High mass loading NiCo2O4 with shell-nanosheet/core-nanocage hierarchical structure for high-rate solid-state hybrid supercapacitors
Wang Yang,Liqiang Hou,Peng Wang,Yun Li,Rui Li,Bo Jiang,Fan Yang,Yongfeng Li
State Key Laboratory of Heavy Oil Processing,China University of Petroleum (Beijing),Changping,102249,China
Abstract Rational design of advanced structure for transition metal oxides (TMOs) is attractive for achieving high-performance supercapacitors.However,it is hampered by sluggish reaction kinetics,low mass loading,and volume change upon cycling.Herein,hierarchical NiCo2O4 architectures with 2D-nanosheets-shell and 3D-nanocages-core(2D/3D h-NCO)are directly assembled on nickel foam via a facile one-step way.The 2D nanosheets are in-situ generated from the self-evolution of initial NCO nanospheres.This 2D/3D hierarchical structures ensure fast ion/electron transport and maintain the structural integrity to buffer the volume expansion.The 2D/3D h-NCO electrode with an ultrahigh mass loading (30 mg cm-2) achieves a high areal capacity of 4.65 C cm-2 (equivalent to 1.29 mAh cm-2) at a current density of 4 mA cm-2,and retains 3.7 C cm-2 even at 50 mA cm-2.Furthermore,the assembled solid-state hybrid supercapacitor yields a high volumetric energy density of 4.25 mWh cm-3 at a power density of 39.3 mW cm-3,with a high capacity retention of 92.4%after 5000 cycles.Therefore,this work provides a new insight to constuct hierarchical electrodes for energy storage application.
Keywords: Transition-metal oxides;Hierarchical structure;High mass loading;Free-standing;Supercapacitors
1.Introduction
The urgent demands for renewable energy and high-power energy storage devices drive the development of supercapacitors.Particularly,the pseudo-capacitive/battery-type electrode materials,which can greatly enhance the energy densities,have aroused intense attention [1-4].Transition metal oxides (TMOs),such as nickel cobaltite (NiCo2O4),manganese oxide(MnO2)and so on,have exhibited significant predominance because of their abundant resources,easy-synthesis process,low cost,and high theoretical capacitance [5-9].These TMOs electrodes store charges by the reversible Faradaic redox reactions with electrolyte ions[10,11].Usually,the pseudocapacitive behavior is highly dependent on the surface or near-surface properties and structures of the electrode materials[12,13].The underneath parts of active species can hardly participate during the charge storage process,especially for large-size bulk materials,thereby impairing the energy storage capacity.Besides,the intrinsically sluggish electron transport and large volume changes during the fast charge/discharge processes easily lead to inferior rate capability and electrochemical stability [14-16].Therefore,it is highly desirable to rationally design various nanostructures of TMOs electrodes for achieving improved pseudocapacitive performances.
Structure engineering is an effective strategy to construct advanced TMOs electrodes[17-21].For instance,hierarchical heterostructure electrodes with NiO nanosheets anchored on the needle-like NiCo2O4have indeed exhibited enhanced electrochemical property with long-term stability[13].This is because that the hierarchical architectures can synergistically combine the advantages of different dimensional structures[22-25].In general,two-dimensional (2D) nanosheets with high surface-to-bulk ratio can possess shorter diffusion paths of ions and abundant electroactive surface sites [26-28].Three-dimensional (3D) nanocages with interior space can have sufficient ions-accessible areas and buffer the large volume expansion [29,30].Despite the merits of these unique structures,it still remains a great challenge to facilely fabricate desired hierarchical structures.Usually,these proposed synthetic methods require two separate steps,in which the second structure grows on the as-prepared first one via hydrothermal or electrodeposition or chemical bath deposition method[10,31-33].Besides,the involved architecture building blocks often require the introduction of elaborately designed templates,thereby inevitably brings cumbersome removal processes.More importantly,the unmatched structures and weak connection between each others can also increase the internal contact resistance and be detrimental to the stability [23].Therefore,it is significant,though challenging,to in-situ construct hierarchical nanostructure that renders the TMOs electrodes with high energy storage capacity in supercapacitors.
To realize the practical applications of TMOs electrodes,the high mass loading is always a critical requirement for supercapacitors.Unfortunately,the mass loading of active materials is usually less than 3 mg cm-2,which is far away from the need of a practical device due to the small total amount of energy stored[18,34,35].Since a high mass loading level inevitably leads to thick electrode thickness and clogged pores,the electron conduction and ion diffusion thus become sluggish,which results in compromised electrochemical behavior[36,37].Recently,an emerging solution strategy is to directly construct TMOs nanomaterials on the conductive substrates as integrated electrodes for supercapacitors [19,38-40].For example,a multiscale architecture composed of arranged polypyrrole-coated MnO2nanofibers on carbon cloth has simultaneously achieved high mass loading(16 mg cm-2)and excellent rate capability for electrochemical capacitors[41].Compared to the conventional slurry-derived electrodes,the binder-free electrodes can effectively reduce the “dead surface” and avoid using of conductive agent and adhesives[11,36].Ultimately,this configuration can maximize the utilization rate of active materials and promote charge/ion transport ability.Inspired by the proof-of-concepts,it is reasonable to expect that the growth of TMOs materials on nickel foam with desired hierarchical structures can balance the high mass loading and electrochemical performances in supercapacitors,but research in this regard is still a longlasting and formidable task.
Here,we demonstrate a simple one-step annealing method with nitrates as precursor to directly grow highly loaded NiCo2O4with hierarchical structures on Ni foam (NF) backbones.The hierarchical architecture is formed through the 3Dto-2D self-transformation process.The self-formed 2D-nanosheets shell and 3D-nanocages core structures guarantee the as-obtained electrodes with appropriate channel networks and high ion-accessible surface area,which can simultaneously improve its capacity,ion/electron transfer rates,and mechanical stability.When employed as binder-free electrodes for supercapacitors,the 2D/3D h-NCO delivers a high areal capacity of 4.65 C cm-2(equivalent to 1.29 mAh cm-2) at a current density of 4 mA cm-2,and remarkable rate capability(3.7 C cm-2even at 50 mA cm-2),which is among the highest values reported for TMOs materials.Moreover,it exhibits an excellent stability with capacity retention of 113.7% after 5000 cycles.As a proof of concept demonstration,the assembled solid-state hybrid supercapacitor delivers a remarkable volumetric energy density of 4.25 mWh cm-3at a power density of 39.3 mW cm-3,and 92.4% capacity retention after 5000 cycles.The performance is superior to most of the state-of-the-art supercapacitors.
2.Experimental section
2.1.Synthesis of 2D/3D hierarchical NiCo2O4(2D/3D h-NCO) on NF
In a typical synthesis,a piece of NF (about 1 × 1 cm2,1.5 mm of thickness) was immersed in a mixed solution(5 mL) containing cobalt nitrate (140 mmol L-1) and nickel nitrate (70 mmol L-1).After the precursors were dried at 100°C in an oven,the obtained nitrates/NF was further subjected to heating at 310°C with a ramping rate of 10°C min-1in a muffle furnace.With a calcination time of 1 min at 310°C,the initial 3D NCO nanospheres were prepared.The 2D/3D h-NCO materials were obtained with the prolonging of time to 120 min.The as-synthesized samples were collected and washed several times with water and ethanol,respectively,and then dried at 80°C for overnight.The mass loading of 2D/3D h-NCO active materials on NF was about 30 mg cm-2.
For comparison,the active component of 2D/3D h-NCO was scraped off from the nickel foam with strong external mechanical force to demonstrate the role of self-standing electrodes.The mixture of peeled-off material,carbon black and polytetrafluoroethylene(binder)(weight ratio/80:10:10)was grounded well in ethanol to form a slurry.Finally,the slurry was coated onto a nickel foam and dried at 80°C for 12 h in vacuum oven.The mass loading of active material in slurry-coated electrode was about 26.4 mg cm-2.
2.2.Material characterization
X-ray diffraction (XRD) patterns of the as-obtained samples were recorded by a Bruker D8 diffractometer with Cu Kα radiation (λ=0.1540598 nm).Thermogravimetric analysis(TGA) was carried out using a SDTA851E instrument in a temperature range of 30-600°C with the heating rate of 10°C min-1in air atmosphere.The morphologies of samples were measured by the scanning electron microscope (SEM;FEI Quanta200F) and transmission electron microscope(TEM,FEI,Tecnai G2 F20).The Brunauer-Emmett-Teller surface area and pore size distribution of samples were examined by Micromeritics(ASAP 2020)with N2at 77 K.Xray photoelectron spectroscopy(XPS)tests were performed on a Thermo Fisher K-Alpha spectrometer.EPR measurements were carried out in the X-band (9.45 GHz) with 5.00-G modulation amplitude and a magnetic field modulation of 105Hz using a Bruker A300 spectrometer.
2.3.Electrochemical measurements
The electrochemical performances of all electrodes were conducted by using a CHI 760E electrochemical workstation(Chenhua,Shanghai).For the three-electrode setup,the obtained electrodes were used as working electrodes,whereas the Pt plate and Hg/HgO electrodes were employed as counter and reference electrodes,respectively.CV (at different scan rates),GCD(at different current densities),and EIS tests(from 0.01 Hz to 100 kHz with potential amplitude of 10 mV)of the electrodes were all measured in a 6 mol L-1KOH aqueous electrolyte.For the solid-state hybrid supercapacitors,the devices were constructed with the 2D/3D h-NCO as positive electrode and FeOOH foam as negative electrode,and solid PVA/KOH gel as an electrolyte.The prepared process of solid PVA/KOH gel electrolyte was described as follows.The PVA was firstly dissolved in deionized water and then heated up to 80°C for 1 h under vigorous stirring.After the completely dissolved of PVA,the KOH was added in above solution and kept stirring for 1 h to obtain the PVA/KOH gel electrolyte.Finally,the devices were sealed with plastic films.Here,the working area of the ASCs was 1.0 cm2.The detailed calculations for areal capacity,energy density,and power density were shown in Supporting Information.
3.Results and discussion
The preparation process of 2D/3D h-NCO on NF is schematically illustrated in Fig.1a.Briefly,a piece of cleaned NF is placed in a homogenous mixture of cobalt and nickel nitrates aqueous solution.After thoroughly drying,the whole skeletons of NF are coated with nitrates precursors(Fig.S1).For the next calcination treatment,the optimized temperature (310°C) is determined by thermogravimetric analysis curve (Fig.1b).At this temperature,the nitrates precursors can be completely transformed into NiCo2O4species coated on the NF.Initially,3D NiCo2O4nanoparticles are obained,denoted as 3D NCO.With further prolonged the calcination time,different 2D/3D h-NCO heterostructures can be producted.

Fig.1.(a) Schematic illustration for synthesis process of the 2D/3D hierarchical NiCo2O4 architecture on nickel foam.(b) Thermogravimetric analysis of the nirates precursor in air.(c) Comparison of the XRD patterns of samples with different heating treatment time.
To uncover the growth mechanism of the 2D/3D hierarchical structure,the samples are prepared under different calcination time(10 min,60 min and 120 min),named as 2D/3D h-NCO-1,2D/3D h-NCO-2 and 2D/3D h-NCO-3,respectively.For all samples,the obtained X-ray diffraction (XRD)patterns manifest typical diffraction peaks cor111responding to the spinel NiCo2O4phase (JCPDS No.73-1702) (Fig.1c),demonstrating that the further calcination process does not alter the initial phase component.The structural evolutions are monitored by scanning electron microscopy (SEM) in details.In the 3D NCO sample (Fig.2a-c),dense and randomly stacked NCO nanospheres are located on the skeletons of NF.Interestingly,a few NCO nanosheets start to appear onto the surfaces of pristine nanospheres,as marked by red arrows(Fig.2d-f).This phenomenon is like the growth process of seed germination,in which the nanosheets sprout from the inside of nanospheres.With the increase in holding time to 60 min,the whole surfaces are covered with nanosheets,while these nanosheets seem to have not really completed the metamorphosis.As expected,the nanosheets continue to grow with prolonged growth time.Ultimately,when it further extends to 120 min,the SEM images (Fig.2j and k) well manifests pronounced and densely grown vertical nanosheets on the surfaces of nanospheres.By contrast,these formed interconnect nanosheets display much thinner thickness.More interestingly,Fig.2l shows an SEM cross-section of the 2D/3D h-NCO-3 establishing a distinct hierarchical structure on NF with a few micrometers thickness.The cor111responding element mapping images show homogeneous distributions of Ni,Co and O on NF(Fig.S2),again confirming the existence of NCO components.
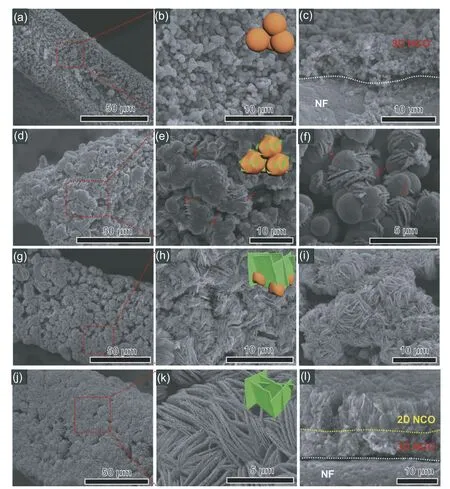
Fig.2.Morphology characterization of the as-prepared samples.SEM images of 3D NCO(a-c),2D/3D h-NCO-1(d-f),2D/3D h-NCO-2(g-i)and 2D/3D h-NCO-3 (j-l).
To achieve more insights into the nanostructure of 2D/3D h-NCO-3 sample,transmission electron microscopy (TEM)analysis is further performed.It is difficult to peel off the coated NCO film from NF,especially separate the 2D and 3D parts.By virtue of external mechanical forces,the dropped NCO powder is further subjected to grinding and ultrasonic processing for following tests.Both 3D nanospheres and 2D nanosheets structures can be clearly observed in the NCO powders (Fig.S3).The TEM image in Fig.3a shows an integrated nanosheet structure even though through grind and ultrasound treatments,indicating a robust property of this structure.This is especially favorable for the electrochemical energy storage.The yellow dotted circle looks like the interfaces between the 2D and 3D structures (inset of Fig.3a).Interestingly,this 2D morphology is similar to a desert plant called tumbleweed (Fig.S4),which has strong ability to absorb water and nutrients from soil.Likewise,this tumbleweed-like 2D structure can be able to facilitate electrons transfer and ions diffusion,which will be discussed later.The nanosheets are actually composed of many interconnected branches with nanorods structure (Fig.3b and c).The highresolution TEM (HR-TEM) image in the selected area displays an interlayer spacing of 0.245 nm,which agrees well with the (311) plan of NCO (Fig.3d and e).As displayed in the selected area electron diffraction (SAED) image (Fig.3f),the numerous concentric diffraction rings are also well matched with the phase of NCO.Besides,the internal layers are indeed consisted with 3D nanospheres,as exhibited in Fig.3g and h.More interestingly,these individual nanospheres exhibit a hollow structure,which is organized by smaller nanoparticles subunits.The lattice fringe spacings in HR-TEM images (Fig.3j and k) and representative SAED pattern(Fig.3l) both verify the component of NCO,which is consistent with XRD results.As for the hollow structure,the growth mechanism may be attributed to the released gas bubbles from the decomposition of nitrates,which forms gassolid interfaces as templates (Fig.S5) [42].Importantly,such hollow structure can provide short ion diffusion paths and plentiful buffer space to release the strain created during charge-discharge processes [30].

Fig.3.Nanostructure characterization of the 2D/3D h-NCO-3 sample.(a-d) TEM images of the 2D NCO parts.Intensity profile plots of the calibration for measuring the interlayer spacing (e) and cor111responding SAED pattern of 2D NCO (f).(g-j) TEM images of the 3D NCO parts.Intensity profile plots of the calibration for measuring the interlayer spacing (k) and cor111responding SAED pattern of 3D NCO (l).
The observations described above establish a 2D/3D NCO hierarchical architecture.The key is the external 3D nanospheres transforming into 2D nanosheets.But what triggers this fascinating self-transformation process? To explore the reasons behind this phenomenon,two contrast tests have been conducted.First,a carbon cloth is adopted as the substrate to prepare NCO materials under the same conditions.However,the obtained NCO presents nanoparticle morphology with much smaller sizes,and the 3D-to-2D transformations have not taken place(Fig.S6).This demonstrate that the framework of NF can affect this process.Furthermore,if the initial NF has been pressed to destroy the porous framework,the transformations do not happen as well (Fig.S7).The as-prepared NCO shows a sintered shape without obvious nanospheres.Recently,a strategy for synthesis of 2D zeolite nanosheets with nanoparticles as seeds has been reported [43].It reveals that suitable size and shape of nanoparticles should be acquired to enable the intergrowth of nanosheets on extended flat surfaces.Therefore,in this work,the formation large size nanospheres induced by the NF frameworks may promote the transitions from 3D to 2D.Indeed,3D-to-2D transformations arise spontaneously from the intrinsic NCO nanospheres.The transition structures have been also observed,in which some cracks appears in the 3D NCO and some 2D nanosheets poke through the surface of 3D NCO (Fig.S8).This may be attributed to the dominant negative surface energy in large size 3D nanosphere,which can reduce the barrier and drive the 3Dto-2D transformations [44].
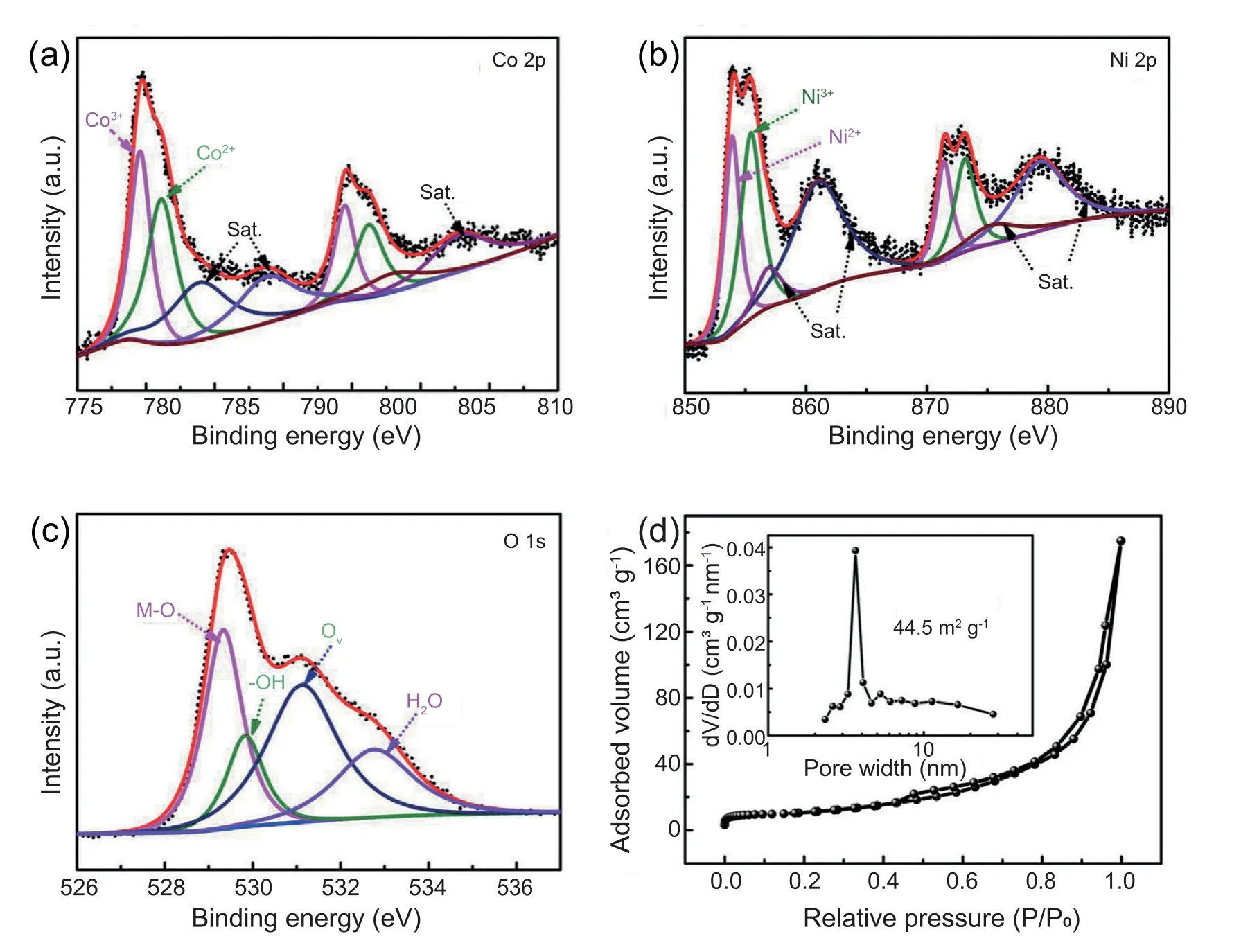
Fig.4.XPS high-resolution spectra of Co 2p(a),Ni 2p(b)and O 1s(c)of 2D/3D h-NCO-3 sample.(d)The N2 adsorption-desorption isotherms(inset:pore size distribution) of 2D/3D h-NCO-3 sample.
X-ray photoelectron spectroscopy (XPS) test is performed to reveal the surface chemical state.The XPS survey spectrum demonstrates the presence of Co,Ni and O in 2D/3D h-NCO-3 sample(Fig.S9).The high-resolution Co 2p spectrum exhibits two spin-orbit doublets at 779.6(Co3+)and 781.1 eV(Co2+),and additional signal of the satellite peaks (Fig.4a) [45].Similarly,in the Ni 2p spectrum(Fig.4b),three obvious spinorbit doublets with binding energies of 853.9,855.5,861.2 eV are observed,which should be related to the Ni2+,Ni3+,and satellite peak,respectively[45].In the O 1s spectrum(Fig.4c),typical metal-oxygen bonds appears at 529.3 eV.These above analyses demonstrate the successful fabrication of NCO specie.Moreover,the peak at 531.1 eV is the characteristic of oxygen vacancy (Ov),which is regared as effective sites to enhance the electrochemical reaction activity,thus leading to a high pseudocapacitance.Besides,the Ovcan also improve the electrical conductivity [46,47].The strong signal peak at g value of 2.001 in the electron paramagnetic resonance (EPR)spectrum also further confirms the formation of Ov(Fig.S10)[48].The causes of Ovmay be attributed to the produced lattice boundaries and defects of NCO.N2adsorption/desorption isotherms are adopted to evaluate the porosity of scraped-off powders (Fig.4d).According to the Brunauer-Emmett-Teller(BET)method,the specific surface area of 2D/3D h-NCO-3 is evaluated to be 45.5 m2g-1,which is higher than that of 3D NCO (41.0 m2g-1) (Fig.S11).The slightly increased porosity can be attributed to the formation of 2D nanosheets.The pore size distribution curve demonstrates a mesoporous feature with mostly centered at around 3-5 nm.Such relatively rich porous nanostructure is attractive for electrolyte permeation and active sites utilization,thus enhancing the pseudocapacitive energy storage performance[49,50].
The electrochemical properties of the resultant electrodes have been evaluated by cyclic voltammetry (CV) and galvanostatic charge/discharge (GCD) in the standard three-electrode system.All the tested electrodes show a pair of redox peaks in CV curves,attributed to the faradaic redox reactions(Fig.S12).The closed curve area of 2D/3D h-NCO-3 is larger than that of other three electrodes (Fig.5a),revealing an enhanced electrochemical performance.Likewise,in the GCD profiles,the 2D/3D h-NCO-3 achieves a more prolonged discharge time than those of 3D NCO,2D/3D h-NCO-1 and 2D/3D h-NCO-2,confirming its enhanced capacity (Fig.5b).By contrast,the capacity contribution of the blank Ni foam can be ignored (Fig.S13).The nonlinear shape with visible voltage plateaus testifies a high reversibility battery-type electrochemical behavior (Fig.5c and Fig.S14) [13,51-53].Specifically,as calculated from GCD curves,the 2D/3D h-NCO-3 delivers the highest areal capacity of 4.65 C cm-2at 4 mA cm-2(equivalent to 1.29 mAh cm-2),which is much higher than those of 3D NCO(2.45 C cm-2),3D/2D h-NCO-1(3.1 C cm-2) and 3D/2D h-NCO-2 (3.55 C cm-2) (Fig.5d).The 2D/3D h-NCO-3 electrode exhibits an excellent rate capability with distinguished capacity retention of 80% as the current density increases from 4 mA cm-2to 50 mA cm-2.It can be clearly found that the areal capacity increases along with the increase of heating time.Likewise,the gravimetric capacities of the electrodes present the same tendency(Fig.S15).Moreover,the variation of the IR drop in GCD curves at 50 mA cm-2suggests that the 2D/3D h-NCO-3 has the smallest internal resistance (Fig.S16),which can guarantee a fast current-voltage response.These above results well manifest that the 3D-to-2D transformations play a pivotal role for the boosted capacities and redox reaction kinetics.This is because that the in-situ generated hierarchical architecture with nanosheets and hollow nanospheres can be able to provide much large ion-accessible surfaces and allow faster ion/electron transportation.More impressively,the capacity and rate capability of 2D/3D h-NCO-3 are substantially better than those of recently reported TMOs-based materials,especially in terms of the high mass loading (Table S1).
The cycling stability of the electrodes is another important factor to be considered when applied in practice,which has been evaluated in Fig.5e.In addition,the cor111responding first five and last five charge-discharge curves are also provided(Fig.S17).For original 3D NCO,the specific capacity drops sharply in the first 500 cycles,and then cycling retention after 1000 cycles is only 74.8%.Interestingly,with the transformations from 3D nanospheres to 2D nanosheets,the formed lamellar structure has greatly enhanced the stability.For instance,the capacity retention of 2D/3D h-NCO-2 is over 95.2% after 2000 cycles.More impressively,the 2D/3D h-NCO-3 electrode exhibits the most superior cycle stability.In the initial stage,the value of the 2D/3D h-NCO-3 increases and reaches 117.2% of initial capacity at 2000th cycle.This can be attributed to the electrochemical activation of active materials.It can still deliver high capacity retention of 113.7%even after 5000 cycles.Such prominent stability should be ascribed to the formation of dense and interconnected 2D nanosheets shells,which can retain structural integrity and effectively mitigate volume expansion as well as internal strain during charge/discharge [17,35].Evidently,there are no obvious morphology and phase changes for the 2D/3D h-NCO-3 electrodes after cycling(Fig.S18).By contrast,the 3D NCO electrodes show a significant structural change with a monolithic block derived from the redox reactions of nanoparticles (Fig.S19).That is to say,the generated 2D/3D hierarchical architecture indeed possesses a robust structural stability for the electrochemical energy storage.
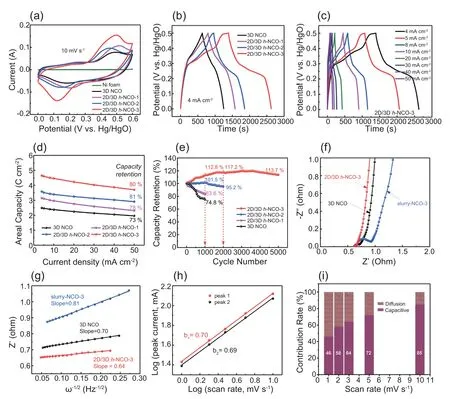
Fig.5.CV curves(a)and GCD profiles(b)of the 2D/3D h-NCO-3,2D/3D h-NCO-2,2D/3D h-NCO-1,and 3D NCO electrodes.(c)The GCD profiles of the 3D/2D h-NCO-3 electrodes at different current densities.Specific capacities as a function of current density(d)and cycling performance(e)for the 2D/3D h-NCO-3,2D/3D h-NCO-2,2D/3D h-NCO-1,and 3D NCO electrodes.Nyquist plots(f)and plots of Z′ versus ω-1/2(g)for the 2D/3D h-NCO-3,slurry-NCO-3,and 3D NCO electrodes.The peak current against the scan rate (h) and the capacitive contribution from capacitive and diffusion-controlled contribution (i) for the 2D/3D h-NCO-3 electrode.
The self-standing feature of electrodes plays a critical role in determining the excellent pseudocapacitive performance as well.For comparison,the scraped-off 2D/3D h-NCO-3 powder is turned into a slurry with the addition of carbon black and binder,and then casted on the NF,named as slurry-NCO-3.As expected,the slurry-NCO-3 presents a much poorer performance (Fig.S20).The slurry-derived electrodes can increase the “dead volume” and reduce the utilization of active materials.To further elucidate the enhanced electrochemical behavior,electrochemical impedance spectroscopy (EIS) tests are carried out.The equivalent circuit for the analyzing of EIS has been provided in the inset of Fig.5f,including the internal resistance (Rs),charge transfer resistance (Rct),Warburg resistance (Zw),limit capacitance (Cps) and double-layer capacitance(CPE).For the three electrodes,the Nyquist plots display an incomplete semicircle in the high-frequency region followed by a straight tail in the low-frequency region,which is related to the Rctand ionic diffusion process,respectively(Fig.5f) [54,55].Evidently,the 2D/3D h-NCO-3 has the smallest Rctand Rs(the intercept on real axis).Furthermore,based on the analysis of Warburg region by replotting Z′as a function of ω-1/2(Fig.5g),the 2D/3D h-NCO-3 electrode shows the lowest slope among three electrodes,indicating the smallest ion diffusion resistivity [37,56].The characteristic frequency f0at the phase angle of -45°also confirms its faster charge/discharge rate (Fig.S21).These above results demonstrate that the 2D/3D h-NCO-3 with hierarchical structures can provide “super highways” for the fast electron transportation and abundant channels to enable the fast ion diffusion kinetics.Besides,this unique structure can boost the exposure of electro-active sites,which has been supported by Trasatti method (see the details in Supporting Information)[57].At the scan rate of 1 mV s-1,the ratio of practical charge storage to theoretical charge is 70% for 2D/3D h-NCO-3,while it is 62% and 49% for 3D NCO and slurry-NCO-3,respectively (Fig.S22),indicating that the 2D/3D hierarchical structure possesses a higher accessible active area.To obtain insight into the energy storage mechanism of 2D/3D h-NCO-3,the capacitive and diffusive contribution are further investigated by the Dunn's method (see the details in Supporting Information) [58].The b-values are confirmed to be 0.70 and 0.69 for the peak 1 and peak 2 (Fig.5h),respectively,revealing the coexistence of diffusion and capacitive-controlled behavior[59,60].Moreover,as the scan rate is increased from 1 to 10 mV s-1(Fig.5i),the capacitive contribution increases from 46%to 85%.Therefore,it verifies that the capacitive contribution occupies the major portion of the whole charge storage at a high scan rate,thus providing superior rate capability.
To evaluate the possibility of 2D/3D h-NCO-3 materials for practical applications,solid-state hybrid supercapacitors(HSCs) are assembled with the 2D/3D h-NCO-3 as positive electrode and FeOOH foam electrodes as negative electrode using a polyvinyl alcohol (PVA)-KOH electrolyte (Fig.6a).The preparation method and detailed characterizations for the FeOOH foam electrodes are described in the Supporting Information (Figs.S23-24).From the CV curves recorded at different voltage windows,the stable operating voltage of HSCs can be extended up to 1.65 V (Fig.6b).With a gradual increase of scan rates from 5 to 100 mV s-1,the shape of CV curves is still preserved,indicating a fast charge/discharge behavior and good rate capability (Fig.6c).Based on the collected GCD curves (Fig.6d),the HSCs exhibit high volumetric capacity (18.55 C cm-3) and areal capacity(3.89 C cm-2) at 10 mA cm-2(Fig.S25).Even at the higher current density of 50 mA cm-2,the volumetric capacity can be retained as high as 15.91 C cm-3with a high capacity retention rate of 85.6%,which further evidences the high capacity and excellent rate behavior.Fig.6e shows the Ragone plots of our HSCs compared with the representatives of recently reported devices.The HSCs deliver a high volumetric energy density of 4.25 mWh cm-3at a power density of 39.3 mW cm-3,and still remain a remarkable value of 3.64 mWh cm-3even at 196.4 mW cm-3,which is comparable to most state-of-the-art supercapacitors (Table S2).Long-term cycling is another critical criterion for devices.Strikingly,the HSCs deliver a robust cycling stability with capacity retention of 92.4%after 5000 charge/discharge cycles(Fig.6f).The increased capacity in the initial 500 cycles may be attributed to the activation process,in which the electrolyte ions will gradually penetrate into the narrow pores of electroactive materials.In addition,a commercial red light-emitting diode (LED) is successfully lighten by two 2D/3D h-NCO-3//FeOOH foam HSCs devices connected in series,indicating the feasibility and potential application of the devices.

Fig.6.(a)The sketch map of the assembled solid-state hybrid supercapacitor(HSCs).(b)CV curves of the 2D/3D h-NCO-3 positive and FeOOH foam negative electrode material at 50 mV s-1.(c)CV curves at various scan rates and(d)GCD profiles at different current densities of the HSCs.(e)The Ragone plots of the HSCs with comparison to previous literatures.(f) Charge-discharge cycling of the HSCs at a constant current density of 10 mA cm-2.
4.Conclusions
In summary,we have demonstrated an in-situ 3D-to-2D self-transition process to prepare hierarchical NiCo2O4architecture with 2D-nanosheets shell and 3D-nanocages core on nickel foam backbones.Benefiting from the merits of different dimensional structures,this resultant hierarchical structure increases the number of electroactive sites for fast Faradaic reaction and ensure facile ion/electron transport in the integrated binder-free electrode even with high mass loading(30 mg cm-2).The as-produced 2D/3D h-NCO delivers a remarkable areal capacity of 9.3 F cm-2(equivalent to 4.65 C cm-2) at a current density of 4 mA cm-2,and a highrate capability (80% capacity retention when the current density increases from 4 to 50 mA cm-2).To the best of our knowledge,such performance outperforms all the state-of-theart TMOs-based electrodes with comparable or less mass loadings.More importantly,this newly-formed 2D nanosheets wrapped on the surfaces can alleviate the structural strain or volume expansion during the charge/discharge processes,leading to a satisfactory cycling stability.Furthermore,the assembled solid-state hybrid supercapacitors(2D/3D h-NCO//FeOOH foam) achieve a high volumetric energy/power density(4.25 mWh cm-3at 39.3 mW cm-3),and a high capacity retention of 92.4% after 5000 cycles.This research opens up new opportunities to design advanced and high loading TMOsbased electrodes for the high-performance energy storage applications.
Conflict of interest
The authors declared that they have no conflicts of interest to this work.
Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos.21908245 and 21776308),Science Foundation of China University of Petroleum,Beijing (No.2462018YJRC009),and China Postdoctoral Science Foundation (No.2018T110187).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gee.2020.11.014.
 Green Energy & Environment2022年4期
Green Energy & Environment2022年4期
- Green Energy & Environment的其它文章
- Multivariate MOF for optimizing atmospheric water harvesting
- Lignin-based carbon fibers: Formation,modification and potential applications
- Charactering and optimizing cathode electrolytes interface for advanced rechargeable batteries: Promises and challenges
- Metal-organic frameworks-derived metal phosphides forelectrochemistry application
- Surface-mediated iron on porous cobalt oxide with high energy state for efficient water oxidation electrocatalysis
- Oxygen-deficient SnO2 nanoparticles with ultrathin carbon shell for efficient electrocatalytic N2 reduction
