One-pot green mass production of hierarchically porous carbon via a recyclable salt-templating strategy
Chnge M,Jing Gong,Shung Zho,Xiogung Liu,Xueying Mu,Ynhui Wng,,Xueheng Chen,,To Tng,
a State Key Laboratory of Polymer Physics and Chemistry,Changchun Institute of Applied Chemistry,Chinese Academy of Sciences,Changchun,130022,China
b University of Science and Technology of China,Hefei,230026,China
c Key Laboratory for Material Chemistry of Energy Conversion and Storage,Ministry of Education,School of Chemistry and Chemical Engineering,Huazhong University of Science and Technology,Wuhan,China
d Faculty of Chemical Technology and Engineering,West Pomeranian University of Technology,Piast′ow Ave.42,Szczecin,71-065,Poland
Abstract Porous carbon materials have exhibited a series of promising applications in supercapacitors and other research fields,yet still confronting the complicated synthetic procedures and massive usage of toxic reagents.Herein,we propose a green and one-pot method to produce heteroatomdoped hierarchical porous carbon materials in large-scale without any toxic reagents employed.Eventually,the as-prepared nitrogen-doped porous carbon (NPC) displays a high specific surface area of 2018 m2 g-1 together with abundant heteroatom dopants (14.8 wt% O and 1.03 wt%N).The potassium carbonate template can be recycled via a simple rinsing and re-precipitation process.Furthermore,the as-prepared nitrogen-doped porous carbon delivers a high specific capacitance of 361 F g-1 at 0.5 A g-1 and excellent rate capability of 240 F g-1 at 20 A g-1 (66.5% capacitance retention).Finally,considering the low-price raw materials and facile green synthesis procedure,the present approach can be easily scalable to prepare biomass-derived heteroatoms doped porous carbon,which is not only applicable for supercapacitor but also for other research fields.
Keywords: Porous carbon;Supercapacitors;Green;Salt template;Recyclable
1.Introduction
Nowadays,porous carbon materials have gained great attention in numerous fields,such as catalyst/catalytic carrier[1],adsorption and separation [2,3],solar steam evaporation[4],batteries[5],and supercapacitors[4,6].This is mainly due to its fascinating characteristics,such as adjustable pore structure,ultrahigh specific surface area (SSA),broad light absorption characteristic,excellent electronic conductivity,and stable chemical properties [7].By now,a variety of methods have been developed to fabricate carbon materials,such as hydrothermal carbonization [8],chemical vapor deposition (CVD) [9],catalytic carbonization [10],and template carbonization [11,12].However,the above-mentioned methods usually require complicated synthesis processes,consume large amounts of organic solvents,carbon deposition template,and corrosive acids for etching templates,leading to high production costs and huge environmental pressure for mass production.Therefore,the development of green and cost-effective methods to prepare value-added porous carbon materials is still highly desired.In recent years,“salt templating” has been applied to prepare carbon materials,owing to the low cost and easy removal in the subsequent purification process [13-16].Among various salts,potassium carbonate(K2CO3),served as an excellent template and activator,exhibits great potential in the preparation of hierarchically porous carbon materials.Compared with other templates,such as CaCO3[17],SiO2[18],NaCl [19],MgO [20],etc.,K2CO3can act as both template and activator simultaneously to prepare hierarchical pore structure carbon materials through onestep carbonization strategy,while other template methods usually require additional high-temperature activation process.Besides,during the process of carbon material purification,some templates (CaCO3,CaCl2,SiO2,FeCl3,MgO,et al.)need to consume large amounts of acid solution [18,20-23],which is not environmentally friendly.However,there are still some obstacles needed to be addressed.For example,the issues of templates recycling have not been considered in the literatures [24].Additionally,the freeze-drying method is not easy to realize mass production of porous carbon materials in industry [25].Besides,the prepared carbon materials usually suffer from unsatisfactory electrochemical performance [25-27].Furthermore,the hydrochloric acid(HCl) is directly used to remove templates from pyrolysis products,resulting in extensive utilization of corrosive acid and the non-recyclability of templates [25].Therefore,it is still imperative to develop a simple and sustainable strategy with salt templates for mass production of hierarchically porous carbon material.
Due to the traditional fossil energy crisis and the cor111responding environmental issues,the development of green energy and relevant energy storage technologies is particularly imperative in our modern society [7,28].Among various energy storage devices,electrical double layer capacitors(EDLCs),also called supercapacitors,exhibit broad application prospects owing to their short charge/discharge time,large power density,wide working temperature window,and superior cyclic stability[7].In general,the commercially activated carbon (AC) is extensively utilized as electrode materials in EDLCs.Nevertheless,the dominate microporous features and curved channel structure of AC normally slow down the ionmigration kinetics and decrease the ion-accessible specific surface area,resulting in undesirable electrochemical behaviors [29,30].Therefore,developing high-performance carbon materials for supercapacitors has aroused widespread interest in the field of energy storage [31,32].
So far,many literatures have reported various preparation methods of carbon materials to improve the electrochemical performance in recent decades [33-37],which can be mainly summarized into two categories.One strategy is to prepare hierarchically porous carbon materials with a well-balanced macro-/meso-/micropore distribution [38-42].In detail,the large amounts of micropores can provide extremely high specific surface area for charge adsorption and storage.Meanwhile,the meso-/macropores can provide effective mass transport of electrolytes [43-45].Furthermore,the other effective way to enhance the electrochemical properties is to dope heteroatoms into carbon skeleton[46].Until now,a wide variety of heteroatoms (e.g.,N,B,O,P and S) have been successfully doped into carbon matrix to improve the capacitive performance [47-54].Generally,it is believed that heteroatom doping can not only lead to additional pseudocapacitance,but also ameliorate the wetting properties between the electrode materials and electrolytes,thereby improving the accessibility of electrolyte ions [9,55].Therefore,designing hierarchical pore nanostructures with suitable heteroatoms doping can combine the above advantages,realizing the goal of preparing high-performance electrode materials.
Herein,N,O co-doped porous carbon material has been successfully fabricated through one-step pyrolysis of the mixture of glucose/urea/potassium carbonate in large quantities.In this study,potassium carbonate can not only act as an excellent template to produce macroporous structures,but also works as a mild activation reagent to create micropores and mesopores,resulting in the formation of hierarchically porous structure.Besides,the addition of urea can not only introduce nitrogen atoms into the carbon skeleton,but also contributes to a well-developed pore structure owing to the produced ammonia from the urea decomposition.Owing to the synergy effects of hierarchical pore structure and heteroatom doping,the resultant carbon material delivers excellent electrochemical performance in supercapacitors.More importantly,during the whole synthetic process,no toxic organic solvents or corrosive acid were used,and only water and a small amount of environmentally friendly acid (citric acid) were involved in subsequent purification of carbon materials,which thus can be regarded as a green synthetic method.Compared with the traditional carbonization-activation strategy,it simplifies the synthesis process,reduces the energy consumption,and saves the production cost.Besides,the most common chemical activation reagents (e.g.,KOH and H3PO4) are not employed at all,thus resulting in less corrosiveness to the reaction equipment.Finally,the pyrolysis products are dispersed in water and subsequently filtered to remove the majority of salt template.More importantly,after a process of“introducing CO2into filtrate-crystallization-heat treatment at 200°C”,K2CO3can be obtained again to realize the recycling of template.Consequently,this research proposes a green and sustainable strategy to prepare porous carbon materials with promising applications in supercapacitors.
2.Experimental
2.1.Preparation of NPC and PC
In a typical synthesis,2 g glucose,2 g urea and 10 g K2CO3template were firstly ground for 10 min in agate mortar until they were mixed uniformly.Then the mixture was put in a corundum crucible and transferred to a tube furnace.The sample was firstly heated at 650°C for 2 h and then heated to 800°C for 1 h with a ramp rate of 5°C min-1under N2protection.After cooling to room temperature,the product was firstly washed with deionized water to remove the majority of potassium compounds.Then the obtained carbon material was washed with diluted citric acid solution to purify carbon product.Finally,the obtained carbon material labeled as NPC is placed in a vacuum oven overnight and dried for subsequent tests.Similarly,PC sample was also synthesized in the above method without adding urea.For comparison,we also carbonized glucose and urea directly without adding potassium carbonate,and the sample is named PC-0.The detailed synthesis process is depicted in Scheme 1.

Scheme 1.Schematic diagram of the synthesis process for porous carbon material.
2.2.Characterization
The morphology and microstructure of the prepared samples were investigated by transmission electron microscopy(TEM,JEM-1011),scanning electron microscopy (SEM,XL30ESEM-FEG),and high-resolution transmission electron microscopy (HRTEM,FEI Tecnai G2 S-Twin).X-ray diffraction(XRD)was used to characterize the phase structure on Bruker D8 Advance diffractometer with Cu-Kα radiation.The Elemental Analyzer (Vario EL III) was used to analyze the element content.X-ray photoelectron spectroscopy (XPS)was measured by an ESCALABMK II spectrometer using Al Kα X-ray source.Elemental Analyzer(Vario EL III)was used to determine the element content.Raman tests was conducted on a Raman spectrometer (T6400) with a laser wavelength of 514.5 nm.The contact angle tester(KRUSS DSA30)was used to measure the contact angle of prepared electrodes.N2sorption/desorption analyzer (Quantachrome NOVA 1000 system) was adopted to characterize the specific surface area and pore parameters at 77 K.The specific surface area and pore diameter distribution were evaluated by BET and DFT method,respectively,and the surface area and pore volume of micropore were calculated by t-plot method.The detailed information of electrode preparation and electrochemical characterization is provided in the supporting information.
3.Results and discussion
In this research,N,O co-doped porous carbon material has been synthesized through one-step pyrolysis of the mixture of glucose,urea,and potassium carbonate.In order to explore the role of urea on the electrochemical performance of carbon material,a controlled experiment without the usage of urea was carried out under the same pyrolysis conditions.The experiment data indicates that the carbon material prepared with urea added displays better electrochemical properties,demonstrating that the low-cost urea can be served as an excellent additive to elevate the electrochemical performance.Furthermore,the experiment results also suggest that K2CO3can act as an excellent template to produce hierarchically porous structure.In detail,the macropores mainly derive from the salt template,while the micropores and small mesopores can be created owing to the activation of potassium carbonate.The activation mechanism is ascribed to the chemical reaction of carbon with K2CO3and its pyrolysis products (K2O and CO2),and the reaction occurred above 700°C [56].As a result,partial carbon atoms are etched away to create micro/mesopores.During this process,the lost carbon atoms will be converted into CO gas and escape.The detailed reactions are listed below [57].


Fig.1.SEM and TEM images of PC(a and c)and NPC(b and d).(e)Photograph of pyrolysis products from the boat crucible without removing salt template.(f)Comparison of the initially added salt and the recycled salt template.
As shown in Fig.1,after removing the salt template,a three-dimensional carbon material with an open porous architecture can be obtained.Fig.1a and c display the SEM and TEM images of PC sample,respectively.Similarly,Fig.1b and d present the morphology of NPC sample.Intuitively,both of them exhibit an irregular macropore structure without any obvious visual differences.In fact,the main difference between the two samples lies in the micro-/mesopore content and the heteroatoms species,and the detailed distinctions will be discussed in the following text with cor111responding data.Moreover,the existence of micropores can be demonstrated by HRTEM images (see Fig.S1 in supporting information).Additionally,EDX mapping results in Fig.S2 indicate that C,N and O elements are equably distributed in NPC sample,indicating nitrogen and oxygen atoms have been successfully doped into carbon skeleton.The SEM images of K2CO3particles after grind are provided in Fig.S3.After pyrolysis at high temperature,the white powder mixtures become a hard black lump,which can be taken out directly from the boat crucible (Fig.1e).The pyrolysis products can be directly dispersed into water to remove the vast majority of salt template without consuming extensive corrosive acids.Meanwhile,we can regain potassium carbonate after a process of crystallizing the filtrate and subsequently heating at 200°C,and 95% of the salt template can be recycled (Fig.1f).The crystal structure of the initial and recycled K2CO3template was also characterized by XRD methods (Fig.S4),demonstrating the successful recovery of salt template.
As shown in Fig.2a,XRD patterns exhibit two diffraction peaks at approximately 21°and 43°,responding to (002) and(101) planes of carbon material[58].The(002) plane migrate to a lower value compared to carbon material without K2CO3added (2Θ=25.1°,d=0.354 nm) (Fig.S5a),implying a larger interlayer spacing (d=0.422 nm) owing to the activation of K2CO3.Specially,we have tested the XRD patterns of the pyrolysis products without removing the salt template and the cor111responding results are in Fig.S5b.From the results,we can infer that the main form of salt in the pyrolysis product is K2CO3.This is because only part of K2CO3decomposes to form K2O,and it is easy to react with CO2in air and generate K2CO3again.Raman spectra in Fig.S6 display two characteristic bands.In general,the D peak at 1329 cm-1can be ascribed to the defective or amorphous structure of carbon materials.The G peak at 1606 cm-1implies sp2hybridized carbon of graphitic nature.The defect degree of graphite structure can be characterized by the ratio of D to G band intensity (ID/IG) [59].NPC sample exhibits a higher ID/IGvalue (1.86) than PC sample (1.78),indicating that more disorder structures exist in NPC sample.By comparison,the value of ID/IGfor PC-0 is 1.59,which is lower than PC and NPC samples,indicating the defects are caused by K2CO3activation,and the defects can act as crucial active sites to enhance capacitor properties [60].In Fig.2b,C 1s (285 eV)and O 1s (533 eV) peaks can be noticed obviously from the XPS survey spectra of both samples.Comparatively,the N 1s peak in NPC locating at 401.0 eV is relatively weak,indicating the content of N atom in carbon material is much less than that of O atom.Besides,we have adopted the elemental analyzer to determine the element content,and the results indicate that the O and N contents in NPC sample are 14.8 wt%and 1.03 wt%,respectively,while the O content in PC sample is 14.5 wt%.Furthermore,N-doping in NPC was proved in the form of graphitic-N (N-Q),pyrrolic-N (N-5),and pyridinic-N (N-6)with the high-resolution N 1s XPS spectrum (Fig.2c).In general,pyrrolic-N and pyridinic-N are generally considered to make great contributions to the improvement of capacitance[61].Besides,high-resolution O 1s XPS spectra of the samples are divided into three peaks(Fig.2d and Fig.S7),referring to carbonyl or anhydride groups (O-1),phenol groups or ether groups (O-2),and carboxyl groups (O-3).Similarly,oxygen doping can also contribute to additional pseudocapacitance owing to the redox reactions between oxygen-containing functional groups and electrolyte ions.The specific redox reaction can be referred to the following literature [62-64].Moreover,heteroatom (N and O) doping can ameliorate the surface wettability of carbon materials,which supplies a larger accessible surface area for the adsorption of electrolyte ions and promotes the improvement of electrochemical performance [65-68].Specially,we have measured the contact angles of the prepared electrode surface in water (Fig.S8).The NPC electrode displays a smaller contact angle of 23°than the PC electrode (64°),reflecting a better wettability of NPC electrode.The excellent hydrophilicity is mainly attributed to the rich heteroatom doping.Therefore,it is expected that the coexistence of N-and O-containing functional groups endow NPC sample with superior electrochemical performance.
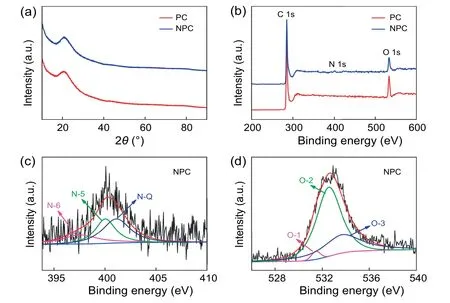
Fig.2.(a) XRD patterns and (b) XPS spectra of PC and NPC,and high-resolution XPS spectra of (c) N 1s and (d) O 1s for NPC.
Fig.3 exhibits the N2adsorption/desorption measurements of carbon materials.As observed in Fig.3a,both samples exhibit high adsorption features in the low-pressure area (P/P0<0.1),indicating the existence of abundant micropores.Specially,NPC displays a higher N2adsorption capacity than PC,suggesting the higher content of micropores.Moreover,the increase of adsorption amount at a high relative pressure(P/P0>0.9) reflects the macropores nature,which is originated from the removal of K2CO3template.Additionally,unobvious adsorption-desorption hysteresis loops appearing at a moderate relative pressure region (P/P0=0.4-0.9) implies the existence of mesopores.Similarly,the pore size distribution curves in Fig.3b also confirm the co-existence of micropores and mesopores in the two samples.In particular,the content of micropores with a pore size distribution of 0.73-1.4 nm and small mesopores centered at 2.00-2.74 nm in NPC sample are much higher than that of PC sample,and previous study have reported that the existence of micropores contributes to the improvement of electrochemical properties significantly since micropores can act as active sites to absorb charged ions [69].In detail,the NPC sample displays larger specific surface area of 2018 m2g-1and pore volume of 1.19 cm3g-1than the PC sample with the cor111responding values of 1526 m2g-1and 1.03 cm3g-1,respectively.Moreover,the micropore specific surface area (1503 m2g-1)and pore volume (0.63 cm3g-1) of NPC are also larger than those of PC (950.3 m2g-1and 0.34 cm3g-1,respectively)(Fig.3c and d).The detailed parameters are presented in Table S1.In view of the above results,we can infer that the addition of urea has significant impacts on the specific surface area and pore structure.In general,the gases (ammonia and carbon dioxide) produced by urea decomposition have a certain activation effect on carbon materials.Additionally,the volatilization of produced gases helps to develop a more-developed porous structure,thereby the NPC sample implies more promising application prospects in supercapacitor than the PC sample.
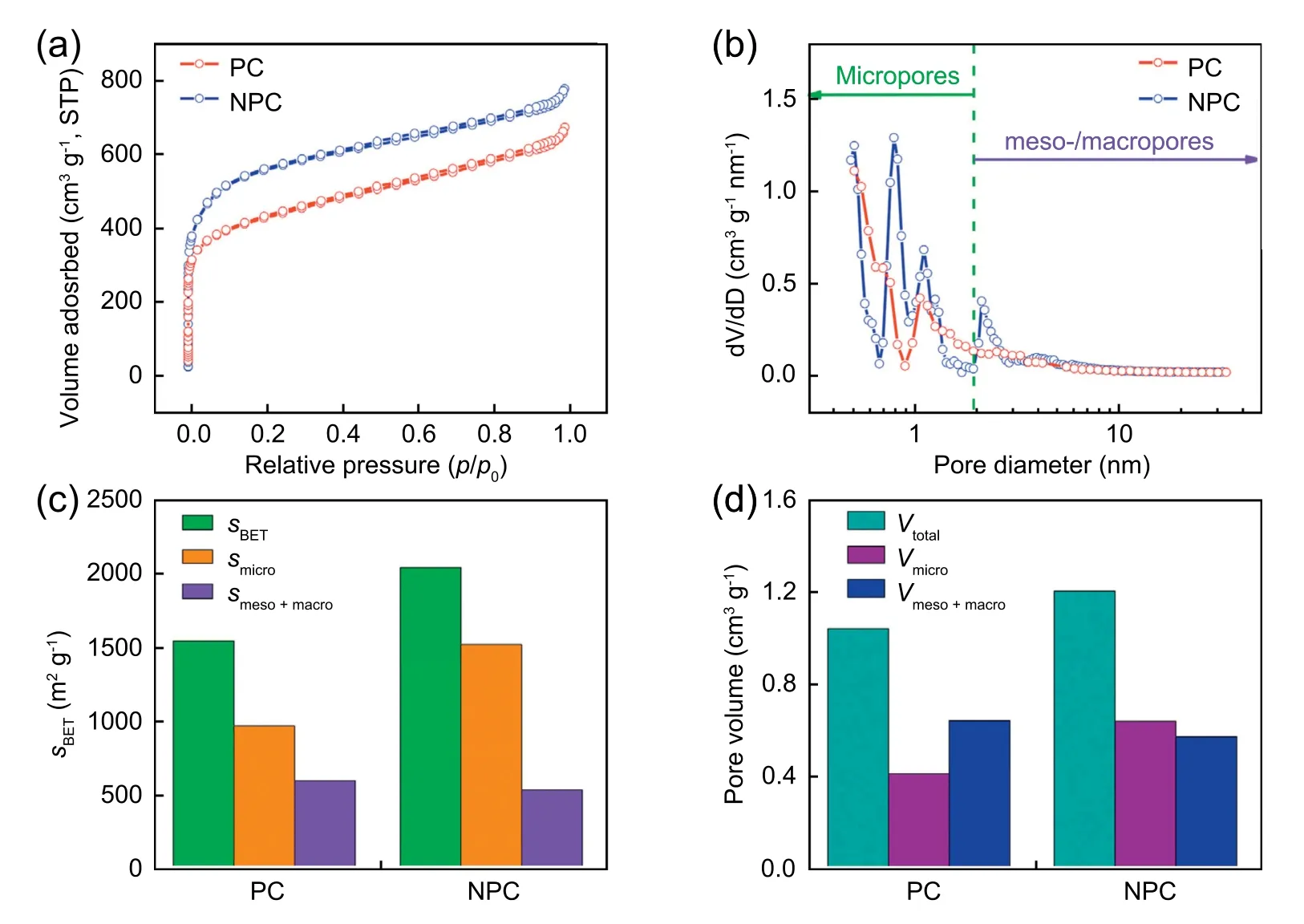
Fig.3.(a) N2 adsorption-desorption isotherms and (b) pore size distributions plots of PC and NPC.Comparison of (c) specific surface area of micropores and meso/macropores and (d) the total pore volume and the micropores pore volume between PC and NPC.
In order to appraise the electrochemical properties of the carbon materials,the three-electrode test was firstly carried out in 6 mol L-1KOH electrolyte,and the cor111responding results are displayed in Fig.4.When tested under the same conditions,NPC exhibits a larger integral area and a longer discharge time,implying better electrochemical performance than PC sample (Fig.4a and b).The inherent reason can be ascribed to the more abundant micropores and additional N doping in NPC sample.Fig.4c and d exhibit the CV curves at 10-200 mV s-1and GCD curves at 0.5-20 A g-1of NPC.Specially,the CV curves remain a nearly rectangle-like shape within the scanning rate (10-100 mV s-1),and the profiles deform slightly at a higher scanning rate of 200 mV s-1,implying ideal capacitance performance and good rate capability [52] Moreover,all the GCD curves in Fig.4d displays symmetrical triangle-like shape even at a larger current density of 20 A g-1,also demonstrating the superior electrochemical performance [70].Similarly,the CV curves and GCD curves for PC are listed in Fig.S9 in supporting information.The specific capacitance calculated by GCD methods is displayed in Fig.4f.In detail,NPC displays a high gravimetric specific capacitance of 361 F g-1at 0.5 A g-1.Furthermore,when tested at a higher current density of 20 A g-1,the cor111responding value is 240 F g-1with a capacitance retention of 66.5%.In comparison,PC sample exhibits 122 F g-1at a large current density of 20 A g-1,59.5% capacitance retention of 205 F g-1at 0.5 A g-1.Specially,we have calculated the volumetric capacitance and areal capacitance,and the cor111responding data is displayed in Fig.S10.Based on the above results,NPC sample is superior to PC sample in terms of specific capacitance and rate performance,which is mainly attributed to the produced well-developed pore structure,larger specific surface area,and additional nitrogen doping.Considering that the content of N heteroatom in NPC is only 1.03 wt%,we believe that the increase of specific area and well-developed pore structure caused by urea decomposition is the main reason that improve the performance of NPC.Correspondingly,we have compared the performance of the prepared carbon materials with those in the literatures (Table S2),and the electrochemical properties of NPC are superior to many porous carbons reported previously.
EIS measurement was conducted to evaluate the electron and ion transportation,and the related results are displayed in Fig.4e.Evidently,the Nyquist plot consists of three sections[71].In general,the relatively small diameters of semicircle in the high frequency range imply low charge transfer resistance.In the medium frequency area,the sloping part of the curves(about 45°) can be considered as the Warburg impedance,reflecting the frequency dependence of electrolyte ion diffusion.Moreover,the vertical line in the low-frequency part reflects the ideal capacitive properties of EDLCs [52].In addition,ESR values can be reflected by the intersection of the curve and real axis,and they are consisted of contact resistance,inherent resistance of the electrode material,and electrolyte resistance[63].ESR values for NPC and PC are 0.76 Ω and 0.97 Ω,respectively,and the relatively smaller ESR value for NPC imply higher electric conductivity of the cor111responding electrode with better properties in supercapacitors.
Fig.5a displays curves that reflect the relationship between the total electrochemical capacitance(Cm)and the square root of the half-cycle time(t1/2).As can be seen from the formula(5),the specific capacitance Cmis comprised of a rate-independent part k1and a diffusion-limited part k2t1/2(t is the discharge time).In detail,k1is mainly ascribed to EDLC(CE),while k2t1/2is related to pseudocapacitance(CP)and determined by the current density[72,73].The formula is listed as follows:

In general,from the intersection point of the fitting line and vertical axis,we can calculate the EDLC contribution is 256.3 F g-1for NPC,and the heteroatoms thus generate at least 104.7 g-1at 0.5 A g-1.While for the PC sample,the values of CEand CPare 135.2 F g-1and 69.9 F g-1,respectively.The detailed results are displayed in Fig.5b.The above results demonstrate that the addition of urea can promote the enhancement of the EDLC capacitance and pseudocapacitance,which can be attributed to the change of pore structure,heteroatom doping and and surface wettability.

Fig.4.The electrochemical properties of carbon samples in 6 mol L-1 KOH electrolyte in a three-electrode system.(a)CV curves at 100 mV s-1.(b)GCD curves at 1 A g-1.(c)CV curves of NPC tested at different scan rates.(d)GCD curves at various current densities.(e)Nyquist plots(inset implies the magnified curves in high-frequency region).(f) Rate performance of PC and NPC,inset implies the capacitance calculated at 0.5 A g-1 and rate capability at 20 A g-1.
The good electrochemical properties of NPC in the threeelectrode system spurred us to evaluate its actual performance in symmetric capacitor.The capacitor was firstly assembled with 6 mol L-1KOH electrolyte and tested in a voltage window of 0-1 V.The near-ideal rectangular shape of CV curves in Fig.6a demonstrates a typical EDLCs behavior.Further increase the scan rate to 200 mV s-1,the CV profiles can still maintain quasi-rectangular shape well with no obvious deformation,suggesting its excellent rate performance.Similarly,GCD tests at 0.5-20 A g-1are displayed in Fig.6b.As we can see,all the GCD curves present approximately equilateral triangle shape with a negligible distortion even at 20 A g-1,also indicating the good electrochemical properties.Specially,we have calculated the gravimetric specific capacitance of NPC and the results are presented in Fig.6c.NPC electrode delivers a gravimetric capacitance of 232 F g-1at 0.5 A g-1,and the capacitance can maintain 164 F g-1even at 20 A g-1(70.7% capacitance retention).In practical applications,cycle stability is also a significant index to appraise the final performance of devices.Fig.6d displays the long-term charge/discharge test at 10 A g-1.In detail,after 5,000 cycles,the capacitance loss is only 5.8%,and a high capacitance retention of 91.4% can be maintained even after 10,000 cycles,demonstrating the superior cycling stability of the capacitor device.
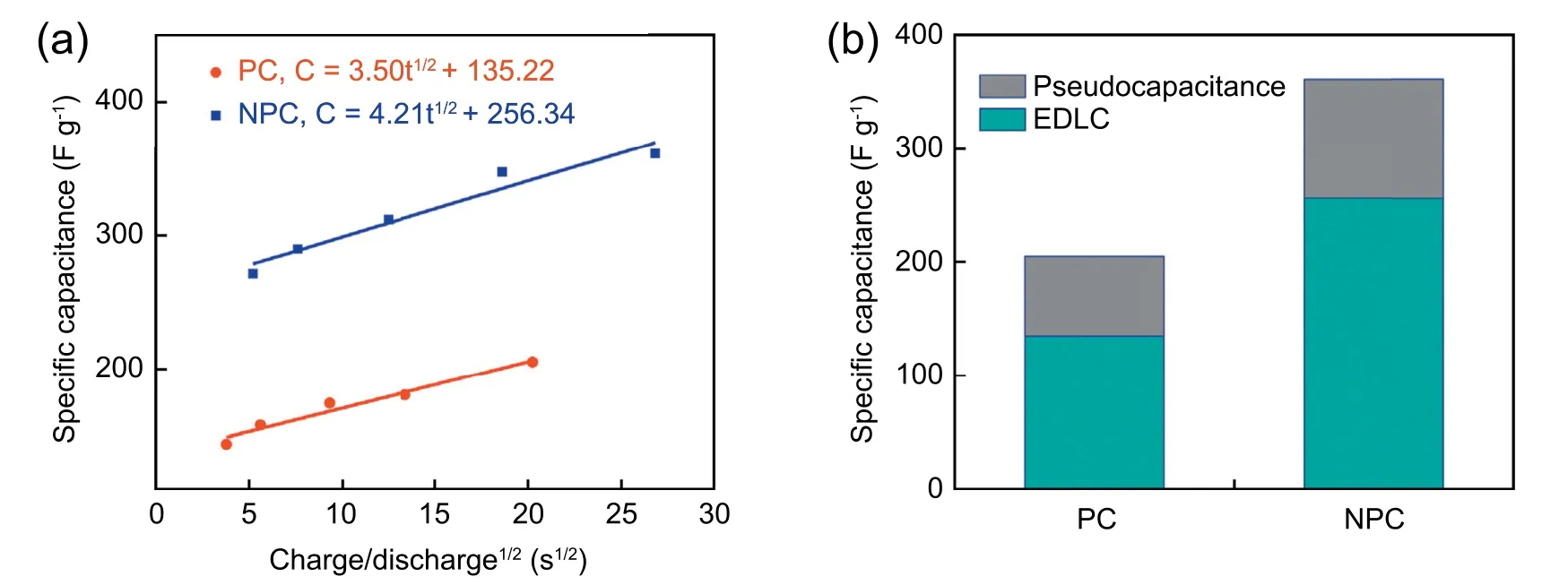
Fig.5.(a)The specific capacitances of PC and NPC versus the square root of the half-cycle time.(b)The contribution value from EDLC and pseudocapacitance at 0.5 A g-1.
In order to enlarge the voltage range and further enhance the energy density,NPC sample was assembled into symmetric capacitor device with neutral electrolyte of 1 mol L-1Na2SO4.Due to the low content of H+and OH-in neutral solution,the overpotential for gas evolution reaction is high,thus the working voltage can be increased.As shown in Fig.7a and b,CV curves tested at various scan rates remain approximately rectangular shape in the voltage range of 0-1.6 V.Moreover,GCD profiles exhibit symmetrical triangular even at a larger current density of 20 A g-1.The above results indicate ideal electrochemical behaviors.Additionally,the gravimetric specific capacitance calculated from GCD tests is depicted in Fig.7c.In detail,the capacitance of electrode is 192 F g-1at 0.5 A g-1.When tested at a higher current density(20 A g-1),a capacitance of 120 F g-1is still retained with good rate capability of 62.5%.Long-term cyclic performance was also estimated through the GCD tests at 10 A g-1,and the cor111responding data are displayed in Fig.7d.After 5,000 cycles,the capacitance loss is only 7.7%.Moreover,a relatively high capacitance retention of 88.1% can be maintained even after 10,000 cycles,reflecting excellent cycle stability.

Fig.6.Electrochemical behaviors of NPC in 6 mol L-1 KOH in a two-electrode system.(a) CV curves tested at 10-200 mV s-1.(b) GCD curves tested at 0.5-20 A g-1.(c)The specific capacitance calculated by GCD methods.(d)The cycle stability test at 10 A g-1,inset indicates the charge/discharge curves of the 1st and 10,000 th cycle.

Fig.7.Electrochemical performance of NPC in 1 mol L-1 Na2SO4 electrolyte in a two-electrode system.(a)CV curves at 10-200 mV s-1.(b)GCD curves at 0.5-20 A g-1.(c) Rate performance.(d) Cycling stability test at 10 A g-1.
As mentioned before,EIS is an effective method to investigate the electron/ion transport process in electrode.Fig.8 implies the Nyquist plot of the symmetric cell and the cor111responding equivalent circuit.The nearly vertical straight line in the low frequency region indicates good EDLC behavior(Cdl).The Warburg impedance (W) reflects the ions diffusion behavior and corresponds to the 45°slope line.In the high frequency,the intercept at the Z’ axis corresponds to solution resistance (Rs),while Rct represents the charge transfer resistance.In detail,the estimated value of Rs and Rct in KOH electrolytes are 0.82 Ω and 0.29 Ω respectively,the estimated value of Rs and Rct in Na2SO4electrolytes are 1.24 Ω and 4.6 Ω respectively.
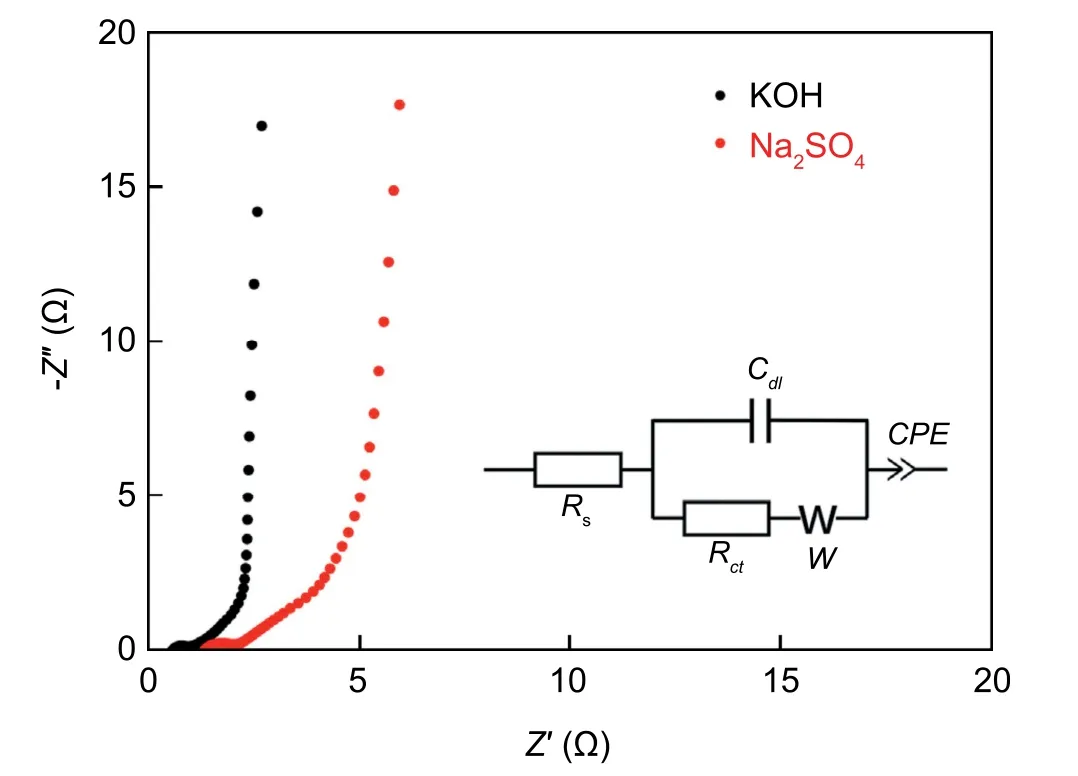
Fig.8.Nyquist plots based on symmetric supercapacitors (inset implies the equivalent circuit).
Ragone plots of NPC based symmetric capacitors tested in KOH electrolyte and Na2SO4electrolyte are depicted in Fig.9a.In KOH electrolyte,the device displays a maximum gravimetric energy density of 8.1 Wh kg-1at a power density of 125.2 W kg-1.Furthermore,the energy density can retain 5.7 Wh kg-1even at a higher power density of 4996.1 W kg-1.Comparatively,since a higher working voltage of 1.6 V can be achieved in Na2SO4electrolyte,the cor111responding capacitor device exhibits larger energy/power density than that of KOH electrolyte according to the equation(E=0.5CcellV2).In detail,the maximum energy density can reach at 17.1 Wh kg-1at a power density of 207.9 W kg-1.More importantly,the energy density can retain 10.7 Wh kg-1at a higher power density of 8195.7 W kg-1.This performance is comparable to or even higher than those of other recently reported carbon-based supercapacitors [74-78],such as nitrogen-doped porous carbon nanosheets (17.3 Wh kg-1at 450 W kg-1)[74],biomass-activated porous carbons(13.5 Wh kg-1at 360.1 W kg-1) [76],sorghum stalk derived porous carbon(9.8 Wh kg-1at 225 W kg-1)[75],and porous carbon prepared from rape pollen (10.5 Wh kg-1at 100.4 W kg-1)[77].Similarly,we have also calculated the energy density and power density based on volume.The device assembled in Na2SO4electrolyte also exhibit larger volumetric energy density and power density than that of KOH electrolyte,and the detailed results are displayed in Fig.9b.Therefore,the above analyses further demonstrate that the adoption of neutral electrolyte with enlarged voltage window can further improve the energy density and power density.

Fig.9.Ragone plots of symmetric capacitor calculated based on mass (a) and volume (b) in two types of electrolytes.
4.Conclusions
In summary,a facile and green method was developed to prepare N/O co-doped porous carbon material in a large scale.No any toxic organic reagents or corrosive acid are involved in the whole synthesis process,and this approach only requires one-step carbonization process.Compared with the traditional carbonization-activation strategy via two-step high-temperature process,it will greatly save energy consumption.Moreover,the effects of added urea on the pore structure and surface functional groups of carbon materials are also analyzed in detail,which is the main reason for the difference of electrochemical properties between the two samples.Besides,when tested in 6.0 mol L-1KOH electrolyte in a three-electrode system,the prepared porous carbon displays a specific capacitance of 361 F g-1at 0.5 A g-1along with superior rate capability (240 F g-1at 20 A g-1,66.5%capacitance retention).More importantly,the symmetric device in Na2SO4aqueous electrolytes can achieve a high energy density of 17.1 Wh kg-1at the power density of 207.9 W kg-1.Therefore,the present study develops a simple and environment-friendly method to prepare porous carbon material for supercapacitors,and opens up a way for the largescale production of porous carbon in the future.
Conflict of interest
The authors declare no conflict of interests.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (No.51303170),and the National Science Centre,Poland within BEETHOVEN UMO-2016/23/G/ST5/04200.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gee.2020.12.004.
 Green Energy & Environment2022年4期
Green Energy & Environment2022年4期
- Green Energy & Environment的其它文章
- Multivariate MOF for optimizing atmospheric water harvesting
- Lignin-based carbon fibers: Formation,modification and potential applications
- Charactering and optimizing cathode electrolytes interface for advanced rechargeable batteries: Promises and challenges
- Metal-organic frameworks-derived metal phosphides forelectrochemistry application
- Surface-mediated iron on porous cobalt oxide with high energy state for efficient water oxidation electrocatalysis
- Oxygen-deficient SnO2 nanoparticles with ultrathin carbon shell for efficient electrocatalytic N2 reduction
