Sun et al.,2021). However,a number of issues related to the potential risk of huMSCbased therapies remain unresolved,such as the purity and identity of huMSCs,the risk of tumor formation,unwanted immune responses,and adventitious infection (Herberts et al.,2011; Das et al.,2019). In this study,our findings from bothin vitroandin vivopreclinical studies suggest that huMSCs do not display immunogenic toxicity or tumorigenicity up to 12 months after implantation. Furthermore,huMSCs exhibit good immunomodulatory function by decreasing pro-inflammatory cytokine concentrations and increasing anti-inflammatory cytokine levelsin vivo,which may play an important role in controlling inflammatory responses after TBI.
Compared with bone marrow-derived MSCs,huMSCs have similar proliferative and multi-lineage differentiation potentials (Baksh et al.,2007),but have many advantages,such as a wide variety of sources,easy harvesting,less risk of rejection after transplantation,low immunogenicity,and no ethical controversy. Therefore,huMSCs are considered an ideal alternative to bone marrow-derived MSCs. However,efficiently isolating huMSCs from human umbilical cord tissues and identifying them remain key tasks for the development of huMSC-based therapies. Currently,MSC isolation/identification has mainly relied on surface markers detected by flow cytometry,adherent properties,and differentiation potential (Bernardo et al.,2009). Our results show that the third passage huMSCs have a morphology resembling long whirling spindles. Flow cytometry revealed that cells with characteristic expression of CD29,CD44,CD73 and CD105 accounted for more than 95% of the total cell population,indicating that our protocol for huMSC isolation and identification was appropriate,guaranteeing the purity of huMSCs for experiments. This is another advantage of huMSCs over bone marrow-derived MSCs,as the latter contain multiple types of stem cells,such as hematopoietic stem cells,which can cause undesirable effects. To be defined as MSCs,cells must be positive for a number of surface markers,such as CD73,CD90,CD166,CD44 and CD29. Furthermore,they must be negative for CD34,CD31,CD14 and CD45 (at least in case of BM-derived cells),as well as HLA complex surface molecules (Le Blanc et al.,2003). The HLA complex,also called the HLA gene complex,is primarily located on chromosome 6p21,encoding the MHC cell-surface antigens responsible for graft-versus-host disease and transplant rejection. There are three major categories of HLAs: MHC class I (A,B and C),MHC class II (DP,DM,DOA,DOB,DQ and DR),and MHC class III. MHC class II plays the major role of presenting antigens on the surface to T-lymphocytes (Tan et al.,2017). In our study,we found that the huMSCs expressed no MHC class II antigens,making them unable to present host antigens to the T-cells. Therefore,the problems of graft-versus-host disease or transplant rejection can be mitigated by huMSC therapy.
A molecular war begins between anti-inflammatory and pro-inflammatory cytokines after TBI (Helmy et al.,2011a). Pro-inflammatory cytokines,including IL-6,IL-12 and TNF-α,which are mainly produced by microglia,but also by endothelial cells,neurons and astrocytes,activate glial cells,inducing further cytokine production and astrogliosis (Lau and Yu,2001; Konsman et al.,2007; Ziebell and Morganti-Kossmann,2010). Anti-inflammatory cytokines such as TGF-β and IL-10 have the ability to counteract and downregulate inflammatory and cytotoxic reactions (Cederberg and Siesjö,2010). It was reported that pro-inflammatory cytokines are rapidly upregulated following brain injury,and some peak as early as 2 hours after TBI (Helmy et al.,2011b). These proinflammatory cytokines stimulate the injected huMSCs to exhibit an immunosuppressive effect (Ren et al.,2008). Our results show that levels of serum pro-inflammatory cytokines (IL-6,IL-12 and TNFα) and anti-inflammatory cytokines (IL-10 and TGF-β) in the TBI model group were higher than those in the sham group. Significantly decreased pro-inflammatory cytokine levels and increased anti-inflammatory cytokine levels were observed shortly after huMSCs transplantation,in agreement with another study (Zhang et al.,2013). In that study,IL-6 at 24 and 72 hours,and TNF-α at 24 and 72 hours were all significantly decreased in the MSC-treated group compared with the control group. Moreover,levels of the anti-inflammatory cytokines IL-10 at 24 and 72 hours,and TGF-β at 24 and 72 hours after TBI were increased in the MSC-treated group compared with the control group. The benefit of stem cell therapy for TBI may primarily arise from the paracrine or systemic secretion of anti-inflammatory chemokines and various growth factors,and by the inhibition of proinflammatory cytokines,rather than their differentiation into neural cells (Uccelli et al.,2008; Scuteri et al.,2011; Lo Sicco et al.,2017).
Since the fluorescence of CFSE labeled cells is halved every generation,immunofluorescence staining of CD29,a surface molecule maker of huMSCs,was implemented to batter track the transplanted huMSCs. CFSE and CD29 co-localization in brain sections,combined with live images,showed the presence of huMSCs in brain tissue during the first week during which huMSCs secrete anti-inflammatory chemokines and various growth factors to repair brain tissue. Because of the apoptosis or differentiation of huMSCs,the fluorescence gradually decreased,and was metabolized primarily in the liver in our study. EGFRvIII,which is a specific EGFR mutation,is often used to detect brain tumors (Kuan et al.,2001; Gong et al.,2014). PCNA is a DNA clamp that acts as a processivity factor for DNA polymerase δ in eukaryotic cells and is essential for replication. PCNA is expressed in the nuclei of cells during the DNA synthesis phase of the cell cycle,and its abnormal increase is related to certain neoplasms,such as breast carcinomas and astrocytomas (Leonardi et al.,1992). Imaging of antibody labeling for PCNA can be used to distinguish the early,mid and late S phase of the cell cycle (Schönenberger et al.,2015). Neither expression of EGFRvIII nor PCNA increased,even 12 months post-transplantation,which aligns with another study that evaluated the tumorigenicity of transplanted stem cells (Garitaonandia et al.,2016). However,the follow-up time of that study was 9 months.
The routes of delivering stem cells to the host,which commonly include intravenous,intra-arterial and intracranial delivery methods,play an important role in the success of stem cell therapies. The intravenous method seems to be the most attractive for clinical applications,whereas the intracranial method is the most frequently used. When we designed our study,two groups,theIn Situand Tail Vein groups,were created to allow a direct comparison of the two delivery methods. Some interesting results are worth noting. The huMSCs injected into the Tail Vein group primarily accumulated in the liver,while those in theIn Situgroup primarily accumulated in the brain,which was expected. Additionally,our results indicated that huMSC injection increased levels of serum anti-inflammatory cytokines and decreased the concentrations of pro-inflammatory cytokines in rats with TBI. However,there were no statistically significant differences in pro-inflammatory cytokine levels between theIn Situand Tail Vein groups. Third,the number of macrophages was greater in theIn Situgroup than in the Tail Vein group. Macrophages are important cells of the immune system that respond to an infection or to the accumulation of damaged or dead cells. Injected huMSCs gradually undergo apoptosis and might be cleared away by macrophages.
There are some limitations to this study. First,some biological properties,such as population doubling time,clonogenicity,and differentiation ability of the huMSCs were not examined. Second,only CD29,CD44,CD73 and CD105 were assayed. Third,we focused on the safety evaluation of the injected huMSCs,but not on efficacy assessment. It would have been informative to examine whether the transplanted huMSCs differentiated into glial cells,such as astrocytes or oligodendrocytes,or into neuron-like cells by co-labeling for the appropriate markers,in vivoandin vitro. Furthermore,it would have been interesting to explore whether the huMSCs could have differentiated into macrophage-like cells. It could have provided information on their immunoregulatory role,particularly as some classes of macrophages can reduce inflammation.
In summary,in this study,we comprehensively evaluated the safety of huMSCs for treating TBI in rats,and the findings suggest that allogeneic huMSC therapy may be a good strategy for treating TBI without major risk of immune attack or rejection. Given the encouraging safety data obtained in this study,we propose further efficacy evaluation of huMSCs for human application.
Author contributions:Study design,data analysis and interpretation,manuscript review: BD; animal,ELISA,live imaging,and slice experiments,data collecting and assembling,manuscript writing: DQY,HLW,YPL,GW; PCR and western blot assay: ZLY,LC; cell experiment: QY,YSG. All authors contributed to this study and approved the final manuscript.
Conflicts of interest:All the authors have declared that they have nocompeting interests.
Financial support:The work was supported by the General Project of Hubei Health Committee of China,No. WJ2019M263 (to GW). The funder had no roles in the study design,conduction of experiment,data collection and analysis,decision to publish,or preparation of the manuscript.
Institutional review board statement:The study was approved by the Ethics Committee of Wuhan General Hospital of PLA (approval No. 20160054) on November 1,2016.
Declaration of patient consent:The authors certify that they have obtained the consent forms from the donor. In the form,the donor has given her consent for the images and other clinical information to be reported in the journal. The donor understand that her name and initials will not be published.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Elena Abati,Facolta di Medicina e Chirurgia,Italy; Fei Gao,University of Pittsburgh,USA.
Additional file:Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- Deciphering the role of PGC-1α in neurological disorders: from mitochondrial dysfunction to synaptic failure
- Dying by fire: noncanonical functions of autophagy proteins in neuroinflammation and neurodegeneration
- Transcranial magnetic stimulation in animal models of neurodegeneration
- SYNGR4 and PLEKHB1 deregulation in motor neurons of amyotrophic lateral sclerosis models: potential contributions to pathobiology
- Cholesterol synthesis inhibition or depletion in axon regeneration
- Challenges in developing therapeutic strategies for mild neonatal encephalopathy

