Engineering electronic structures of titanium vacancies in Ti1-xO2 nanosheets enables enhanced Li-ion and Na-ion storage
Huiqin Wng,Fenghu Zhng,Jing Xi,Fei Lu,Bo Zhou,Ding Yi,Xi Wng,
a Key Laboratory of Luminescence and Optical Information,Ministry of Education,Department of Physics,School of Science,Beijing Jiaotong University,Beijing,100044,China
b Institute of Molecular Plus,Tianjin University,Tianjin,300072,China
c Chemistry and Chemical Engineering Guangdong Laboratory,Shantou,515031,China
Abstract Up to now,three kinds of ion-storage mechanisms are summarized towards anode materials in lithium/sodium-ion batteries,but they have low capacity and poor cyclic performance.Therefore,it is necessary to develop a new approach to optimize ion storage.Herein,we report an adsorption/desorption storage route through engineering electronic structure of cation-deficient Ti1-xO2 nanosheets.Ti1-xO2 nanosheets indeed exhibit higher capacity (332.1 mA h g-1 vs.137.7 mA h g-1 for LIBs,195.7 mA h g-1 vs.111 mA h g-1 for SIBs),and more stable cyclic performance (296 mA h g-1 vs.99 mA h g-1 for LIBs,178.1 mA h g-1 vs.80.2 mA h g-1 for SIBs after 100 cycles) at 0.1 A g-1 than TiO2 nanosheets.Kinetics analysis and density functional theory (DFT) calculations reveal that electronic structures of vacancy within Ti1-xO2 nanosheets encourage a novel adsorption-desorption storage route.These results highlight the benefits of the engineered electronic structures within electrode material and implement novel ion-storage mechanism towards broad energy storage applications.
Keywords: Titanium vacancy;Titania nanosheet;Electronic structure;Ion storage;Storage mechanism
1.Introduction
In recent years,there is a surging demand for electrochemical energy storage with the rapid development of highpower equipment [1].The growing demands for energy storage are currently met by lithium-ion batteries (LIBs) and sodium-ion batteries(SIBs).As we all know,anodes materials in LIBs/SIBs are classified into three types according to their storage mechanisms: intercalation/de-intercalation (e.g.graphite,TiO2),alloying/de-alloying(e.g.Si,Sn,Sb,Se-based material) and conversion reaction mechanism (e.g.Co3O4,Fe2O3) [2-10].The intercalation/de-intercalation mechanism can be summarized as follows: Li-ion/Na-ion anode materials are intercalated and deintercalated into the layered compounds during the charging and discharging,respectively.For example,the commercialized graphite anode allows a maximum interlayer of one Li-ion cor111responding to six carbon atoms,which means that the theoretical capacity can reach 372 mA h g-1[11].In alkali metals,K+with a large-size can be intercalated in graphite [12-14].Still,Na-graphite compounds have the highest formation energy and are not thermodynamically stable,resulting in lower sodium capacity[15].Therefore,graphite is not suitable for Na+,and it is necessary to find a new type of anode material to realize the possibility of high-efficiency Na+storage.The alloy/de-alloy mechanism can be summarized as follows: M+yLi+/Na++ye-↔LiyM/NayM.For example,Si anode holds a high theoretical capacity of 4200 mA h g-1in LIBs [16],but suffers from a vast volume expansion during the alloying reaction,leading to large capacity fading and poor cyclic performance[17].As for the conversion mechanism,it can be expressed as:MX+2yLi+/Na++2ye-↔M+yLi2X/Na2X,which experiences disadvantages similar to the alloying/dealloying process.The huge volume change during conversion reaction and electrode materials' powdering results in a dramatic capacity fading.Therefore,it is necessary to develop a new route to optimize ion storage performance.
Inspired by the water reservoir,the introduction of vacancies within anode material could be an effective way to adsorb/insert more Li+/Na+and further increase the capacity of LIBs/SIBs.Therefore,we introduced Ti vacancies as storage sites for Li+/Na+and demonstrated a novel Li+/Na+adsorption/desorption storage route through engineering electronic structure of vacancy sites in cation-deficient titania(Ti1-xO2) nanosheets.A top-down exfoliation process fabricated the Ti1-xO2nanosheets [18].Note that the Ti vacancies'size is estimated to be 0.30×0.38 nm[19],which is employed as the Li+/Na+storage reservoir.As tested as the anode materials in LIBs/SIBs,Ti1-xO2nanosheets delivered better cycling stability and rate capability than the stoichiometric TiO2nanosheets.Kinetics analysis results demonstrated two storage mechanisms in the Ti1-xO2nanosheets:the adsorption/desorption mechanism in the Ti vacancies,and the intercalation/deintercalation mechanism between the restacked nanosheets layers.DFT calculation results further clarified that the electronic structures of Ti vacancies guaranteed the novel adsorption-desorption storage route.Thus,the engineered Ti vacancies substantially inspired a new ion-storage mechanism,which also provided a new idea for developing high-efficient Li-ion and Na-ion anode materials.
2.Material and methods
2.1.Synthesis of Ti1-xO2 nanosheets
Ti1-xO2nanosheets were synthesized using a top-down exfoliation process.CsCO3(99%,Alfa Aesar) and TiO2(rutile,99%,Alfa Aesar) (molar ratio of 1:5.3) were ground and calcined at 800°C for 20 h to produce Cs0.7Ti1.825□0.175O4(□: vacancy) (Supporting Information,Fig.S1a).After calcination,Cs0.7Ti1.825□0.175O4exchanges with 1 mol L-1diluted hydrochloric acid (HCl) three times to obtain H0.7Ti1.825□0.175O4(Supporting Information,Fig.S1b).The obtained H0.7Ti1.825□0.175O4was washed with deionized water to remove excess HCl and vacuumdried.Finally,in order to obtain Ti1-xO2nanosheets,tetramethylammonium hydroxide solution (C4H9)4NOH,(10%,Aladdin) was added to the H0.7Ti1.825□0.175O4with a molar ratio of 1:1.
2.2.Material characterization
The morphology of the as-obtained samples was observed using the scanning electron microscopy (SEM,JEOL JSM 6700F) and the high-resolution transmission electron microscope (HR-TEM,JEOL JEM-2100F).X-ray diffraction(PANalytical B.V.Empyrean)was performed investigating the structural details of the samples.X-ray photoelectron spectroscopy (XPS,ESCALAB250XI) was used to determine the chemical and valence states.The atomic force microscopy(AFM) was conducted to measure the morphology and thickness of Ti1-xO2nanosheets.HAADF-STEM (FEI Tecnai G2 F20)measurement was corrected by a spherical aberration corrector.
2.3.Electrochemical tests
2.3.1.Electrochemical tests of SIBs
The electrochemical performance of all samples was measured in the CR2032 coin cells.The electrodes were prepared by dispersing the active material,polyvinylidene difluoride (PVDF) binder and acetylene carbon black with a weight ratio of 8:1:1 in N-methyl-pyrrolidone (NMP) solvent to form a slurry,where the active material is either Ti1-xO2or TiO2.The slurry was pasted onto a copper foil and vacuumdried at 110°C for 12 h.The as-dried electrode was cut into disks with a diameter of 1 cm by the slicer.Sodium metal was used as a reference electrode and counter electrode,the Whatman glassy fibre membrane was used as the separator,and 1 mol L-1NaClO4dissolved in a mixed solvent of ethylene carbonate (EC) and propylene carbonate (PC) (1 : 1 by volume ratio) with 5 wt% fluoroethylene carbonate (FEC)additive as the electrolyte.The sodium half-cells were assembled in an argon-filled glovebox.The galvanostatic charge/discharge tests of all electrodes were evaluated on a multichannel battery testing system(LAND CT2001A,China)between the voltage range of 0.01 V and 3.0 V.The CV was carried out by a 760E electrochemical workstation (Chenhua,China).
2.3.2.Electrochemical tests of LIBs
LIBs were assembled by a procedure similar to SIBs,except that lithium metal was used as a reference electrode and counter electrode,Celgard 2500 microporous membrane was used as the separator,and 1 mol L-1LiPF6dissolved in a mixed solvent of dimethyl carbonate (DMC),ethyl methyl carbonate (EMC) and ethylene carbonate (EC) (1:1:1 by volume ratio) as the electrolyte.
2.4.DFT calculations
Density functional theory (DFT) calculations were implemented to calculate the adsorption energy of both the TiO2nanosheet and the Ti1-xO2nanosheet via Vienna Ab initio Simulation Package (VASP) [20].All calculations use the Perdew-Burke-Ernzerhof (PBE) [21] type exchange correlation functional and the projector augmented wave(PAW)[22]method under generalized gradient approximation(GGA),and plane wave expansion cuts energy to 400 eV.The Monkhorst-Pack k-point sampling of 3×3×1 was used for Brillouin zone integration.The vacuum region was set as 16 Å to eliminate the artificial interaction between adjacent unit cells caused by periodic boundary conditions.All atoms were allowed to relax completely until the changes of Hellmann-Feynman force on each atom were less than 0.01 eV Å-1,and spin polarization was taken into account in the calculations.
The Li+/Na+adsorption energy on stoichiometric TiO2nanosheet or Ti1-xO2nanosheet was defined as
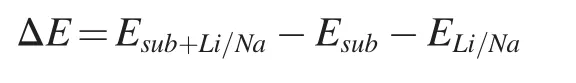
where Esub+Li/Naand Esubare energies of stoichiometric TiO2nanosheet or Ti1-xO2nanosheet with and without Li or Na adsorption.ELi/Nais the energy of one Li or Na atom in the bulk.
3.Results and discussion
3.1.Synthesis and characterizations of Ti1-xO2 nanosheets
The preparation method of Ti1-xO2is shown in Fig.1a,and the titania nanosheets with abundant Ti vacancies (Ti1-xO2) were synthesized by a top-down exfoliation process.The obtained Ti1-xO2monolayer nanosheets are composed of edge-sharing TiO6octahedra,in which part of the Ti4+ions have been emptied to form net negative charge.Ti1-xO2nanosheets could be well dispersed in an aqueous solution to create a stable colloidal suspension due to the electrostatic reaction.
Transmission electron microscopy (TEM) and Scanning electron microscope (SEM) images revealed the flake-like shape and ultra-thin thickness of the obtained Ti1-xO2sample(Fig.1b and Supporting Information,Fig.S2a).Atomic force microscopy (AFM) was further employed to determine the monolayered characteristics of the obtained nanosheet(Fig.1c),in which a thickness of about 1.1 nm and a lateral size from submicron to several microns were observed [23].The monolayer nanosheets can enlarge the contact area between the active material and the electrolyte,thus facilitating the diffusion of Li+/Na+in the host materials.Besides,the large exposed surface of Ti1-xO2nanosheets could maximize the active sites towards Li+/Na+storage.As shown in the Xray powder diffraction (XRD) pattern of Ti1-xO2nanosheets(Supporting Information,Fig.S3),the main diffraction peaks located at low angles could have well corresponded to (010)serial peaks [24].Note that there are two additional peaks at about 48°and 62°,which can be ascribed to (200) and (002)plane of the in-plane structure of the titania nanosheet,respectively [25].The X-ray photoelectron spectroscopic(XPS)analysis further specifics the electronic structure of Ti1-xO2nanosheets,as shown in Fig.S4.The XPS spectrum for Ti 2p displays doublets located at energies of approximately 463.69 and 458 eV,cor111responding to Ti 2p1/2and Ti 2p3/2,respectively.The high-angular annular dark field-scanning electron microscopy (HAADF-STEM) test was conducted to gain a refined structure of the specific Ti vacancy(Fig.1d).
The bright spots in Fig.2d represent Ti atoms,and the defects are cor111responding to Ti vacancies.The engineered Ti vacancies could be employed to boost the ion-storage capacity[26-30].
3.2.Electrochemical properties
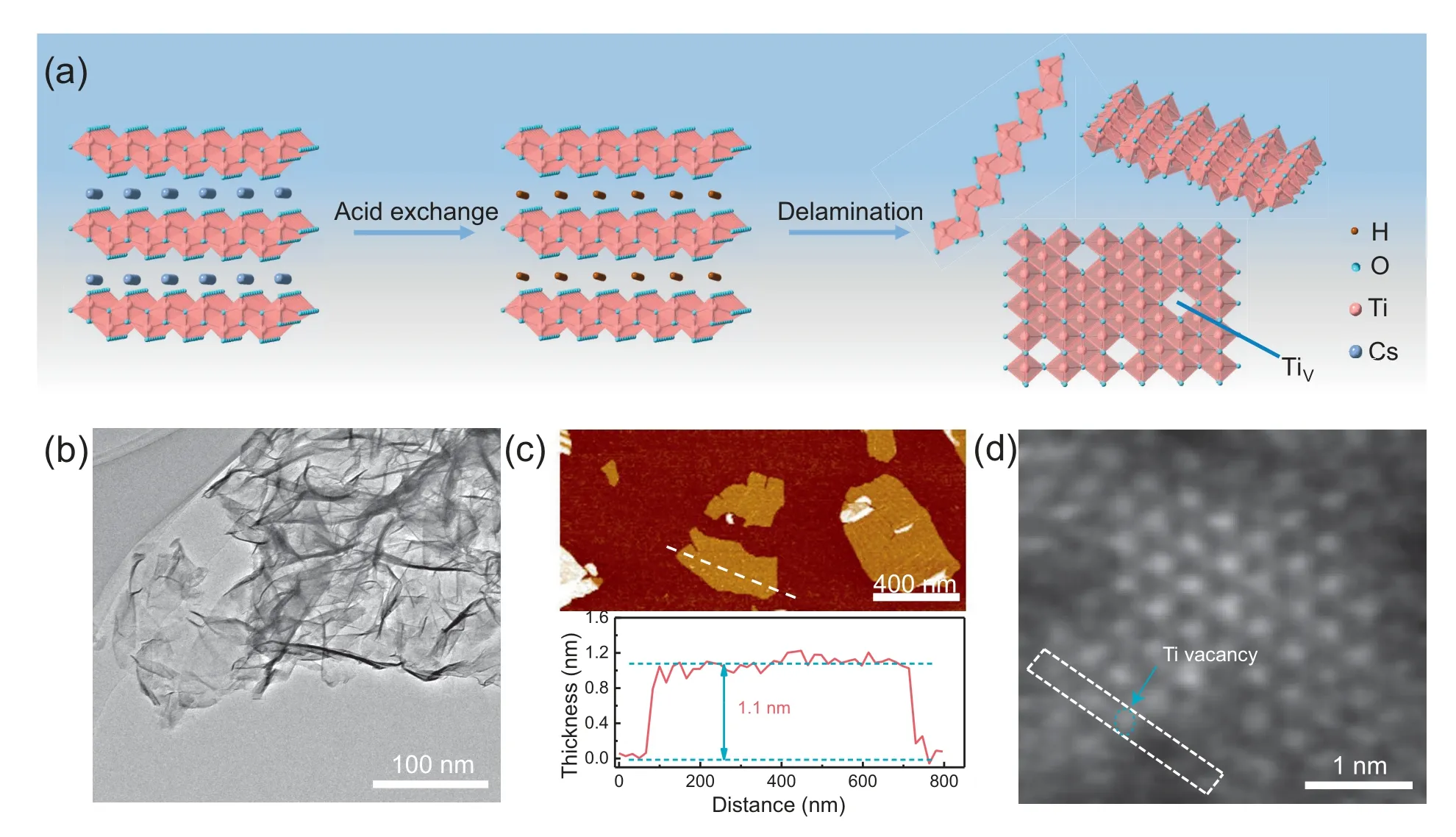
Fig.1.(a)Schematic illustration of the synthesis processes of the Ti1-xO2 nanosheets;(b)TEM image of Ti1-xO2 nanosheets;(c)AFM image and thickness profile of Ti1-xO2 nanosheets;(d) HAADF-STEM image of Ti1-xO2 nanosheets.
Fig.2.Galvanostatic discharge-charge curves of (a) TiO2 nanosheets and (b) Ti1-xO2 nanosheets in SIBs at 0.1 A g-1;(c) Cycling performance of Ti1-xO2 nanosheets and TiO2 nanosheets in SIBs at 0.1 A g-1;Galvanostatic discharge-charge curves of (d) TiO2 nanosheets and (e) Ti1-xO2 nanosheets in LIBs at 0.1 A g-1;(f) Cycling performance of Ti1-xO2 nanosheets and TiO2 nanosheets in LIBs at 0.1 A g-1.
In order to verify whether the Ti vacancies in Ti1-xO2can boost the ion-storage capacity,the electrochemical performance of Ti1-xO2and stoichiometric TiO2was compared by button lithium/sodium-ion cells.To sum up,the Ti1-xO2nanosheets afford remarkably better performance than stoichiometric TiO2nanosheets.Fig.2a and b show the 1st,2nd,3rd and 5th discharge/charge profiles of the stoichiometric TiO2nanosheets and Ti1-xO2nanosheets at a current density of 0.1 A g-1in SIBs,respectively.The first reversible capacity of 195.7 mA h g-1was obtained for Ti1-xO2nanosheets,which is higher than that of stoichiometric TiO2nanosheets (111 mA h g-1).The relatively low Coulomb efficiency (28%) is mainly attributed to the formation of solid electrolyte interphase (SEI) layers on the surface of Ti1-xO2nanosheets and the irreversible Na+intercalation layer between the restacked Ti1-xO2nanosheets.It is widely known that it is challenging to achieve long-term cyclic stability for SIBs anode material due to the sluggish Na-ion diffusion kinetics and vast volume expansion caused by the large Na-ion radius[31].With respect to stoichiometric TiO2nanosheets,it delivers a first reversible capacity of 111 mA h g-1and decays to 80.2 mA h g-1after 100 cycles at a current density of 0.1 A g-1(Fig.2c).In contrast,the Ti1-xO2nanosheets show stable long-term cyclability (91% capacity retention after 100 cycles).In order to further evaluate the rate performance of the as-prepared materials in SIBs,galvanostatic discharge-charge tests are performed at various current densities (from 0.1 to 1 A g-1).The Ti1-xO2nanosheets deliver high average discharge specific capacities of 153.4,119.5,91,84.8 and 70 mA h g-1at 0.1,0.2,0.5,0.8 and 1 A g-1,respectively(Supporting Information,Fig.S5a).When the applied current density is returned to the initial 0.1 A g-1,the discharge capacity can quickly recover to 132 mA h g-1.Conversely,the stoichiometric TiO2nanosheets show worse rate performance than Ti1-xO2nanosheets (Supporting Information,Fig.S5b).The electrochemical test results indicate that the Ti vacancies in Ti1-xO2contribute additional Na-ion storage capacity reversibly.Furthermore,the favour of the introduced Ti vacancies is also determined in Li-ion storage.As the trend found for SIBs,Ti vacancies dramatically enhance the electrochemical performance of Ti1-xO2nanosheets in LIBs.Ti vacancies substantially promote the first reversible capacity from 137.7 mA h g-1of stoichiometric TiO2to 332.1 mA h g-1of Ti1-xO2(Fig.2d and e).Meanwhile,the Liion storage capability maintains well during the 100 cycles measurement (Fig.2f).Based on the above electrochemical characterizations,the Ti1-xO2nanosheets show better performance than the stoichiometric TiO2nanosheets,essentially highlighting Ti vacancies’ effect towards lithium/sodium ion storage.
3.3.Electrochemical behaviour
Additionally,the cyclic voltammetry (CV) test of Ti1-xO2nanosheets and stoichiometric TiO2nanosheets were also investigated in the voltage range of 0.01-3 V(vs.Na+/Na)at a scan rate of 0.1 mV s-1for SIBs (Supporting Information,Figs.S6 and S7).A significant reduction peak at~0.8 V in the cathodic scan was potentially attributed to the Na+intercalation process for the restacking Ti1-xO2nanosheets and stoichiometric TiO2nanosheets [32,33].The reduction peaks at 1.5 V could be attributed to titanium vacancies provide sodium-ion storage sites for the Ti1-xO2nanosheets,which indicates the capacity contribution of stoichiometric TiO2is dominated by the intercalation reaction,while the Na+adsorption due to the introduction of Ti vacancies and the intercalation reaction for Ti1-xO2.
CV measurements were conducted at various scan rates from 0.1 to 1.0 V s-1,as presented in Fig.3a and b to gain insight into the electrochemical characteristics of Ti1-xO2nanosheets and stoichiometric TiO2nanosheets.The peak voltage has an inconspicuous change (within 0.05 V) as the scan rate increases,suggesting a small electrode polarization.The Faraday reaction in batteries includes a pseudo-capacitive process that occurs on the surface and a diffusion-control process that occurs by ion intercalation[33-,34].The capacity contribution of the electrode material can be determined by the power-law relationship(i=a vb)between the scan rate(v)and the peak current(i)[35],where a and b are both constants.The b value is between 0.5 and 1.0,which can be determined by the slope of log(i)versus log(v)(log(i)=blog(v)+log(a)).When b value is close to 0.5,the electrode material is dominated by the diffusion control process,while when b value approaches 1.0,the electrode material is dominated by the capacitive process [36,37].Fig.3c shows the linear relationship between log (i) and log (v) of Ti1-xO2and TiO2nanosheets in SIBs,respectively.For Ti1-xO2nanosheets,the b values are calculated to be approximately 0.77 and 0.85 for cathodic and anodic peaks,suggesting that the charge storage kinetics for the Ti1-xO2nanosheets is mostly capacitive process[38].By way of contrast,the b values of TiO2nanosheets for cathodic and anodic peaks are estimated to be 0.5 and 0.52,meaning that the Na-ion storage kinetics for the TiO2nanosheets is mainly affected by diffusion processes.The capacitive process represents the adsorption/desorption process in the Ti vacancies,while the diffusion processes are the intercalation/deintercalation process between the restacked nanosheet layers.Generally,the capacitive process has faster electrochemical kinetics than the diffusion processes.Therefore,the Ti1-xO2nanosheets show better electrochemical performance than stoichiometric TiO2.The following formula can quantitatively distinguish the capacitive contribution and diffusioncontrolled contribution:


Fig.3.(a,b)CV curves from 0.1 to 1 mV s-1 of the Ti1-xO2 nanosheets and TiO2 nanosheets for Na-ion storage;(c)Determination of the b-value of the Ti1-xO2 nanosheets and TiO2 nanosheets for Na-ion storage;(d,e)Contribution of the capacitive process of the Ti1-xO2 nanosheets and TiO2 nanosheets for Na-ion storage;(f) Contribution ratio of diffusion-controlled and capacitive capacities from 0.1 to 1 mV s-1 of the Ti1-xO2 nanosheets and TiO2 nanosheets for Na-ion storage.
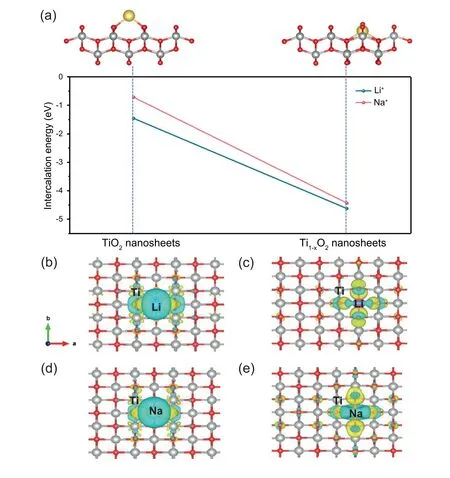
Fig.4.(a)Energy profiles for Na+and Li+intercalation in TiO2 nanosheets and Ti1-xO2 nanosheets;(b,c)Charge density difference(CDD)of Li+intercalation in TiO2 nanosheet (b) and Ti1-xO2 nanosheet (c),respectively;(d,e) CDD of Na+ intercalation in TiO2 nanosheet and Ti1-xO2 nanosheet,respectively,with an isosurface value of 0.0017 e Bohr-3.
where k1v and k2v1/2represent the capacitive process and diffusion-controlled intercalation/deintercalation,respectively.According to Eq.(2),the values of k1and k2can be obtained by the linear relationship between i(V)/v1/2and v1/2.Based on the quantitative analysis,72% of the total capacity(Fig.3d)is provided by the capacitive process for the Ti1-xO2electrode at 1 mV s-1,much higher than 48% of the stoichiometric TiO2(Fig.3e).Fig.3f displays the contribution ratio of the capacitive and diffusion-controlled capacities at various scan rates,respectively.The proportion of the capacitive contribution increases as the scan rate increases.The capacitive contribution of the Ti1-xO2electrode is constantly higher than that of stoichiometric TiO2nanosheets.In order to verify the role of Ti vacancies,we tested the Brunauer-Emmett-Teller (BET) of Ti1-xO2nanosheets and stoichiometric TiO2nanosheets.The test results showed that the specific surface areas of Ti1-xO2nanosheets and stoichiometric TiO2nanosheets were 93 m2g-1and 98 m2g-1,respectively (Supporting Information,Fig.S9).And the morphology of TiO2is the same with Ti1-xO2nanosheets (Supporting Information,Fig.S2b).Therefore,the specific surface areas and morphology of the two are not much different.The Ti vacancies in Ti1-xO2thus substantially contribute a large ratio of pseudo-capacitance for the sodium ions storage,enabling Ti1-xO2to achieve faster electrochemical kinetics.Moreover,the electrochemical behaviour of Ti1-xO2nanosheets in LIBs is also investigated by CV measurements at various scan rates,as presented in Fig.S8.Similar kinetics was observed for Li+,with the Ti vacancy enabling Ti1-xO2is dominated by the adsorption/desorption process (Supporting Information,Figs.S10-S12).It is noted that the b value of LIBs was lower than SIBs,which may be ascribed to the diffusion contribution is increased by Li+is more easily intercalated into restacked nanosheet layers.
Based on these results,we interpret the storage mechanism of Li+/Na+,as adsorption/desorption in the Ti vacancies and the intercalation/deintercalation in Ti1-xO2nanosheets.The chemical equations can be expressed as follow:
Intercalation/deintercalation:
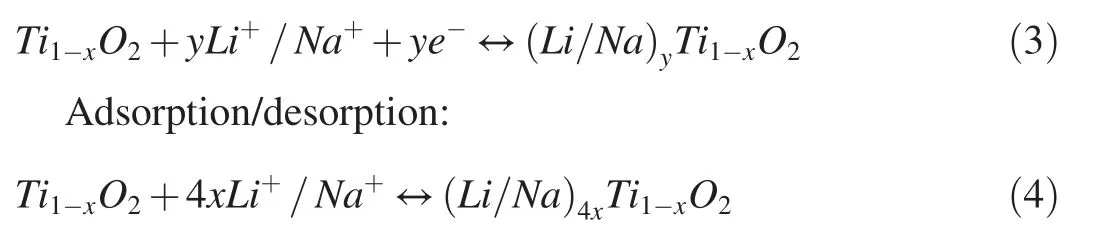
within Ti1-xO2nanosheets,there are x Ti vacancies,producing a ratio of 4 Li+/Na+per Ti vacancy in Eq.(4).Since a large part of the Li+/Na+storage capacity of Ti1-xO2nanosheets depends on the concentration of Ti vacancies,the material's Li+/Na+storage capacity can be optimized by introducing more vacancies,which opens up a new way for the development of advanced electrode materials.Moreover,we find that the introduced Ti vacancies can be used as storage sites for Li+/Na+and thus generate a novel adsorption/desorption storage route.
3.4.Electronic structures
The XPS spectrum is performed to compare Ti 2p ex-situ spectra of Ti1-xO2and TiO2in the pristine,full sodiumation,and full desodiumation states to study the electronic structure changes of Ti1-xO2and TiO2during the electrochemical process (Supporting Information,Fig.S13).As shown in Fig.S12a,the characteristic peaks of Ti 2p have no sign of transition to Ti3+or metallic Ti,indicating that there is no phase transition in the Ti1-xO2nanosheet anode.Compared with the pristine state,after full sodiumation,the Ti 2p peak slightly changes,which should be due to the interaction between the Ti1-xO2nanosheet and Na+.The Ti 2p peak returned to the pristine position after full desodiumation,indicating that the Ti1-xO2electrode has superior electrochemical reversibility.However,the Ti4+in stoichiometric TiO2is reduced to low-value Ti3+or metal Ti during full sodiumation.This result is entirely consistent with the above-mentioned CV results.The decrease in the binding energy of TiO2indicates that the surrounding chemical environment has changed.It may be that electrons are transferred to the Ti element to increase the shielding,which leads to a decrease in the binding energy.In summary,the change in the electronic structures of Ti1-xO2nanosheets indicates that Ti1-xO2nanosheets introduce a new reversible storage path.
The effect of introducing Ti vacancies on electrochemical behaviour was discussed using the DFT method.We calculated the intercalation/adsorption energies of Li+and Na+in stoichiometric TiO2and Ti1-xO2nanosheets.Fig.4a shows the two potential host sites considered: the intercalation sites between layers and the adsorption site of Ti vacancies in Ti1-xO2nanosheets.The calculated intercalation energies of Li+and Na+are -1.816 eV and -1.458 eV,respectively.The adsorption energies of Li+and Na+in Ti vacancies are much lower than that of TiO2,which are-5.534 eV and-4.625 eV,respectively,making it easier for Li+and Na+to be adsorbed into the Ti vacancies.Therefore,the introduction of Ti vacancies could provide more active sites for Li+/Na+storage,providing a new perspective for the development of LIBs and SIBs.Besides,drawn from the charge density difference(CDD) analysis,the electronic structures of Ti1-xO2nanosheets and stoichiometric TiO2nanosheets were calculated.Fig.4b and d show a charge transfer from Li and Na to Ti of TiO2nanosheets,in which the electron density of Li and Na decreases significantly (cyan electron cloud) and the electron density of Ti increases as well(yellow electron cloud).On the contrary,the electron density of Ti has no significant change in Ti1-xO2nanosheets(Fig.4c and e).These results are consistent with the XPS spectra,clarifying the Ti vacancy's electronic structure introduces an adsorption/desorption process,which further optimizes the electrochemical performance and kinetics.Based on the Atom-Realm (AR) effect [39],such an electronic structures can influence the surrounding chemical environment,resulting in the improvement of electrochemical performance.
A brief schematic illustration is given in Fig.5 to better clarify the Li+/Na+storage mechanism of the Ti1-xO2nanosheets.As shown in the figure,there are two reaction mechanisms for Li+/Na+storage in Ti1-xO2nanosheets: the Li+/Na+adsorption/desorption in the Ti vacancies and the intercalation/deintercalation between interlayer.The new adsorption/desorption mechanism enables Ti1-xO2nanosheets to deliver an exceeding theoretical capacity than TiO2nanosheets.
4.Conclusions
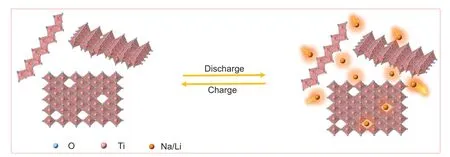
Fig.5.Schematic illustration of the Ti1-xO2 nanosheets reaction mechanisms towards Li+/Na+.
In summary,we have demonstrated that a novel Li+/Na+storage route through engineering electronic structure of cation-deficient sites in Ti1-xO2nanosheets,in which the abundant Ti vacancies could serve as Li+/Na+storage sites.When tested as anode materials,Ti1-xO2nanosheets indeed exhibited superior electrochemical performances than the stoichiometric TiO2.Various measurements and theoretical calculations disclosed that Ti vacancies in Ti1-xO2nanosheets could substantially serve as the extra Li+/Na+storage sites as compared with the stoichiometric TiO2ones.The kinetic analysis uncovered that both the adsorption/desorption at Ti vacancies and the intercalation/deintercalation between the restacked nanosheets layers during ion storage.We believed that engineering electronic structure of vacancy sites in nanosheets enables advanced electrodes for LIBs/SIBs.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (Grant Nos.91961125 and 21905019),“Key Program for International S&T Cooperation Projects of China” from the Ministry of Science and Technology of China (Grant No.2018YFE0124600),“the Fundamental Research Funds for the Central Universities” (Grant No.2018JBZ107),and the Chemistry and Chemical Engineering Guangdong Laboratory (Grant No.1932004).The authors also appreciate the support from the “Excellent One Hundred” project of Beijing Jiaotong University.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gee.2020.11.006.
 Green Energy & Environment2022年4期
Green Energy & Environment2022年4期
- Green Energy & Environment的其它文章
- Multivariate MOF for optimizing atmospheric water harvesting
- Lignin-based carbon fibers: Formation,modification and potential applications
- Charactering and optimizing cathode electrolytes interface for advanced rechargeable batteries: Promises and challenges
- Metal-organic frameworks-derived metal phosphides forelectrochemistry application
- Surface-mediated iron on porous cobalt oxide with high energy state for efficient water oxidation electrocatalysis
- Oxygen-deficient SnO2 nanoparticles with ultrathin carbon shell for efficient electrocatalytic N2 reduction
