Ni2P/MoS2 interfacial structures loading on N-doped carbon matrix for highly efficient hydrogen evolution
Yuelong Xu,Ran Wang,Zhan Liu,Lili Gao,Tifeng Jiaoa,,,Zhenfa Liu,
a State Key Laboratory of Metastable Materials Science and Technology,Yanshan University,438West Hebei Street,Qinhuangdao,066004,China
b Hebei Key Laboratory of Applied Chemistry,School of Environmental and Chemical Engineering,Yanshan University,438West Hebei Street,Qinhuangdao,066004,China
c Hebei Engineering Research Center for Water Saving in Industry,Shijiazhuang,050081,China
d Institute of Energy Resources,Hebei Academy of Sciences,Shijiazhuang,050081,China
Abstract Electrochemical catalysts for the hydrogen evolution reaction(HER)have attracted increasing attentions.Noble metal-free cocatalysts play a vital role in HER applications.Herein,a novel strategy to prepare a Ni2P/MoS2 cocatalyst through a simple hydrothermal-phosphorization method was reported,and the prepared cocatalyst was then loaded on an N-doped carbon substrate with excellent conductive performance.The large surface area of the carbon substrate provided many active sites,and the interface between Ni2P and MoS2 improved the catalytic performance for the HER.Compared with pure Ni2P catalyst and MoS2 catalyst,the prepared Ni2P/MoS2 cocatalyst exhibited enhanced catalytic performance.In addition,the results indicate that the prepared cocatalyst has a wide pH range and low onset potential values of 280,350 and 40 mV in acidic,phosphate-buffered saline and alkaline solutions,respectively,and the cor111responding Tafel slopes are 75,121 and 95 mV dec-1,respectively.Density functional theory(DFT)was adopted to calculate the hydrogen adsorption free energy(ΔGH*).The results showed that the interface between Ni2P and MoS2 reduced ΔGH*,which was beneficial to the adsorption of hydrogen.Present preparation of cocatalysts with unique interfaces provides a new strategy for improving the catalytic performance of HER.
Keywords: MoS2;Ni2P;Hydrogen evolution;Electrochemical performance;Density functional theory
1.Introduction
Because of the growing demand for energy,hydrogen production has evolved as a fossil fuel substitute due to its high heat and lack of environmentally concerning exhaust gases [1-5].At present,as the application of HER,electrochemical catalysis and photoinitiated water splitting have opened up new avenues to obtain clean hydrogen [6-8].Traditional electrochemical catalytic methods require noble metal catalysts with high catalytic performance,which are precious and rare.In the development of electrochemical catalysts for the HER,low-cost and earth-abundant catalysts with high catalytic efficiency have emerged to promote hydrogen evolution under water splitting conditions.Transition metal nitrides [9],phosphides [10],carbides [11] and dichalcogenides [12] have been considered promising replacements for traditional platinum-like catalysts,which exhibited a low free energy of H*(ΔGH*) and excellent conductivity.
The HER mechanism in an acidic solution can be summarized in two steps: the Volmer reactionand the Heyrovsky reaction[13].The adsorption/desorption of H*on the catalytic surface determines the process of hydrogen evolution.Therefore,the lower the ΔGH*is,the higher the catalytic efficiency.Traditional noble catalysts for electrochemical water splitting with a low ΔGH*have been widely reported [14-16].The Pt-like d-band states can facilitate the adsorption/desorption of H*to reduce the ΔGH*.To date,heteroatoms (such as P,S and N) also exhibit a similar activity for the adsorption/desorption of H*[17-19].For example,heteroatoms can induce a change in charge at transition metal atoms on the catalyst surface to form Pt-like dband states.Xia Long et al.reported a novel catalyst of ironnickel sulfide with outstanding activity and stability in an acidic solution [20].The overpotential (OP) was 105 mV at 10 mA cm-2with a Tafel slope (Ts) of 40 mV dec-1.They investigated the catalytic mechanism with DFT calculations,which indicated that the S atom induced a change in charge of the Fe and Ni atoms on the catalyst surface to reduce the ΔGH*.The Pt-like d-band states contributed to the lower energy barrier for H+adsorption and facilitated the HER.
Heretofore,P has been widely studied due to its lone pair electrons[21-23].These paired electrons in the 3p and hollow 3d orbitals can affect H*adsorption,thus benefiting the HER[24-26].Recently,an increasing number of transition metal phosphides have been reported,such as Fe2P,CuxP,CoxP and NixP [22-26].The traditional and widespread preparation method for phosphides is through high-temperature phosphorization with NaH2PO2or NH4H2PO2in a N2atmosphere[27].However,phosphides easily aggregate in the formation process,which decreases the activity of catalysts for the HER.To avoid this problem,many studies about different morphologies have been reported: Ni2P microspheres,Ni2P nanoparticles and porous carbon matrix loaded with Ni2P.Dan Ma et al.investigated Ni2P using carbon-based substrates for HER activity [28].N-doped reduced graphene oxide (N-RGO) was adopted as the substrate for Ni2P nanoparticles and the above construction showed enhanced HER performance.The electron density of Ni was modulated by P and the doped N,and this modulation was beneficial for H*adsorption and a low ΔGH*.The N-RGO substrates alleviated the aggregation of Ni2P and provided a large surface area for active site exposure.
Currently,MoS2has been the most promising candidate for HER catalysts with a similar ΔGH*of H*adsorption to Pt[29,30].The d orbitals of Mo are easily induced by the s and p orbitals of adjacent heteroatoms to expand and form Pt-like d orbitals,thus improving HER activity.However,because of poor conductivity and few active sites,pure MoS2exhibits undesirable catalytic performance.To solve this issue,many efforts have been made to perfect a nanostructure or load other heteroatoms,such as MoS2with a 2D-layered structure,amorphous MoS2and heteroatom-doped MoS2[31-33],which shows excellent HER activity in acidic solutions but demonstrates poor activity in alkaline or neutral solutions.Therefore,developing catalyst with outstanding catalytic performance in alkaline or neutral solutions is a research hotspot.The doping of single Ni atoms can enhance the catalytic performance for the HER in an alkaline or neutral solution.Qi Wang et al.reported a novel catalyst of MoS2decorated with single atoms of Ni,and this catalyst exhibited a low overpotential and Ts in solutions with a wide pH range[34].The single Ni atoms were introduced into the MoS2S-edge and H-sites of the basal plane,which reduced the ΔGH*of H*adsorption and extended the pH range that can catalyze HER.Because of its excellent conductivity and plentiful defects,RGO has been widely reported as a substrate to enhance the catalytic performance of MoS2.Lin et al.have prepared RGO loaded with Ni-doped MoS2composite [35].They found that the doped Ni atoms could facilitate the formation of H*and accelerate the adsorption and desorption of H*on the catalyst surface.In principle,the doping of Ni atoms into MoS2could increase the HER activity at different pH values.
Recently,heterogeneous interfaces between multicomponent catalysts have been proposed [35,36].Because of the synergistic effect between the different components and heterogeneous interfaces,the charge distribution is changed,and active sites are formed,which improves catalytic performance.In this work,a novel Ni2P/MoS2cocatalyst was prepared by using porous N-doped carbon as the substrate (Ni2P/MoS2-CC).The synergistic effect between Ni2P and MoS2was investigated through theoretical calculations and experimental verification.Raman and XPS spectra proved the presence of synergistic effect between Ni2P and MoS2.The results showed that the cocatalysts had an outstanding catalytic performance for the HER not only in acidic solutions but also in alkaline solutions.The onset potential (OP) values were 280,350 and 40 mV in acidic,phosphate-buffered saline and alkaline solutions,respectively.
2.Materials and methods
2.1.Materials
Details about the reagents used in this work are shown in the Supporting Information (SI).
2.2.Preparation of the Ni2P/MoS2-CC catalysts
The detailed preparation route is presented in Scheme 1.Typically,pectin (1.5 g),nickel nitrate (0.87 g,3 mmol),ammonium hypophosphite (1.0 g,12 mmol) and melamine(2.5 g,20 mmol)were placed into 70 mL of deionized water.Then,the solution was placed into a 150 mL hydrothermal reactor.The temperature was set to 150°C and kept for 12 h.The resulting material was dried at 105°C,and then it was carbonized under a N2atmosphere at 900°C to obtain Ni2Ploaded N-doped carbon substrates.The aforementioned Ni2Ploaded N-doped carbon substrates (0.1 g) and ammonium tetrathiomolybdate (0.01 g) were added into a 25 mL hydrothermal reactor with 15 mL deionized water,which was then kept at 150°C for 20 h.The obtained materials were named Ni2P/MoS2-CC catalysts.The CC catalysts,Ni2P-CC catalysts and MoS2-CC catalysts were prepared using the same method without the addition of nickel nitrate,ammonium hypophosphite and ammonium tetrathiomolybdate,respectively.

Scheme 1.Schematic formation process of N-doped porous carbon matrix with Ni2P/MoS2 supported.
2.3.Density functional theory (DFT) calculation
The DFT calculation details,structural details and computational methods are all described in the SI.
2.4.HER electrochemical measurements
The details about the preparation of the working electrode are presented in the SI.The used electrolytes were 0.5 mol L-1H2SO4,1.0 mol L-1PBS and 1.0 mol L-1KOH.The voltages were referenced to a reversible hydrogen electrode (RHE),E (vs.RHE)=E (vs.SCE)+0.224 V.The cyclic voltammetry (CV) curves were obtained from a potential range of 0.0-0.1 V(vs.RHE)at scan rates of 40,60,80,100,and 140 mV s-1.The electrochemical impedance spectroscopy (EIS) performance was tested from a frequency of 0.01-105Hz with potentials of 100,125,135,140 and 150 mV and amplitude of 5 mV.A modified Randles equivalent circuit was adopted to fit the Nyquist plots and obtain the electrolyte resistance (Rs) and charge transfer resistance (Rct).The detailed methods for the above tests are presented in the SI.
2.5.Characterization
The morphologies and microstructures of the as-prepared materials were observed by scanning electron microscopy(SEM),high resolution transmission electron microscopy(HRTEM) and a surface-area analyzer.The crystal structures of the prepared composites were investigated by X-ray diffractometry (XRD) and Raman spectroscopy.The surface components were obtained from X-ray photoelectron spectroscopy (XPS).The detailed methods for the above characterization procedures are presented in the SI.
3.Results and discussion
In order to investigate the morphology of Ni2P/MoS2-CC,scanning electron microscopy (SEM) images were obtained,and the results are presented in Fig.1.It can be clearly seen that the Ni2P/MoS2nanoparticles were uniformly dispersed on the N-doped carbon substrate surface.Compared with Ni2PCC(Fig.1a) and MoS2-CC(Fig.1b),Ni2P/MoS2-CC(Fig.1c and 1d) exhibited more scalloped edges to provide more loading sited.As shown in Fig.1c and 1d,a 3D-interconnected structure could be observed,which provided plentiful defects for active sites.The flower-like structure could reduce the agglomeration of MoS2,which improved catalytic performance of the prepared composite.The EDX images (Fig.1e-1k) corresponded to SEM results indicated the presence of C,N,O,Ni,Mo,P and S,which were uniformly distributed on the carbon substrate surface.
As shown in Fig.2a and b,uniformly distributed Ni2P/MoS2nanoparticles were observed on the substrate surface,and the porous structure could be clearly seen.The d spacing 0.22 nm corresponded to the Ni2P (111) crystal lattice,respectively [37].In addition,the interface between Ni2P and MoS2could be clearly seen from Fig.2b,and Ni2P particles were coated with MoS2sheets.This specific structure provided a synergistic effect that improved HER performance.Moreover,the lattice distance of MoS2cannot be observed due to its amorphous structure [38,39].
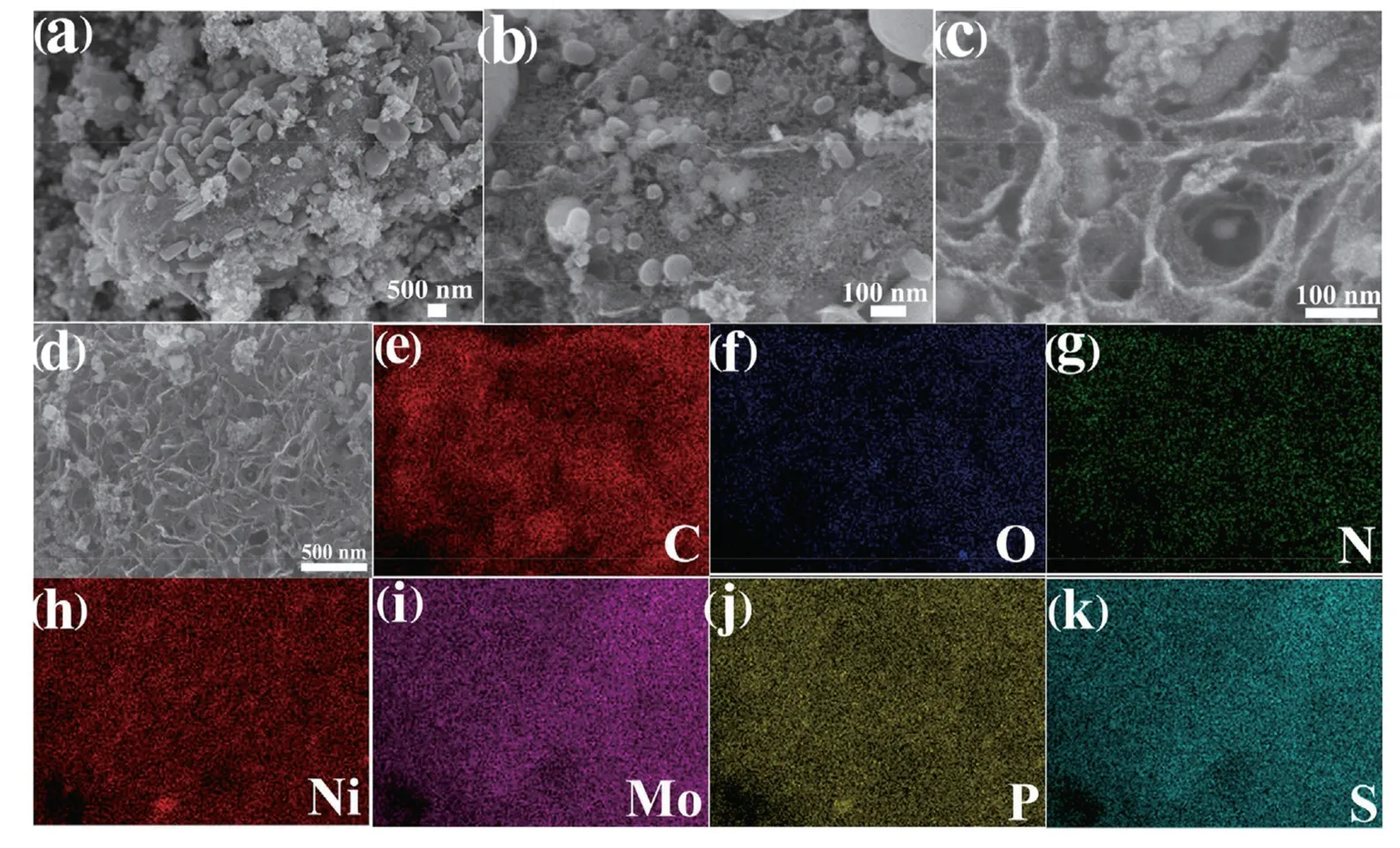
Fig.1.SEM image of (a) Ni2P -CC;(b) MoS2-CC;(c,d) Ni2P/MoS2-CC composite and (e-k) EDX images corresponds to (d).
The crystal structure of the as-prepared catalysts were investigated by X-ray diffractometry (XRD),and the results are shown in Fig.3a.Obviously,the peak at 26°can be attributed to the graphite carbon diffraction (002),indicating that the carbon substrates possessed excellent conductivity for electron transport [37].The peaks of Ni2P could be easily observed at 2θ values of 41°,45°,47°,54°,55°,66°and 75°,which corresponded to(111),(201),(210),(300),(211),(310)and (400) of Ni2P (PDF 01-074-1385).The cor111responding peaks of Ni2P and carbon could be seen in CC,Ni2P-CC,MoS2-CC,Ni2P/MoS2and Ni2P/MoS2-CC composites.In addition,a neglectable shift was observed,indicating that the each other have no effect on the crystal phase.This result was in accordance with the aforementioned analysis.However,the basic peak patterns of MoS2could not be found,which illustrated that the as-prepared MoS2had an amorphous structure.
Raman spectra were obtained to determine the degree of graphitization and the presence of MoS2,and the results were presented in Fig.3b and 3c.From Fig.3b,it can be clearly seen that the characteristic peaks of graphite carbon (G line,1572 cm-1) and amorphous carbon (D line,1344 cm-1),and the value of ID/IGindicates the degree of graphitization.The G band in the Raman spectrum of carbon materials is assigned to the stretching bond of sp2-hybridized carbon.Meanwhile,the D band is attributed to the disorder induced by structural defects and impurities.It could be observed that Ni2P/MoS2-CC exhibited the lowest degree of graphitization (ID/IG=1.24),which indicated the presence of rich structural defects for the electron transport.The peaks of MoS2could be clearly observed at 373 and 400 cm-1,which were assigned toand A1gof the Mo-S phonon mode,respectively [35].This result further suggested that Ni2P/MoS2-CC composite was successfully prepared.A shift in the Raman spectra of Ni2P/MoS2-CC could be clearly observed compared with that of MoS2-CC,and this shift was attributed to the presence of the interface between Ni2P and MoS2.The Ni2P and N-doped carbon structure affected the number of layers along the z orientation and improved the uniform distribution of MoS2[35].All of the above results enhanced catalytic performance for the HER.Fig.3d presented the FTIR spectroscopy of Ni2P/MoS2-CC,Ni2P and MoS2.The characteristic peaks of Ni2P and MoS2could be observed in the Ni2P/MoS2-CC curve,which indicated that Ni2P and MoS2successfully loaded on the N-doped carbon matrix surface.The presence of C-N(1520 cm-1) and C=O (2400 cm-1) were apparent in Ni2P/MoS2-CC,implying the presence of nitrogen and oxygen sources.

Fig.2.(a) TEM image and (b) HRTEM image of the prepared Ni2P/MoS2-CC composite.

Fig.3.(a)XRD patterns of Ni2P/MoS2-CC composite;(b,c)Raman spectra of MoS2-CC and Ni2P/MoS2-CC composite;(d)FTIR spectra of Ni2P,MoS2 and Ni2P/MoS2-CC composite.
X-ray photoelectron spectroscopy (XPS) was used to investigate the electronic states of the elements on the surface of Ni2P/MoS2-CC.As shown in Fig.4,the peaks appeared at 286,532,401,130,855,172 and 233 eV can be attributed to C,O,N,P,Ni,S and Mo elements,respectively [27,35,40].The peaks at 284.7 and 286.0 eV were assigned to C-C or C=C and C-O in Fig.4b,respectively.As shown in Fig.4c,the O 1s spectra were divided into two peaks of 531.0 and 532.4 eV,which were attributed to adsorbed oxygen and hydroxyl oxygen,respectively.In addition,the peaks in Fig.4d were attributed to pyridinic-N (398.7 eV),pyrrolic-N (400.9 eV),oxidized-N (402.8 eV),Ni-N (396.2 eV) and Mo 3p(395.0 eV) [41].The N dopants that were introduced into the carbon substrates and Ni2P/MoS2could serve as electron acceptors to improve the catalytic performance for the HER.Three peaks at 129.5,130.2 and 133.2 eV corresponded to 2p3/2,2p1/2and P-O bonding were observed in the P 2p spectra,respectively.The Ni 2p spectra were divided into six peaks: 853.2 eV (2p3/2),868.4 eV (2p1/2),872.3 eV (Ni-Ox),856.7 eV (Ni-Ox),860.8 eV (satellite) and 879.9 eV(satellite).The presence of Ni-Oxbonding was attributed to the oxidation of surface Ni atoms.A clear negative shift of 0.8 eV could be observed and it was caused by the strong interaction from the MoS2charge density effect.The peaks located at 162.7,161.3 and 169.0 eV corresponded to S 2p1/2,S 2p3/2and SO42-,respectively.The Mo 3d spectra were fitted to Mo4+3d3/2(228.9 eV),Mo4+3d5/2(232.6 eV),Mo6+(232.6 eV)and S 2s(226.1 eV)in Fig.4h.As shown in Fig.4g and 4h,a positive shift to a high binding energy could be observed.It is worth noting that the electronic interaction from the adjacent Ni2P could affect the charge distribution of Mo and S.This result demonstrated that the electronic interaction between Ni2P and MoS2could facilitate the electron transport from MoS2to Ni2P,which enhanced the activity of catalysts for the HER.

Fig.4.XPS spectra of Ni2P/MoS2-CC composite: (a) full-scan spectrum,(b) C 1s,(c) O 1s,(d) N 1s,(e) P 2p peaks,(f) Ni 2p peaks from Ni2P/MoS2-CC composite and Ni2P-CC,(g) S 2p peaks from Ni2P/MoS2-CC and MoS2-CC and (h) Mo 3d peaks from Ni2P/MoS2-CC composite and MoS2-CC.
The catalytic performance for the HER was studied by a three-electrode system in 0.5 mol L-1H2SO4solution,1.0 mol L-1PBS solution and 1.0 mol L-1KOH solution,respectively.The cor111responding result curves of the as-prepared catalysts are presented in Fig.5.The onset potential(Eonset),Cdl and OP values at a current of 10 mA cm-2are listed in Table 1.Additionally,commercial Pt/C was also tested as the reference in this work.The commercial Pt/C exhibited an outstanding performance with a negligible Eonset,and the OP at a current of 10 mA cm-2was 10 mV in the 1.0 mol L-1KOH electrolyte (Fig.5a).As shown in Fig.5a,the pure N-doped carbon substrates showed negligible activity for the HER.Compared with Ni2P-CC,MoS2-CC composite and Ni2P/MoS2,the prepared Ni2P/MoS2-CC composite exhibited a better performance with a lower OP of 170 mV(vs.RHE) at a current of 10 mA cm-2(Table 1),which can be attributed to the synergistic effect between Ni2P and MoS2at their interface and the N-doped carbon matrix.As previously reported,the use of only MoS2revealed a good catalytic performance for the HER [42,43].Clearly,the addition of Ni2P enhanced the activity of catalysts in alkaline and neutral solutions.It is worth noting that the rate-determining step in 1.0 mol L-1KOH was the Volmer reaction(H2O+e-→Hads+OH-).For Ni2P,the Ni atom could play a hydroxide-acceptor role,and the P atom could play a protonacceptor role,which was attributed to the enhancement of the unfilled d-orbital in Niδ+caused by MoS2.The stronger the unfilled d-orbital in Niδ+is,the higher the OH-adsorption ability.As presented in Fig.5b,the as-obtained catalyst also exhibited outstanding catalytic activity for the HER in acidic and neutral solutions with Eonsetvalues of 280 and 310 mV,respectively.The aforementioned results indicated that the asprepared catalyst had a wide pH range for the catalysis of the HER.Compared with previous reports (Table 2),the obtained Ni2P/MoS2-CC composite exhibited a better catalytic performance,indicating that this method opened a novel avenue to prepare catalysts for the HER [44-51].
To investigate the reaction kinetics for the HER process,the Ts was obtained from the equation η=blog(j)+a (where b represents the Ts),and the plots are presented in Fig.5c and 5d.The rate-determining step of the Volmer reaction in 1.0 mol L-1KOH and 0.5 mol L-1H2SO4was the OHproduction(H2O+e-+Cat.→Hads+OH-)and the H+adsorption(+e-+Cat.→Hads+H2O),respectively.As shown in Fig.5c and 5d and Table 1,Ni2P/MoS2-CC possessed lower Ts than the others,which indicated that Ni2P/MoS2-CC had favorable kinetics for H2evolution.The synergistic effect between Ni2P and MoS2at their interface introduced the charge density distribution of the d-orbital,which facilitated OH-adsorption.It should be noted that Ts values of 95 and 75 mV dec-1in alkaline and acidic solutions were lower than 120 mV dec-1,which suggested that the reaction mechanism was the Volmer-Heyrovsky mechanism [17,22].

Table 1 Electrochemical parameters for the HER in 1.0 mol L-1 KOH.
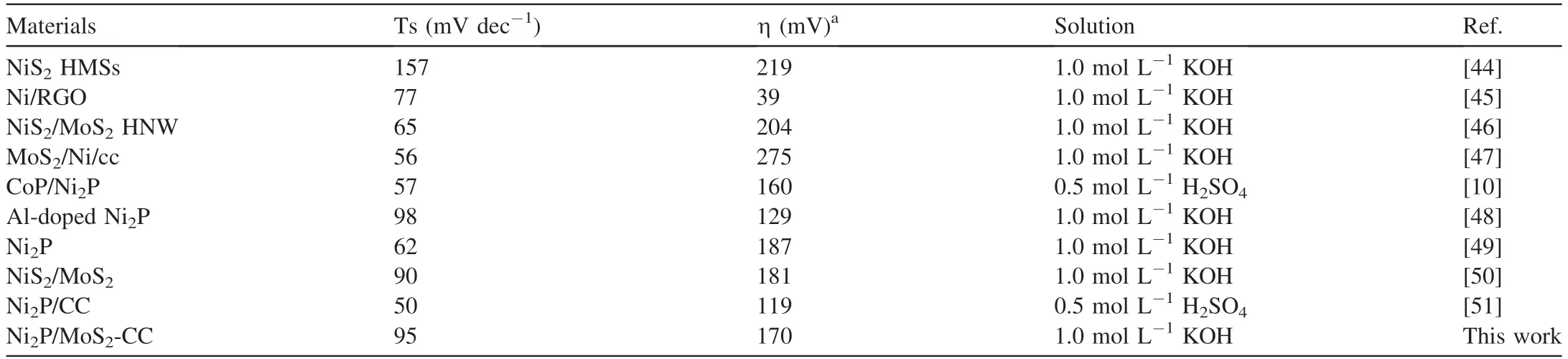
Table 2 Comparison of the prepared catalyst with previous reports.
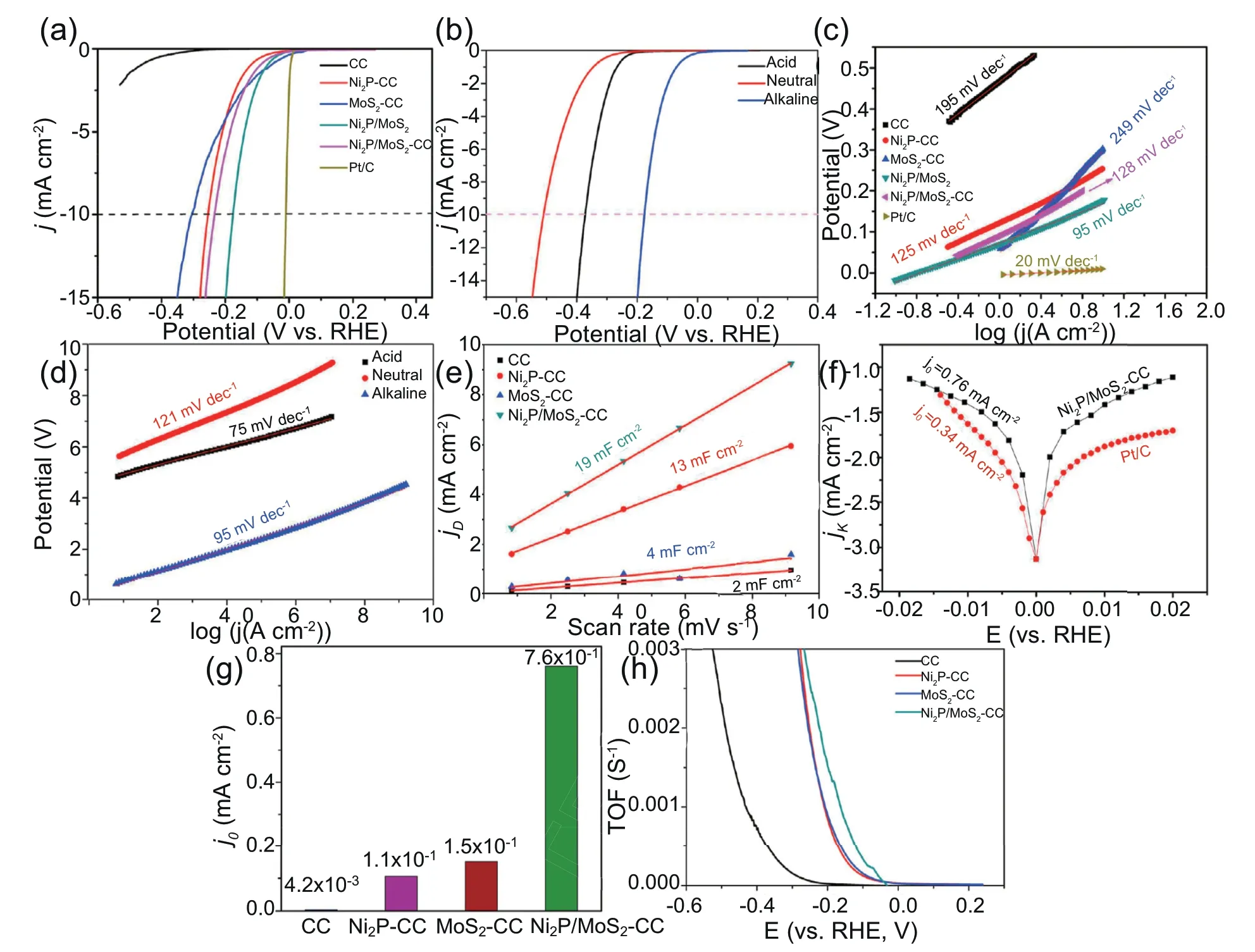
Fig.5.(a) LSV curves of the different catalysts in 1.0 mol L-1 KOH;(b) LSV curves of Ni2P/MoS2-CC composite in different solutions;(c) Tafel plots of the different catalysts in 1.0 mol L-1 KOH;(d)Tafel plots of Ni2P/MoS2-CC composite in different solutions;(e)Capacitive currents as a function of scan rates;(f)Tafel plots for HER/HOR including their Bulter-Volmer fits and the specific exchange current densities from the fitting,(g)Exchange current density of as-prepared samples,(h) TOFs with respect to potential.
In order to investigate the intrinsic catalytic performance of Ni2P/MoS2-CC composite for HER,the kinetic current density for hydrogen evolution/oxidation reactions (HER/HOR) was fitted with simplified Butler-Volmer equation:

where j0represents the exchange current density of intrinsic activity,α represents the transfer coefficient regarding to the symmetry of the HER/HOR.F,R,and T are Faraday's constant(96,485 C mol-1),the universal gas constant(8.314 J mol-1K-1) and the temperature (around 293 K),respectively.η represents the applied overpotential (V).It could be clearly seen from Fig.5f that the fitted curves of Ni2P/MoS2-CC (0.76 mA cm-2) showed a larger exchange current density than Pt/C (0.34 mA cm-2),indicating that Ni2P/MoS2-CC had a faster HER kinetics.These results can be attributed to the H*adsorption/desorption energy on the active sites [52].
To estimate the intrinsic catalytic activity,the electrochemical active surface areas (ECSA) of the catalysts were obtained through the calculation of the double-layer capacitance (Cdl)according to the cyclic voltammetry (CV) curves(shown in SI) at different scan rates,and the results were shown in Fig.5e.As presented in Fig.5e,the prepared Ni2P/MoS2-CC composite had a superior Cdl,up to 19 mF cm-2,which was caused by the synergistic effect between Ni2P and MoS2at their interface and the substrate nanostructure.Additionally,Ni2P/MoS2-CC composite possessed an outstanding specific surface area of 390 m2g-1with a pore size distribution of 4 nm (shown in the SI),which was beneficial for the transport of electrons and Hads.As shown in Fig.5f,5g and 5h,Ni2P/MoS2-CC exhibited a higher TOF values and exchange current density,which also indicates that Ni2P/MoS2-CC composite has higher intrinsic activity[53].
Electrochemical impedance spectroscopy (EIS) was conducted to study the kinetics of the HER,and the cor111responding results are presented in Fig.6.The values of electrochemical impedance are listed in Table 3.As shown in Fig.6a and 6b and Table 3,the catalysts had small solution impedance.It can be observed that Ni2P/MoS2-CC composite exhibited a smaller semicircle in Fig.6a,which suggested a lower Rct value(87.2 Ω) and indicated that this catalyst possessed a higher activity for the HER than the others.The Rct value of Ni2P/MoS2-CC composite(87.2 Ω)was smaller than CC(591.6 Ω),Ni2P-CC (192.1 Ω) and MoS2-CC (490.2 Ω),indicating that the prepared Ni2P/MoS2-CC composite had high interfacial charge transfer efficiency and dynamic velocity.This result was attributed to the porous structure and the synergistic effect between Ni2P and MoS2at their interface,which accelerated the transport of electrons and Hads[54,55].The conductivity of the catalyst was improved by the addition of the N-doped carbon substrate and Ni2P.Compared with the other potentials,the Rct exhibited a clear change.As shown in Fig.6b,the semicircle gradually decreased as the overpotential decreased,indicating that a high overpotential was beneficial for the HER[56,57].The experimental data were well fitted with the Randles equivalent circuit(Fig.6c).The Tafel slope calculated from the electrochemical impedance was 93 mV dec-1,which was similar to the value obtained from the LSV curves.This result reflected outstanding electrode kinetics of the as-prepared catalyst.
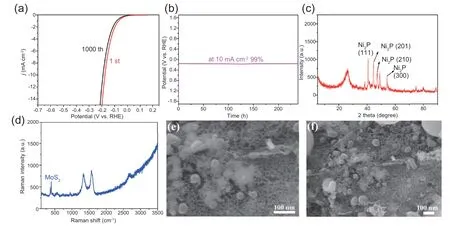
Fig.7.(a)LSV curves before and after 10,000 cycles with Ni2P/MoS2-CC composite;(b)Voltage-time response of Ni2P/MoS2-CC composite;(c)XRD patterns of Ni2P/MoS2-CC composite after the stability test;(d)Raman spectra of Ni2P/MoS2-CC composite after the stability test;(e and f)SEM images of Ni2P/MoS2-CC composite after the stability test.
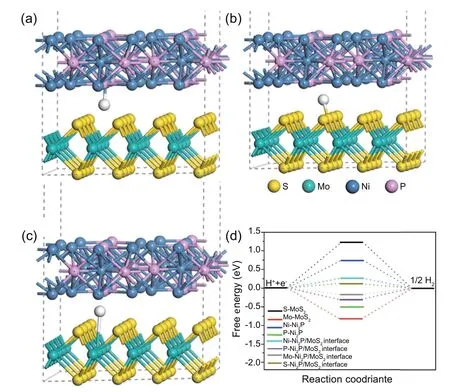
Fig.8.(a)Crystal structures of Ni2P/MoS2 using Ni as the adsorption sites;(b)crystal structures of Ni2P/MoS2 using S as the adsorption sites;(c)crystal structures of Ni2P/MoS2 using Mo as the adsorption sites;(d) HER free energy diagrams of the different catalysts using different adsorption sites.

Table 3 Electrochemical impedance parameters in 1.0 mol L-1 KOH.
To describe the durability of Ni2P/MoS2-CC,the LSV curves before and after 10,000 cycles and the voltage-time response were obtained,and the results are shown in Fig.7.Meanwhile,the structure of Ni2P/MoS2-CC composite after the stability test was also investigated.It can be observed that a negligible change occurred in Fig.7a and 7b,which indicated that the catalyst maintained good stability after a longterm experimental test.The characteristic peaks of Ni2P were observed in the XRD patterns,as shown in Fig.7c,which corresponded to the standard Ni2P.It should be noted that the peaks of MoS2located at 373 and 400 cm-1appeared in the Raman spectra,indicating that MoS2was definitely present on the substrate.There was no change in the structure after analyzing the SEM images,and the porous structure was effectively retained.The aforementioned analysis proved that the catalyst had outstanding durability,which was attributed to the specific substrate structure.
3.1.Density functional theory (DFT) calculation
To shed more light on the synergistic effect between the Ni2P and MoS2at their interface,DFT was adopted to calculate the Gibbs free energy(ΔGH*),and the crystal structures of different catalysts with different H*adsorption sites are shown in Fig.8 and Fig.2S.The perfect activity for the HER is a zero value for ΔGH*,which is caused by the ΔG offset from a proton reduction and ΔG from the removal of adsorbed hydrogen[56,57].As shown in Fig.8d,the H*adsorption sites of Mo and S have high absolute (ΔGH*) values.Clearly,the introduction of Ni2P into the MoS2structure improved HER activity.The absolute ΔGH*value of Ni2P or MoS2was greatly reduced.The minimum value for S at the adsorption sites of Ni2P/MoS2was 0.10 eV,which was much closer to zero,indicating that Ni2P/MoS2has good HER performance.These results proved that the synergistic effect between Ni2P and MoS2at their interface could improve the catalytic performance for the HER,which was also in accordance with the experimental results,demonstrating wide applications in composite materials [58-65].
4.Conclusions
In summary,a Ni2P/MoS2-CC synergistic catalyst was prepared through a novel method.The synergistic interface between Ni2P and MoS2greatly reduced the free energy barrier and improved the catalytic performance for the HER.The addition of N-doped carbon substrates and Ni2P provided excellent conductivity for electron transport,and the porous structure prevented the aggregation of MoS2while also provided more passageways for the desorption of the adsorbed H*intermediate.Compared with Ni2P and MoS2catalysts,the prepared Ni2P/MoS2-CC cocatalyst exhibited obvious improvements with a wide pH range for the HER.In addition,Ni2P/MoS2-CC cocatalyst showed low OP values of 280,350 and 40 mV in acidic,phosphate-buffered saline and alkaline solutions,respectively,and the cor111responding Ts values were 75,121 and 95 mV dec-1,respectively.The DFT results revealed that the interface between Ni2P and MoS2decreased the absolute (ΔGH*) value to accelerate proton/electron transfer.This work has opened a new avenue to prepare a cocatalyst with a specific interfacial structure that facilitates the HER process.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work,there is no professional or other personal interest of any nature or kind in any product,service and/or company that could be construed.
The work described has not been submitted elsewhere for publication,in whole or in part,and all the authors listed have approved the manuscript that is enclosed.
Acknowledgments
We greatly appreciate the financial support of the National Natural Science Foundation of China (No.21872119,22072127),the Natural Science Foundation of Hebei Province(No.B2021203016),the Science and Technology Project of Hebei Education Department (No.ZD2022147),and the Special Project for Local Science and Technology Development Guided by the Central Government of China (No.216Z1301G).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gee.2020.12.008.
 Green Energy & Environment2022年4期
Green Energy & Environment2022年4期
- Green Energy & Environment的其它文章
- Multivariate MOF for optimizing atmospheric water harvesting
- Lignin-based carbon fibers: Formation,modification and potential applications
- Charactering and optimizing cathode electrolytes interface for advanced rechargeable batteries: Promises and challenges
- Metal-organic frameworks-derived metal phosphides forelectrochemistry application
- Surface-mediated iron on porous cobalt oxide with high energy state for efficient water oxidation electrocatalysis
- Oxygen-deficient SnO2 nanoparticles with ultrathin carbon shell for efficient electrocatalytic N2 reduction
