Effects of Temperature, Salinity and Stocking Density on Larval Survival and Growth of Reciprocal Crosses Between Two Strains of Pacific Oysters, Crassostrea gigas
MENG Lingxin, XU Chengxun, and LI Qi, 2), *
Effects of Temperature, Salinity and Stocking Density on Larval Survival and Growth of Reciprocal Crosses Between Two Strains of Pacific Oysters,
MENG Lingxin1), XU Chengxun1), and LI Qi1), 2), *
1),,,266003,2),,266237,
The Pacific oysteris one of the most widely cultivated aquaculture species and contributes signifi- cantly to total seafood production for human beings. However, mass mortality occurred frequently, and in some regions almost all oysters died during seed production and grow-out stage. In order to explore whether hybridization breeding can improve its growth and survival, a complete diallel cross between a selected strain ‘Haida No. 1’ (S) and an orange shell variant (O) ofwas carried out. The larval growth and survival were compared among hybrids and purebred strains at temperatures of 16, 20, 24, 28 and 32℃; salinities of 15, 20, 25, 30 and 35; and stocking densities of 0.5, 1, 2, 4 and 8larvaemL−1. Under different environments, the hybridization between two strains ofshowed the heterosis of growth and survival. The mean shell height and survival rate of the two reciprocal crosses (OS, SO) were significantly higher than those of the two purebred strains (SS, OO) under all environ- mental conditions. In particular, OS showed greater heterosis than the purebred strains and SO progeny.Theresults showed that the productive traits of the ‘Haida No. 1’ could be improved by crossing with the orange shell line.Meanwhile, the results from this study also indicated that hybridization between the two strains ofmay be a promising way for breeding new variety with high survival rate.
; heterosis; larvae; temperature; salinity; stocking density; survival; growth
1 Introduction
Oysters are important species in shellfish industry in Chi- na, accounting for >30% of the total marine mollusc yield with a total production of 5.2 million tons in 2019 (DOF, 2020). The Pacific oyster () is one of the most widely cultivated oyster species in northern China. Due to its high commercial value, a number of selectivebreeding programs ofbased on family or mass se- lection have been launched, leading to significant improve-ments of commercially important traits, such as growthrate (Li., 2011; Xu., 2019) and shell coloration(Han.,2019). Despite these efforts, mass mortalityhas occurred frequently. In some regions, almost all stocks died during seed production and grow-out stage. A large-scale summer mortality of Pacific oysters has become amajor problem affecting oyster aquaculture. Hybridizationbreeding is regarded as an effective method to transferdesirable traits between species, breed new varieties, in-crease environmental tolerance, and produce genetic im-provement of marine shellfish (Hedgecock., 1995;Hedgecock and Davis, 2007). A number of hybridization experiments have been conducted in marine bivalves, suchas scallops (Zheng, 2011), mussels (Matson, 2003) and oysters (Tan., 2020).
In, efforts have been made to produce recipro- cal crosses through interspecific and intraspecific hybridi- zation. Because of the genetic incompatibility between dif-ferent species, only a few studies demonstrated that the be-neficial development traits ofcan be achieved by interspecific hybridization (Xu., 2019; Tan., 2020)On the other hand, more attentions have been focused on the intraspecific hybridization, which generally has higher hatching rate and growth performance compared with in- terspecific hybridization (Hulata, 2001). Our previous stud- ies proved that the high heterosis for growth and survival ofcan be obtained by crossing among different breeding lines (Kong., 2017; Han., 2020). How- ever, most of these investigations put particular emphasis on comparing the differences among hybrids under the identical condition, did not examine the performance in the growth and survival of hybrid progeny under different environments. In order to obtain an accurate evaluation of the performance for hybrids, the complex environmental conditions need to be considered.
The pelagic larval phase is the most vulnerable stage. The growth and survival of the larvae at this stage are possibly most susceptible to the external environmental conditions, such as temperature, salinity and stocking density (Claudi and Evans, 1993). Water temperature and salinity are se- lected by many researchers to study their effects on bivalve ontogeny (Li and Li, 2010; Xu., 2019). The fluctua- tion of temperature and salinity has become major stress factors for many marine organisms in various ways, such as growth performance (Mak and Chan, 2018), disease re- sistance (Fuhrmann., 2016), physiological and bioche-mical responses (Pourmozaffar., 2019), geographic distribution (Matsuda., 2008), and food consumption (Wasielesky., 2003). In addition to the temperature and salinity, one of the principle variables in hatchery con- ditions is stocking density, which is also an important fac- tor for cultivation-related economic variability (Ren., 2017). Inappropriate larval stocking density can lead to sub- optimal growth and survival performance. Thus, favorable stocking density forcultivation is necessary interms of maintaining a positive correlation between den- sity and growth rate.
In our previous study, we have bred a selected strain‘Haida No. 1’ with rapid growth characteristics and an in- breeding line with orange shell color. In the present study, two strains ofand their reciprocal crosses wereevaluated to determine if heterosis exists in the productive characteristics such as growth and survival during larval stage, and compare the heterosis under different tempera- tures, salinities and stocking densities.
2 Materials and Methods
2.1 Broodstock Collection and Larval Rearing
One-year-oldwere collected from a selected strain ‘Haida No. 1’ (S: 12th generation) and an orange shell variant (O: 9th generation). The broodstock of both strainswere grown in the same area located in Rushan, ShandongProvince, China (36.4˚N, 121.3˚E). Both strains were trans- ferred to Laizhou Haiyi hatchery, Shandong Province, and a pool with 24m3of seawater (temperature: 24℃; salinity: 30) was used for 1 month of acclimation prior to experiment. Fifty sexually maturedfrom each strain were in- duced to spawn. A complete diallel cross produced four ex- perimental groups, including two purebred strains of SS (S♀×S♂) and OO (O♀×O♂), and two reciprocal hybrids of OS (O♀×S♂) and SO (S♀×O♂).
After 24-h incubation, D-larvae were collected and trans- ferred into 15-L rearing tanks. A total of 180 tanks were used in the experiment (15 treatments×4 strains×3 replicates). Larvae were fed excessively withthree times a day. For the temperature and salinity experiment, the concentration of 15×103cellsmL−1was maintained un- til the end of the experiment. And for the stocking density experiment, the concentration ofproportionate-ly increased with stocking density to supply larvae in each treatment group with the same amount of algae (Table 1). Before the algae were added to each container, same vo- lume of water was removed to maintain the constant wa- ter volume.
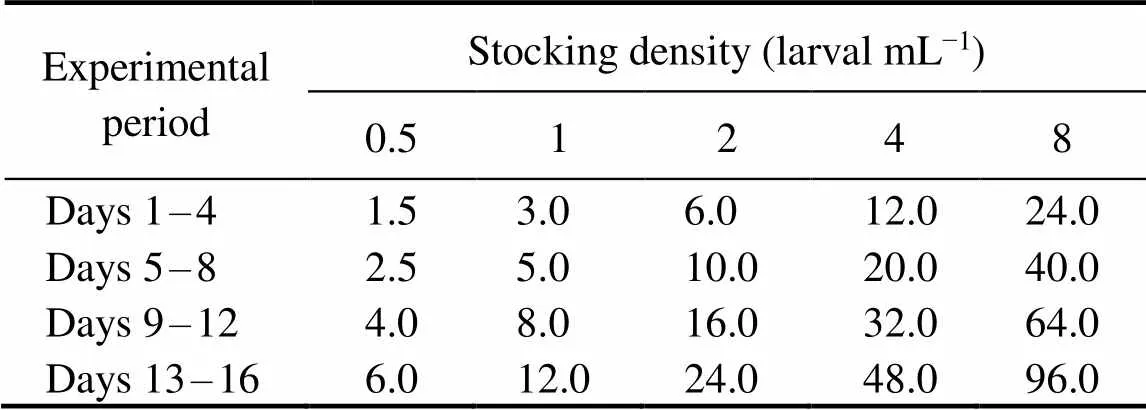
Table 1 Daily algal ratio (×103cellsmL−1) for C. gigas larvae reared under different stocking densities during the experimental period
During the rearing period, larvae containers were slight- ly aerated to increase the oxygen concentration and re- duce the accumulation of organic matter. Every second day morning, 100% of the water was renewed. The fresh sea- water was filtered through sand and nonwoven polypropy- lene fabric and adjusted to the test condition before chang- ing. During water changes, the larvae were transferred onto a 55-μm mesh and gently washed back into containers after the containers had been lightly scrubbed and refilled with fresh seawater.
2.2 Experimental Design
Five experimental temperatures of 16, 20, 24, 28 and 32℃ were selected to evaluate the effect of temperature change on growth and survival oflarvae. The sa- linity was kept stable at 30 and the initial larval density was kept at 2 larvaemL−1. Water temperature was maintained by water bath with immersed heaters or water chiller (HC- 150A, 33ILEA, China) and a temperature regulator. Wa- ter temperature was gradually adjusted to the experimen- tal requirements at a rate of 0.5–1℃ per hour. Five ex- perimental salinities of 15, 20, 25, 30 and 35 were estab- lished to examine the effects of salinity change on growth and survival of larvae. The temperature was kept stable at 24℃ and the initial larval density was kept at 2 larvaemL−1.Water salinity was adjusted by diluting natural seawater with filtered fresh water (filtered through a 50-μm mesh sieve) or adding sea salt and measured by an optical sali- nometer. The initial salinity was raised or lowered at a rate of 2 until desired salinity was obtained. The effects of stock- ing densities were examined at five different densities (0.5, 1, 2, 4, and 8 larvaemL−1). The rearing temperature and salinity were maintained at 24℃ and 30, respectively.
2.3 Measurement and Analysis
In the temperature and salinity experiment, a 50-mL sam- ple was collected randomly. In the stocking density experi- ment, 200-, 100-, 50-, 25- and 10-mL samples were ran- domly taken from containers with stocking densities of 0.5, 1, 2, 4 and 8 larvaemL−1. Subsequently, the samples were fixed by the addition of 1% Lugol’s solution to determine the mean shell height and survival. The shell height of 30 larvae randomly selected in each replicate sample were quantified using a light microscope (160×) equipped with an ocular micrometer. Larval survival was based on an ini- tial density. Larvae were sampled three times to calculate survival rate for each replicate, and the water volume was adjusted according to the survival rate to ensure that each experimental tank always meets the required cultivation density.
The data were analyzed using SPSS 20.0 (IBM SPSS Inc., Chicago, IL, USA). The differences of shell height and sur- vival rate among different strains were analyzed using one- way analysis of variance (ANOVA), followed by the least significant difference (LSD) test and Duncan’s multiplerange tests formean comparisons. Differences were considered statistically significant if<0.05.
Heterosis was calculated to evaluate the aquaculture traits, the equation to determine mid-parent heterosis () was ta- ken from Falconer and Mackay (1996):
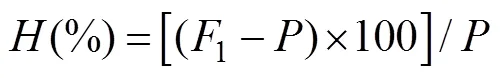
whereis the average phenotypic value of the two paren- tal populations;1is the mean value of one hybrid cross.
3 Results
3.1 Effects of Temperature on Larval Growth and Survival
A significant difference in the shell height appeared start-ing from day 8 (<0.05), and the shell heights of four strains were the largest at 28℃ and the smallest at 16℃ (Fig.1). At the temperature of 24℃and 28℃, the average shell heights of the OS, SO and SS strains were significantly greater than that of the strain OO (<0.05), while no sig- nificant difference was found between the SO and SS strains from day 4 (>0.05). At the temperatures of 16℃, 20℃ and 32℃, the hybrid cross OS showed high growth he- terosis (Table 2), and the mean shell height of OS strain was significantly higher than that of the purebred crosses (OO, SS) and the reciprocal cross SO from day 8 (<0.05).
The larval survival rate at temperature 24℃ was higher than those at other temperatures, and the difference was sig- nificant (<0.05, Table 3). At the temperatures of 16 and 32℃, the survival rate of purebred strains was lower than reciprocal hybrids. The larvae in OO strain all died on day 12, and in SS strain all died on day 16. Therefore, survival data in these treatments were not included in the statistical analyses. The reciprocal hybrids (OS, SO) at temperature 16 and 32℃ occurred the mass mortality, while both strains survived at all the tested temperatures. Overall, during the whole larval stage, the survival rates of reciprocal crosses (OS, SO) were significantly larger than those of purebred strains (OO, SS) (<0.05). And the survival rate was the highestin OS strain and the lowestin OO strain.
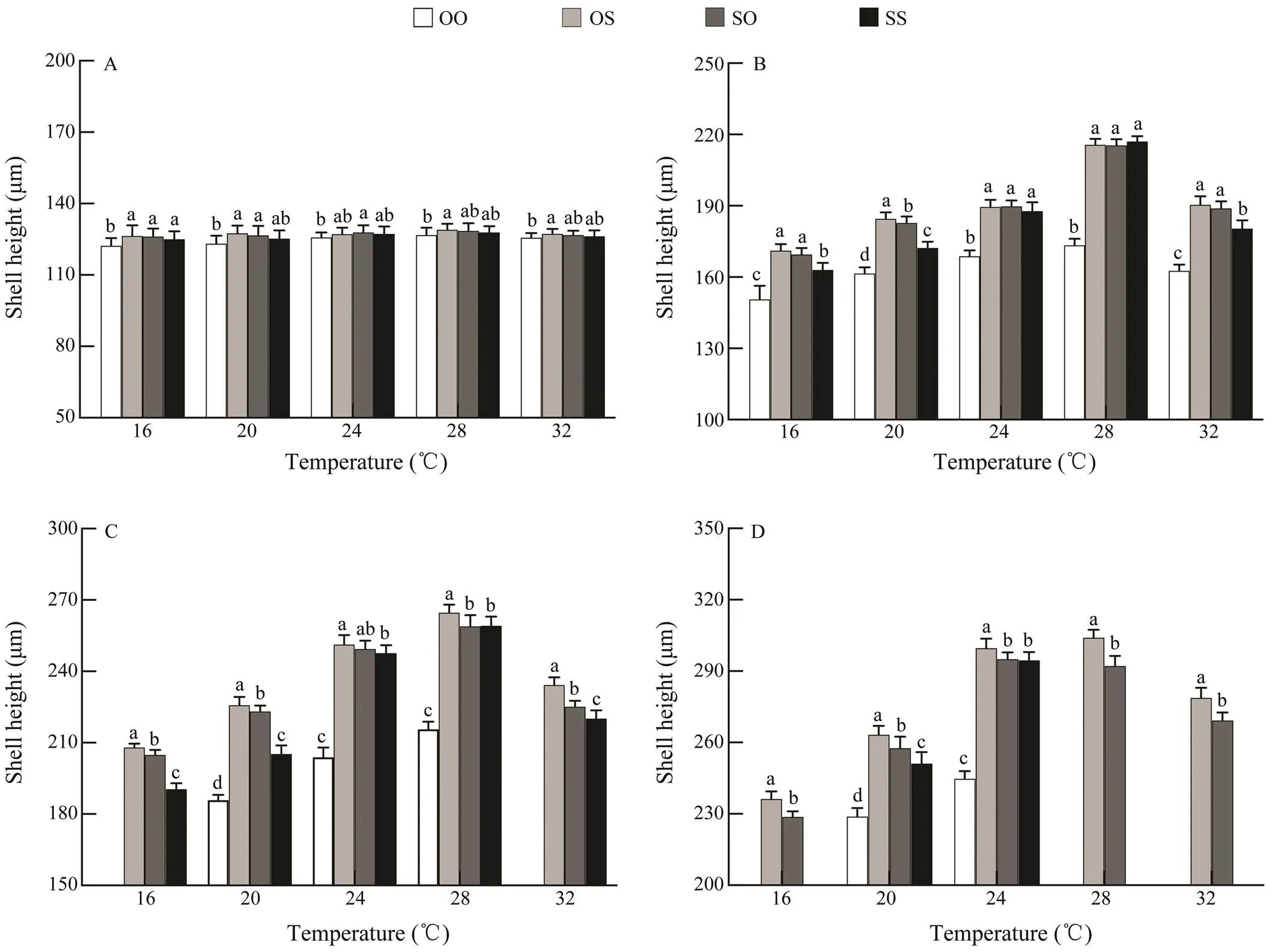
Fig.1 Effects of different temperatures on the larval shell height of the C. gigas in different strains on (A) day 4, (B) day 8, (C) day 12 and (D) day 16. Bars denote standard deviation, different letters show significantly different (P<0.05).
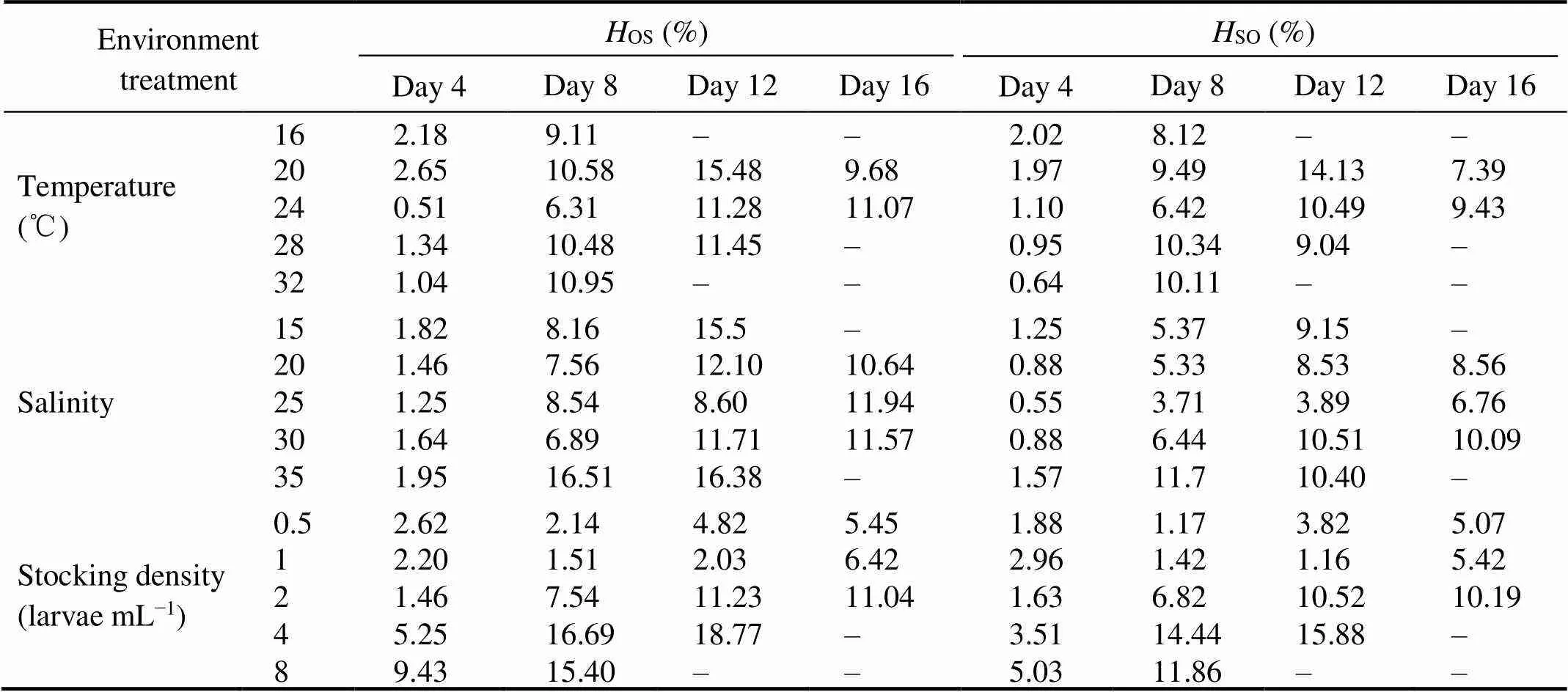
Table 2 Heterosis for larval shell height (HOS and HSO) in two hybrids crosses of C. gigas with different environmental treatments
Note: ‘–’means the value was not available.
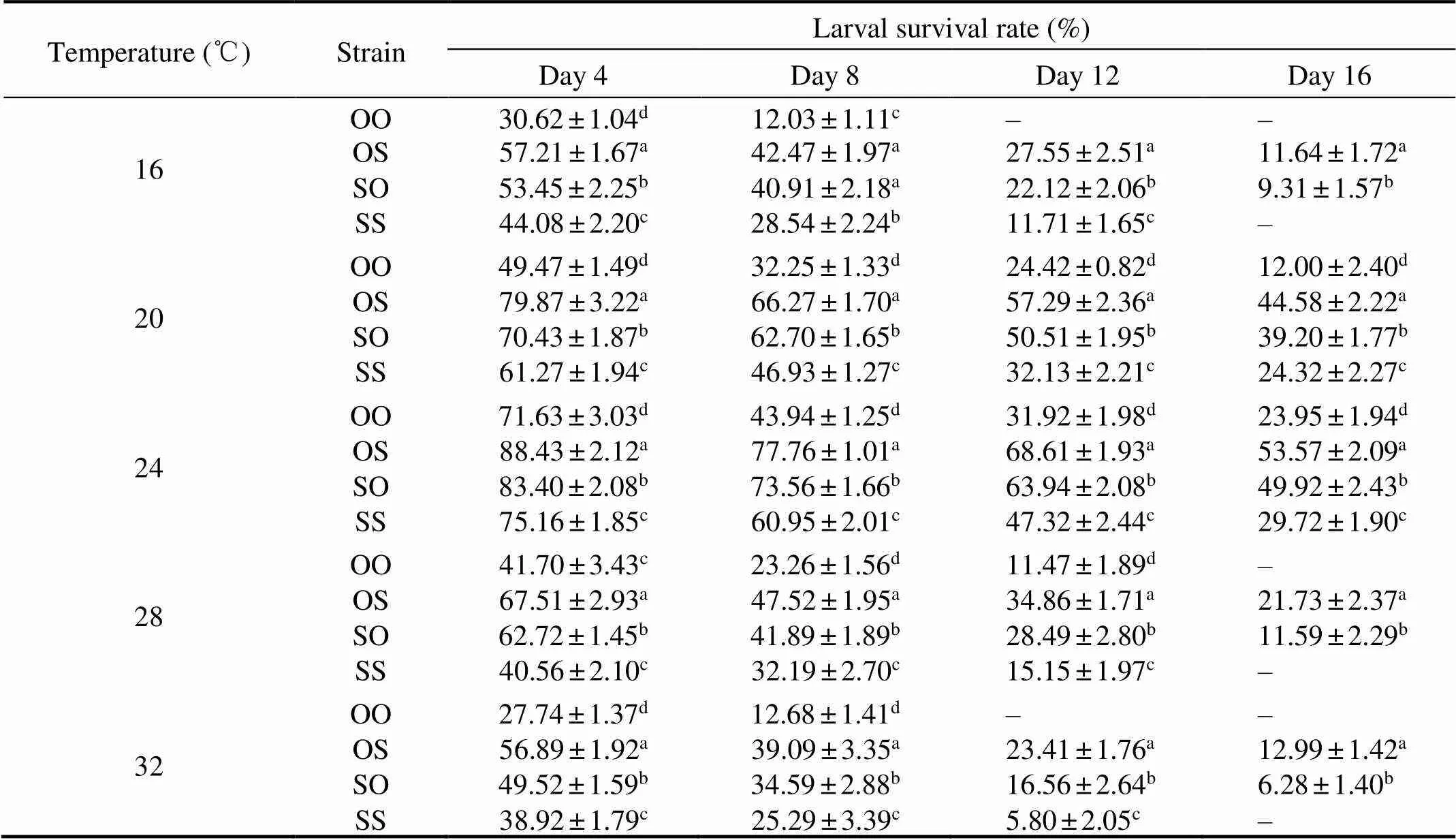
Table 3 Effects of different temperatures on the larval survival rate of C. gigas in different strains
Notes: Different letters show significant difference (<0.05);‘–’means the value was not available.
3.2 Effects of Salinity on Larval Growth and Survival
In the rearing period, the larvae of four strains reared at salinity 25 achieved the highest mean shell height, which was significantly (<0.05) greater than at all the other sa- linity. There were no significant differences in the mean shell height among the purebred strains and their hybrid crosses on day 4, and the shell height of larvae from the four strains was significantly different from day 8 (<0.05). In all salinity treatments, the shell height of the larvae of OS strain was larger than SO and SS strain, while the OO strain was the smallest (Fig.2).
The larval survival rate of the four strains were signifi- cantly affected by salinities (<0.05). The larval survival rate of the four strains were significantly higher at salinity 25 and 30, compared with the other salinity treatments (<0.05, Table 4). In our range of salinity (15–35), all experi-mental animals in the OS, SO, and SS strains survived, and the survival rate of OS strain significantly outperformedall other three strains, which reveals high heterosis for survi- val (<0.05, Table 5). Under the optimal salinity 25 in thepresent experiment, the purebred strain OO showed poor survival performance, which was significantly different from the reciprocal crosses OS and SO (<0.05). At the salinities of 15 and 35, all the larvae from OO strain died on the 16th day.
3.3 Effects of Stocking Density on Larval Growth and Survival
The mean larval shell height of four strains decreased as the stocking density increased. Over the sampling pe- riod from day 4 to 16, the larvae reared at the highest den- sity (8 larvaemL−1) had the smallest height, while those at the lowest density (0.5 larvaemL−1) had the highest height (Fig.3). At low density treatment (0.5 and 1larvaemL−1), the average shell height of the OS, SO, SS strains was sig- nificantly greater than that of the OO strain (<0.05), whileno significant difference was found between the SO and OS strains from day 4 (>0.05).With high density treatment (4 and 8larvaemL−1), the average shell height of the OS strain was the highest among four strains, and the signi- ficant difference was found between the two reciprocal crosses (OS, SO) (<0.05).

Fig.2 Effects of different salinities on the larval shell height of the C. gigas in different strains on (A) day 4, (B) day 8, (C) day 12 and (D) day 16. Bars denote standard deviation, different letters show significantly different (P<0.05).
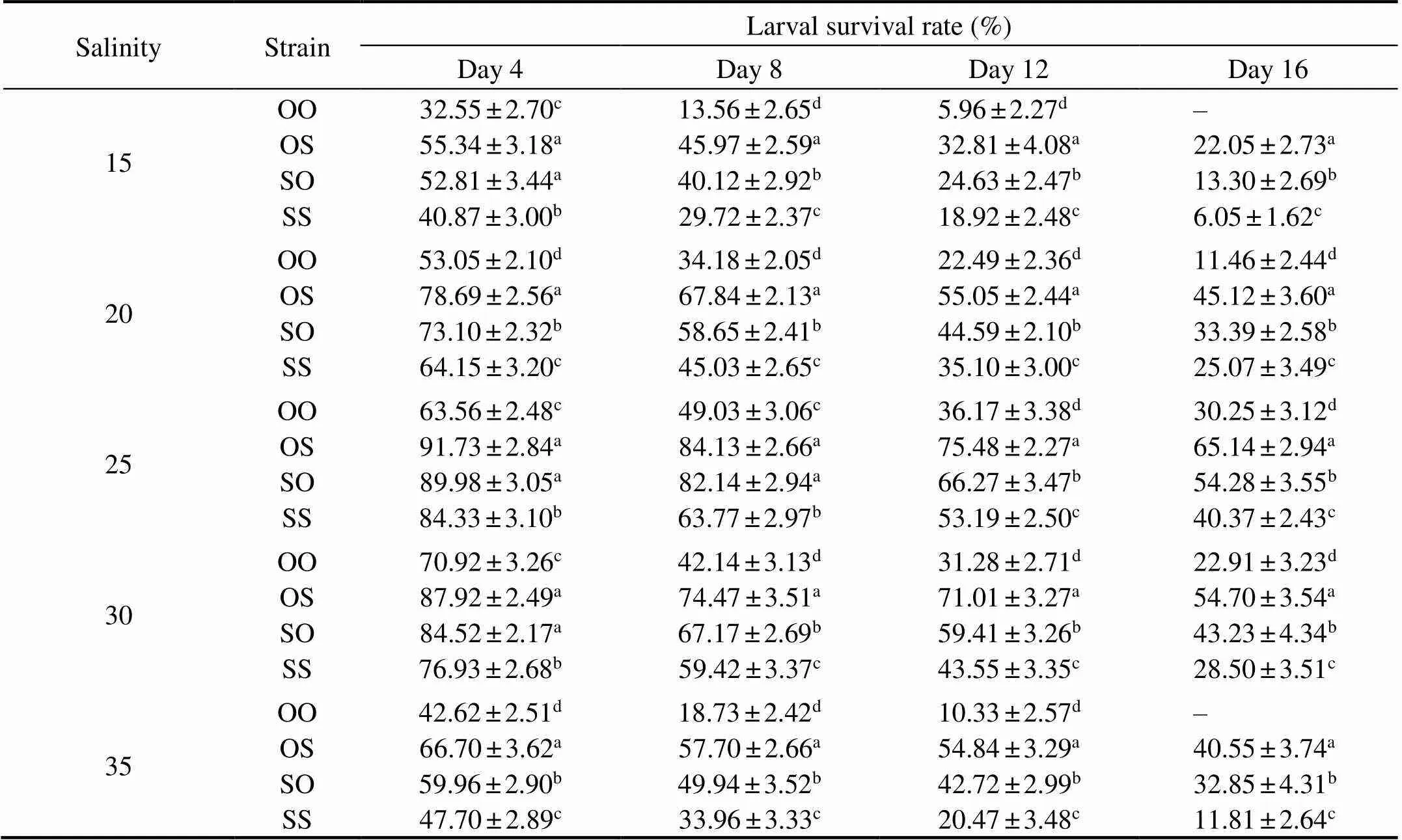
Table 4 Effects of different salinities on the larval survival rate of the C. gigas in different strains
Notes: Same as those in Table 3.
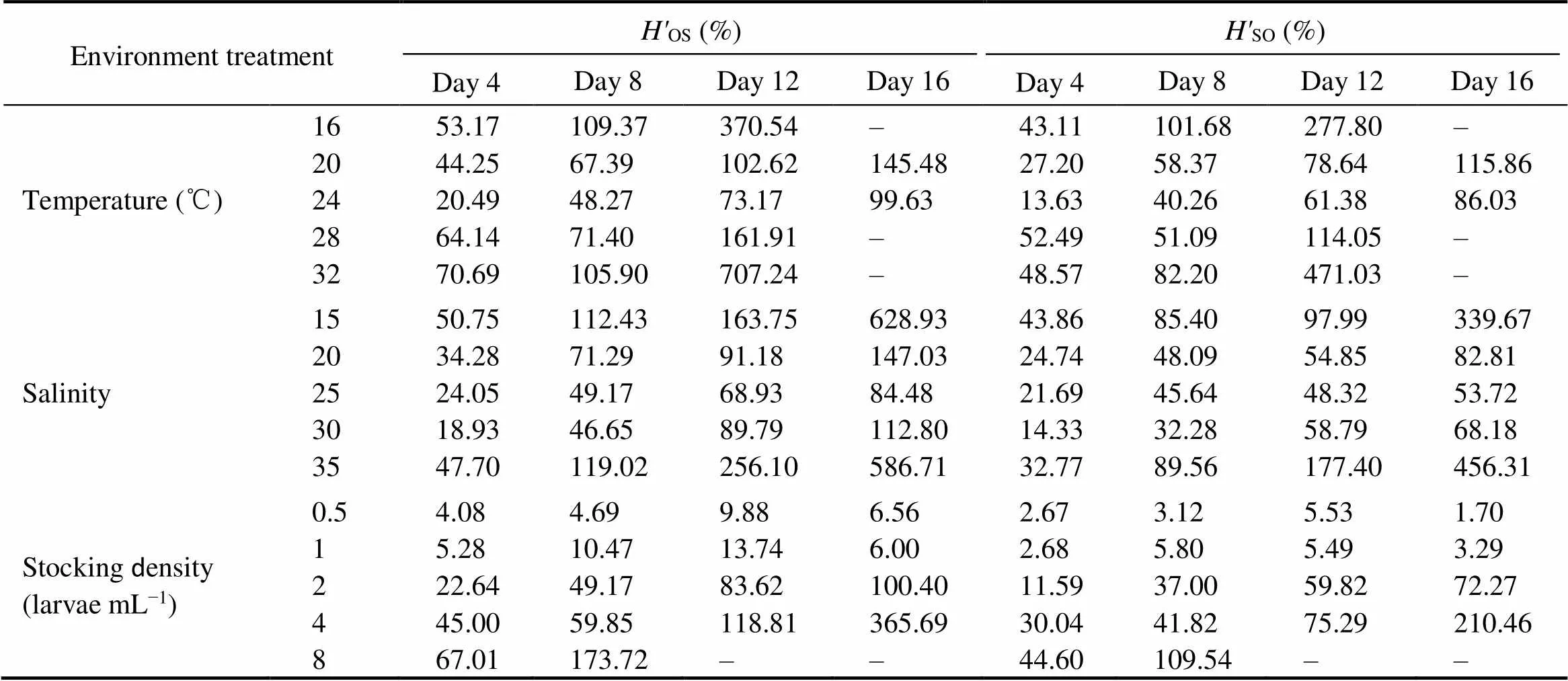
Table 5 Heterosis for larval survival rate (H'OS and H'SO) in two hybrids crosses of C. gigas with different environmental treatments
Note: ‘–’means data are not available.

Fig.3 Effects of different stocking density on the larval shell height of the C. gigas in different strains on (A) day 4, (B) day 8, (C) day 12 and (D) day 16. Bars denote standard deviation, different letters show significantly different (P<0.05).
From day 4, the larval survival rates of four strains with low density treatments (0.5, 1 and 2larvaemL−1) were sig- nificantly (<0.05) higher than those with high density treatments (4 and 8larvaemL−1) (Table 6). On day 4, sur- vival rate of larvae from four strains remained above 65% with an increase in stocking density from 0.5 to 2larvaemL−1; however, a further increase in density of 4 and 8larvaemL−1caused a reduction in survival. The larvae in purebred strains OO and SS reared at 8larvaemL−1died at day 12 of the experiment, and the survival rate of OS strain was higher than that of SO strain. On the 16th day of the experiment, all the reciprocal crosses (OS, SO) rear- ed at 8larvaemL−1had died. Therefore, survival data from these treatments were not included in the statistical analy- sis. In the rearing period, the OS strain showed obvious survival heterosis, and on the 16th day of feeding, the sur- vival rate at 2larvaemL−1was higher than 50% (as shown in Table 6).

Table 6 Effects of different stocking densities on the larval survival rate of the C. gigas in different strains
Notes: Same as those in Table 3.
4 Discussion
4.1 Temperature
The growth performance of oysters was affected by tem- perature, especially at the upper (32℃) and lower (16℃) levels where significant differences in the shell height and survival rate were detected (<0.05). In our study, in- creasing temperature accelerated the larval development. The rapid growth oflarvae in the higher tempera- ture range coincided with the results obtained from other bivalve species such as(Lazo and Pita, 2011),(Nowland, 2019)and(Drent, 2002). As a general rule, an increase in water temperature augments the activity and accelerates the metabolic processes to promote the growth of larvae. On the other hand, a higher mortality rate was caused by the increasing temperature. Due to the fast re- production of bacteria and other microorganisms in thehigher temperature ranges, more oxygen is consumed by these microorganisms and less oxygen can be used by the larvae for their growth, which causes mass mortality in bi- valve hatcheries (Gruffydd and Beaumont, 1972). The mor-tality of larvae at temperature 32℃ is in agreement with the findings of most bivalve larval studies. For example,Nair and Appukuttan (2003) found that there was complete mortality after 24h of the larvae reared at 33℃ and 35℃. Moreover, the survival rate at high temperature (32℃) were much lower than the results of the previous studies aboutKheder. (2010) found the survival rate at theend of larval development was always above 90% at all trial temperatures (17–32℃). The results may be explain- ed by geographical factors, as thein subtropical and tropical areas can have different adaptabilities to tem- perature change (Eirman and Hare, 2013). The lowest tem- perature applied in the present study (16℃) caused a sig- nificant reduction of larval growth, and the mortalities were≥40% after 4 days of rearing. The inability of larvae to grow at low temperature could be due to their inability to digest ingested microalgae(Nair and Appukuttan, 2003). The high- er values for shell height and survival rate were found inthat were held at 24℃, indicating 24℃ is the op- timum growth and survival temperature for larval.
4.2 Salinity
It is well known that salinity is a major environmental parameter and the fluctuation in salinity is the major fac- tor to change the distribution of bivalves. This study de- monstrated that these four strains of larvalcould grow within a salinity range of 15–35. The highest shell height and survival rate were found inthat was held at salinity 25, indicating this as the optimum salinity for larval. Moreover, larval growth and survival were positively correlated with increasing salinity up to 25, from which further salinity increases resulted in decli- ning larval shell height and survival rate. The results of the present study have shown that lowering salinity during lar- val culture had significantly positive effects on larval deve- lopment. Other oyster larvae that display a preference for low salinity include, while the larvae achieved the highest mean shell and maximum survival at salinity 26 (Wang., 2018). Another example is, as the hatchery and nursery cultur- ing of this species at lower salinities was recommended to produce a higher yield (Huo, 2014). At the lowest salinity 15 and the highest salinity 35, the minimum shell height and the lowest survival rate were observed during the rearing period. Previous studies showed that, increased excretion of ammonia and decreased free amino acid con- centration at low salinity led to lower energy for growth (Pourmozaffar., 2019). Additionally, food assimila-tion and feeding efficiency reduced when salinity is be- yond the toleration range (Wang., 2011).
4.3 Stocking Density
In the present study, the shell height and survival rate remained consistent across stocking density at 0.5 and 1larvaemL−1, decreasing significantly at the other tested den- sities of 2, 4, 8larvaemL−1. The result indicated that poor growth performance and higher mortality were observed at densities greater than 4larvaemL−1. High stocking den- sity has been reported to negatively affect larval growth and survival in many bivalves (Daume, 2004; Li and Li, 2010; Ren., 2017). Firstly, the limited growth of shell height at high densities is partly due to the intra-specific competition for food and space. The per capita food supply for high-density individuals is lower than that for low-density individuals (Hadley and Manzi, 1984). In our research, food should not be a limiting factor because al- gal concentrations were adjusted according to larval den- sities to ensure that larvae in each treatment received an equal diet. At high densities, physical contact between in- dividuals can inhibit the food intake, while shells may suf- fer damage and more energy is allocated to repair this da- mage (Holliday, 1991). Secondly, the high mortality of larvae rearing at high densities can also be caused by poor water quality. With increasing stocking densities, more metabolic wastes will be accumulated in the water, result- ing in low oxygen level and high ammonia content (Deng., 2013). Although daily water changes with filtered, UV-sterilized seawater were made to minimize bacterial colonization, it is still inevitable at high density. In addition, whenlarvae were reared at 0.5 and 1larvaemL−1, there was no statistically significant difference in survival rate. Results demonstrated that survival rate was indepen- dent of density when larvae were reared at optimal densi- ties. Similar results also occurred in other studies. For ex- ample, Liu(2006) reported that no correlation has been found between larval survival rate (74.8%–79.1%) and density (5, 10, 20, 40, 60indmL−1) in the treatments. As mentioned previously, high stocking density can cause higher larval mortality while lower stocking density can achieve rapider growth, a moderate density ranging from 1 to 2larvaemL−1is recommended forlarval hat- chery production to achieve rapid growth, high survival rate and maximum economic benefit.
4.4 Heterosis
The objective of selective breeding is to achieve a sta- ble state of excellent production characteristics in differ- ent environments, that is, a variety of offspring should have the properties that can regulate its performance, adapt it to different environments, and maintain its inherent balance of physiological and reproductive traits. In the present study, the selected strain had a greater growth performance and survival ability compared to the orange shell variant. It has documented that the selected strain has an average in- crease in growth of 10% per generation than general oys- ters (Li., 2011). Furthermore, the orange shell vari- ant showed similar performance at optimal conditions and potential disadvantages in suboptimal conditions compared to the general oysters (Han and Li, 2018). Heterosis for growth and survival has been demonstrated in crossbreed- ing of inbred, and the yield increased with ge- neral diallel crosses among inbred parent lines (Hedge- cock., 1995; Hedgecock and Davis, 2007).Accord- ing to our data, the hybridization can promote the growth and survival of oysters, in which the shell height and sur- vival rate of the two reciprocal crosses (OS, SO) were sig- nificantly greater than those of the two purebred strains (SS, OO) in all treatments (<0.05). In particular, OS strain showed greater heterosis compared to the purebred strains and SO progeny as well. In contrast, other similar studies reported that the hybrid larvae showed intermedi- ate growth between the parental species, which was report- ed for×(Huo, 2013),×(Allen Jr. and Gaffney, 1993) andSay×Lamarck (Zhang, 2007). In addition, in many interspecific hybrids, the progeny exhibits slower growth than the parent species (Beaumont., 2004; Zhang, 2012).This study showed undoubtedly the high tolerance of the reciprocal cross OS larvae under extreme environ- ments.If bivalves can tolerate a wide range of environmen- tal fluctuation, it is possible that they become dominant species under challenging environmental conditions.
5 Conclusions
This study firstly examined larval growth and survival of reciprocal crosses between two strains ofat dif- ferent environmental conditions. Results indicated that the larval productive traits of the selected strain ‘Haida No. 1’ could be improved by crossing with the orange shell line. Under different environments, the hybridization be- tween the ‘Haida No. 1’ and the orange shell variant show- ed the heterosis of growth and survival. The reciprocal cross OS reveals higher heterosis for growth and survival compared to the reciprocal cross SO. The environmental parameters, including the temperature of 24℃, the sali- nity of 25, the stocking density of 2larvaemL−1 in presentstudy, provide optimal culture conditions forin the hatchery. These findings can guide future hatchery breed- ing of new variety with high survival rate.
Acknowledgements
This work was supported by the National Natural Sci- ence Foundation of China (Nos. 31972789 and 31772843), the Industrial Development Project of Qingdao City (No. 20-3-4-16-nsh), and the Science and Technology Deve- lopment Project of Weihai City (No. 2018NS01).
Allen Jr., S. K., and Gaggney, P. M., 1993. Genetic confirmationof hybridization between(Thunberg) and(Gould)., 113: 291-300.
Beaumont, A. R., Turner, G., Wood, A. R., and Skibinski, D. O. F., 2004. Hybridisations betweenandand performance of pure species and hybrid veliger larvae at different temperature., 302: 177-188.
Claudi, R., and Evans, D. W., 1993. Chemical addition strategies for zebra mussel () control in once-through service water systems. In:. Nalepa, T. F., and Schloesser, D. W., eds., Lewis Publishers, Boca Raton, FL, 563-574.
Daume, S., Huchette, S., Ryan, S., and Day, R. W., 2004. Nur-sery culture of: The effect of cultured algaeand larval density on settlement and juvenile production., 236: 221-239.
Deng, Y. W., Fu, S., Liang, F. L., and Xie, S. H., 2013. Effects ofstocking density, diet, and water exchange on growth and sur- vival of pearl oysterlarvae., 21: 1185-1194.
Department of Fisheries (DOF), 2020.. China Agriculture Press, Beijing, 387pp (in Chinese).
Drent, J., 2002. Temperature responses in larvae offrom a northerly and southerly population of the Euro- pean distribution range., 275: 117-129.
Eierman, L. E., and Hare, M. P., 2013. Survival of oyster larvae in different salinities depends on source population within an estuary.,449: 61-68.
Falconer, D. S., and Mackay, T. F. C., 1996.. 4th edition. Pearson Education Limited, Es- sex, 480pp.
Fuhrmann, M., Petton, B., Quillien, V., Faury, N., Morga, B., and Pernet, F., 2016. Salinity influences disease-induced mortality of the oysterand infectivity of the ostreid herpesvirus 1 (OsHV-1)., 8: 543-552.
Gruffydd, L. D., and Beaumont, A. R., 1972. A method for rear- inglarvae in the laboratory., 15 (4): 350-355.
Hadley, N. H., and Manzi, J. J., 1984. Growth of seed clams,, at various densities in a commercial scale nursery system., 36 (4): 369-378.
Han, Z. Q., and Li, Q., 2018. Different responses between orange variant and cultured population of the Pacific oysterat early life stage to temperature-salinity combi- nations., 49 (6): 2233-2239.
Han, Z. Q., Li, Q., Liu, S. K., and Kong, L. F., 2020. Cross- breeding of three different shell colour lines in the Pacific oys- ter reveals high heterosis for survival but low heterosis for growth., 529: 735621.
Han, Z. Q., Li, Q., Liu, S. K., Yu, H., and Kong, L. F., 2019. Genetic variability of an orange-shell line of the Pacific oys- terduring artificial selection inferred from microsatellites and mitochondrial COI sequences.,508: 159-166.
Hedgecock, D., and Davis, J. P., 2007. Heterosis for yield and crossbreeding of the Pacific oyster., 272: S17-S29.
Hedgecock, D., McGoldrick, D. J., and Bayne, B. L., 1995. Hy- brid vigor in Pacific oysters: An experimental approach using crosses among inbred lines., 137 (1-4): 285-298.
Holliday, J. E., Maguire, G. B., and Nell, J. A., 1991. Optimum stocking density for nursery culture of Sydney rock oysters ()., 96 (1): 7-16.
Hulata, G., 2001. Genetic manipulations in aquaculture: A review of stock improvement by classical and modern technologies., 111: 155-173.
Huo, Z. M., Wang, Z. P., Liang, J., Zhang, Y. H., Shen, J. P., Yao, T.,., 2014. Effects of salinity on embryonic development, survival, and growth of., 13 (4): 666-670.
Huo, Z. M., Wang, Z. P., Yan, X. W., and Gaffney, P. M., 2013. Fertilization, survival, and growth of♀×♂ hybrids in northern Chi- na., 32 (2): 377-385.
Kheder, R. B., Moal, J., and Robert, R., 2010. Impact of tempera- ture on larval development and evolution of physiological in- dices in., 309 (1-4): 286-289.
Kong, L. F., Song, S. L., and Li, Q., 2017. The effect of inter- strain hybridization on the production performance in the Pa- cific oyster., 472: 44-49.
Lazo, C. S., and Pita, I. M., 2011. Effect of temperature on sur- vival, growth and development oflarvae., 43 (8): 1127-1133.
Li, L., and Li, Q., 2010. Effects of stocking density, temperature, and salinity on larval survival and growth of the red race of the sea cucumber(Selenka)., 18 (3): 447-460.
Li, Q., Wang, Q. Z., Liu, S. K., and Kong, L. F., 2011. Selection response and realized heritability for growth in three stocks of the Pacific oyster., 77 (4): 643-648.
Liu, B. Z., Dong, B., Tang, B. J., Zhang, T., and Xiang, J. H., 2006. Effect of stocking density on growth, settlement and survival of clam larvae,., 258 (1-4): 344- 349
Mak, K. Y., and Chan, K. Y. K., 2018. Interactive effects of tem- perature and salinity on early life stages of the sea urchin., 165 (3): 1-11.
Matson, S., Davis, J. P., and Chew, K. K., 2003. Laboratory hy- bridization of the mussels,and: Larval growth, survival and early development., 22 (2): 423-430.
Matsuda, M., Shinagawa, A., Higano, J., Fujii, A., Hirano, K., and Ishimatsu, A., 2008. Effects of low salinity on survival, hemo- lymph osmolality and tissue water content of the Manila clam., 56 (1): 127-136.
Nair, R. M., and Appukuttan, K. K., 2003. Effect of temperature on the development, growth, survival and settlement of green mussel(Linnaeus, 1758)., 34 (12): 1037-1045.
Nowland, S. J., O’Connor, W. A., Penny, S. S., Osborne, M. W. J., and Southgate, P. C., 2019. Water temperature and salinity synergistically affect embryonic and larval development of the tropical black-lip rock oyster., 27 (5): 1239-1250.
Pourmozaffar, S., Jahromi, S. T., Rameshi, H., Sadeghi, A., and Lazarjani, S. A., 2019. The role of salinity in physiological re-sponses of bivalves., 12 (3): 1548-1566.
Ren, Y. C., Liu, W. S., and Pearce, C. M., 2017. Effects of stock- ing density, ration and temperature on growth, survival and metamorphosis of auricularia larvae of the California sea cucumber,., 49 (1): 517-525.
Tan, K., Liu, H. X., Ye, T., Ma, H. Y., Li, S. K., and Zheng, H. P., 2020. Growth, survival and lipid composition of,and their reciprocal hybrids cultured in southern China., 516: 734524.
Wang, T., Li, Q., Zhang, J. X., and Yu, R. H., 2018. Effects of salinity, stocking density, and algal density on growth and sur- vival of Iwagaki oysterlarvae., 26 (4): 947-958.
Wang, Y. J., Hu, M. H., Wong, W. H., Cheung, S. G., and Shin, P.K. S., 2011. Combined effects of dissolved oxygen and salinity on growth and body composition of juvenile green-lipped mus- sel., 30 (3): 851- 857.
Wasielesky, W., Bianchini, A., Sanchez, C., and Poersch, L., 2003.The effect of temperature, salinity and nitrogen products on food consumption of pink shrimp., 46 (1): 135- 141.
Xu, C. X., Li, Q., and Chong, J. D., 2019. Combined effect of temperature, salinity, and rearing density on the larval growth of the black shell strain and wild population of the Pacific oys- ter., 28 (1): 335-
347.
Xu, C. X., Li, Q., Chong, J. D., Liu, S. K., and Kong, L. F., 2019. Mass selection for growth improvement in black shell line of Pacific oyster, 18 (6): 1411-1416.
Xu, H. Q., Li, Q., Han, Z. Q., Liu, S. K., Yu, H., and Kong, L. F., 2019. Fertilization, survival and growth of reciprocal cross- es between two oysters,and., 507: 91-96.
Zhang, H. B., Liu, X., Zhang, G. F., and Wang, C. D., 2007. Growth and survival of reciprocal crosses between two bay scallops,Say andLamarck., 272: S88-S93.
Zhang, Y. H., Wang, Z. P., Yan, X. W., Yu, R. H., Kong, J., Liu, J.,., 2012. Laboratory hybridization between two oysters:and., 31 (3): 619-625.
Zheng, H. P., Xu, F., and Zhang, G. F., 2011. Crosses between two subspecies of bay scallopand het- erosis for yield traits at harvest., 42: 602-612.
December 22, 2020;
March 4, 2021;
June 22, 2021
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2023
. E-mail: qili66@ouc.edu.cn
(Edited by Qiu Yantao)
 Journal of Ocean University of China2022年4期
Journal of Ocean University of China2022年4期
- Journal of Ocean University of China的其它文章
- The Role of Bottom Currents on the Morphological Development Around a Drowned Carbonate Platform, NW South China Sea
- Controls on the Gas Hydrate Occurrence in Lower Slope to Basin-Floor, Northeastern Bay of Bengal
- Effective Elastic Thickness of the Lithosphere in the Mariana Subduction Zone and Surrounding Regions and Its Implications for Their Tectonics
- Geophysical Evidence for Carbonate Platform Periphery Gravity Flows in the Xisha Islands, South China Sea
- Seismic Characteristics and Hydrocarbon Accumulation Associated with Mud Diapir Structures in a Superimposed Basin in the Southern South China Sea Margin
- Simulation and Analysis of Back Siltation in a Navigation Channel Using MIKE 21
