层状六边形Co1-xS修饰氮掺杂碳纳米管用于锂硫电池的硫载体
王相文
(德州职业技术学院粮食工程系,德州253034)
Currently,with the depletion of conventional fossil energy and the explosion of environmental pollution issues,clean and renewable energy sources are urgently exploited,including solar and biomass,and so forth.However,the prerequisite for using these renewable new energy sources is the development of advanced and economical energy storage systems.However,traditional lithium-ion batteries have limited capacity and high cost,and cannot meet this demand in terms of specific energy.And Li-S batteries are recognized as a promising system for energy storage.Since the theoretical specific energy density of Li-S batteries(LSBs)is as high as 2 600 Wh·kg-1,which is five times that of commercial lithium-ion batteries,and practical energy densities of beyond 500 Wh·kg-1.Besides,sulfur element is abundant,so it has become one of the most promising positive electrode active materials because of its high theoretical specific capacity(1 675 mAh·g-1)[1-2].
Despite the outstanding advantages,many issues cannot be ignored for the LSBs.For instance,the high capacity of LSBs is based on the reversible conversion of sulfur and Li2S in a redox reaction,yet sulfur and Li2S are insulated.In addition,the intermediate lithium polysulfide(LiPSs)formed during the reaction is soluble in liquid electrolytes.And LiPSs can shuttle through the separator and deposit on the anode during the charging process,which leads to the loss of active material(S).Moreover,the large volume change(about 80%)of the cathode during the electrochemical process is also a serious problem.All these issues inevitably lead to the rapid capacity decay and poor Coulombic efficiency of the LSBs,which hurdle their wide applications.Therefore,effective strategies are urgently needed to improve these unfavorable situations.
In recent years,researchers have made strenuous efforts to solve the shuttle effect of LiPSs,but passive restriction or retention strategies cannot fundamentally prevent the dissolution of polysulfide into the electrolyte.Therefore,it is urgent to introduce effective electrocatalytic means and adjust the structure of sulfur cathode,including structural confinement design,conductive composite material fabrication,electrolyte modification,multifunctional polar binders,and interlayer configurations[3-5].A series of polar hosts such as metal oxides,metal sulfides,metal carbides,metal nitrides,and metal phosphides hosts have been found to have catalytic activity for the conversion of LiPSs.Compared with these materials,metal sulfides have superior catalytic activity in promoting the redox reaction kinetics of LiPSs,owing to the metal—S bond that can anchor lithium polysulfide through dipole interaction[5].Co1-xS has attracted widespread attention due to its excellent catalytic performance.However,nanoscale Co1-xS is easy to agglomerate,and some conductive materials such as carbon can be used as substrates to ensure cycle stability[6-7].
So far,many reports have testified that carbonsulfur hybrid electrodes have favorable properties,such as the presence of pores in the carbon structure,which can promote the wettability of the electrolyte,enhance the transport rate of lithium ions and electrons,and improve the specific capacity and cycling stability[6,8-10].Such good performance is usually obtained at low current densities,and there is a significant degradation in the capacity as well as cycling behavior when the current density increases.Especially,the weak physical adsorption to polar LiPSs of nonpolar carbon-based materials limits their application as sulfur hosts.Therefore,decorating the surface of the carbon-based hosts with polar materials to capture polar LiPSs and inhibit the“shuttle effect”is considered a good choice[7,11-12].
This work combined the advantages of a porous conductive frame with the advantages of Co1-xS for anchoring and catalyzing polysulfides.A new type of layered hexagonal Co1-xS modified N-doped carbon nanotube(CNT)composite sulfur host material was prepared by a simple solvothermal method.Co1-xS-CNT/S can effectively adsorb polysulfides and catalyze their conversion.Moreover,the CNT conductive frame can improve the conductivity of the Co1-xS-CNT/S cathode,provide enough space to buffer the large volume expansion of S,and play a role in the polysulfide′s physical limitation.Due to these multifunctional advantages,the obtained Co1-xS-CNT/S composite cathode exhibited a satisfactory electrochemical performance,especially the outstanding cycle stability.After 170 cycles,Co1-xS-CNT/S can maintain a discharge capacity of 405.6 mAh·g-1at 0.5C,with a stable Coulombic efficiency(over 99.2%).
1 Experimental
1.1 Synthesis of materials
Polypyrrole(PPy)nanotubes were prepared through a previously reported method[13].The CNT template was obtained through sintering PPy at 700℃for 3 h in an Ar flow.At first,65 mL of deionized water and 15 mL of ethylene glycol were mixed by magnetic stirring,and then 6 mmol·L-1CoCl2·6H2O,24 mmol·L-1thiourea,and 0.1 g PPy nanotubes were added to the solution in sequence,followed by stirring and ultrasonic dispersion for 30 min.Next,the mixture was transferred into a Teflon-sealed autoclave and reacted at 180℃for 15 h.In this solvothermal process,hexagonal nanosheets grew on the CNT surface.After being cooled to room temperature,the mixture was centrifuged and washed with ethanol and deionized water,respectively.The Co1-xS-CNT host was obtained after drying overnight at 50℃.The preparation process of the Co1-xS-CNT host was shown in Fig.1.

Fig.1 Preparation process of the Co1-xS-CNT host
The prepared samples,polyvinylidene fluoride(PVDF),and acetylene black were dispersed inNmethyl pyrrolidone(the mass ratio was 7∶1∶2)to form a uniform slurry after ball milling for 4 h.Then the slurry was coated onto an Al foil and dried at 50℃for 12 h under a vacuum.The diameter of each electrode was 1.2 cm and the sulfur loading wasca.1.5 mg·cm-2.Li metal and polypropylene were utilized as reference electrodes and separators,respectively.The electrolyte was 1.0 mol·L-1LiTFSI and 1.0% LiNO3in a solution of 1,3-dioxolane/ethylene glycol dimethyl ether(1∶1,V/V).The electrolyte dosage was accurately controlled with the ratio of electrolyte/sulfur ratio ofca.65 μL·mg-1under normal conditions.
1.2 Material characterization
Electrochemical performances were tested in standard CR2032 simulated batteries.Galvanostatic chargedischarge measurements were performed on a Neware battery testing system with a potential window of 1.7-2.8 V.Electrochemical impedance spectra(EIS)test was conducted on a CHI760E electrochemical workstation,the frequency ranged from 100 kHz to 0.01 Hz and the amplitude was 5 mV.The X-ray diffractometer(XRD)test of CoNiP-rGO was conducted by using CuKαradiation(Shimadzu XRD-6100AS),λ=0.154 16 nm,the operating voltage was 40 kV and the operating current was 30 mA,and the 2θranged from 10° to 60°.Morphological characterization of the synthesized samples was measured with a Hitachi S-4800 scanning electron microscope(SEM,3.0 kV).Raman spectra were investigated by a LabRam HR confocal laser micro-Raman spectrometer at room temperature.
2 Results and discussion
2.1 Morphology and structure characterization
The morphologies of CNT and Co1-xS-CNT were investigated by SEM.SEM images revealed that the CNT exhibited an obvious tubular structure.And the diameter of CNT wasca.300 nm(Fig.2a and 2b),and the surface of the tube was rough.The SEM images of Co1-xS-CNT are shown in Fig.2c and 2d,the hexagonal nanosheets interspersed together were evenly wrapped on the surface of CNT.After Co1-xS encapsulation,the diameter of the nanotube grew toca.600 nm.The unique structure of Co1-xS-CNT can provide a fast and continuous transmission path for Li+and electrons.Most importantly,Co1-xS has excellent catalytic and chemical adsorption for LiPSs[3-4].
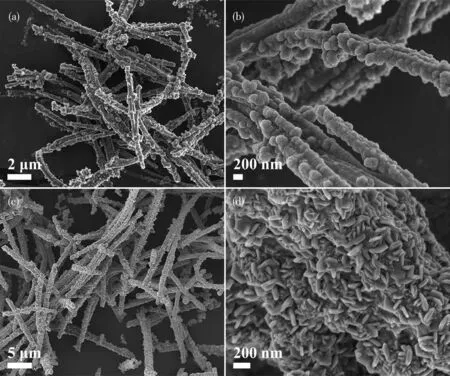
Fig.2 SEM images of(a,b)CNT and(c,d)Co1-xS-CNT
The crystal structure of as-prepared Co1-xS-CNT hosts was analyzed by XRD.All the diffraction peaks of the composite are matched well with the Co1-xS(PDF No.42-0826),without other impurities(Fig.3a).No carbon peak was detected in the XRD data,indicating that the carbon nanotubes are amorphous.There are two wide peaks at 1 355 and 1 599 cm-1in the Raman spectrum of Co1-xS-CNT,which are assigned to carbon′s D band and G band,respectively(Fig.3b),and the intensity ratio ofID/IGreflects the defective and graphitic structure in carbon materials.TheID/IGin Co1-xS-CNT was 1.11,the high value indicates that there are plentiful defects in the Co1-xS-CNT structure[14].
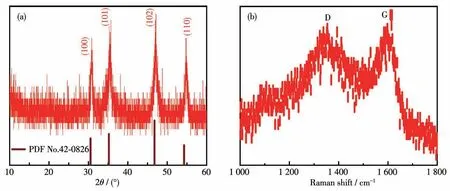
Fig.3(a)XRD pattern and(b)Raman spectrum of Co1-xS-CNT
2.2 Electrochemical performance
The EIS results of the Co1-xSCNT/S and CNT/S electrodes are shown in Fig.4a and 4b.According to the result,the Co1-xS-CNT/S electrode presented a lower charge-transfer resistance and a faster Li+diffusion rate.And the result also indicates that the e-/Li+diffusion rate and lithiation/delithiation kinetics of the CNT/S electrodes are enhanced by introducing the Co1-xS[15-18].The long cycle stability of the Co1-xS-CNT/S electrode with a sulfur loading of 1.5 mg·cm-2has been investigated at different rates(Fig.4c and 4d).The initial and the 170th discharge capacities of the Co1-xSCNT/S electrode at 0.5C were 785.8 and 405.6 mAh·g-1,respectively(Fig.4c).The initial and the 380th discharge capacities of the Co1-xS-CNT/S electrode at 1C were 504.3 and 326.4 mAh·g-1,respectively(Fig.4d).By contrast,under the same condition,the CNT/S electrode exhibited a much lower capacity.In addition,the Co1-xS-CNT/S electrode maintained a stable Coulombic efficiency of over 99.2% at 0.5C and 97.7% at 1C,which is much higher than that of the CNT/S electrode(89.4% and 84.0%).It proves the superior cycle performance and practical application potential of the Co1-xSCNT/S electrode[19].The excellent cycle stability of the Co1-xS-CNT/S electrode is attributed to the presence of Co1-xS,which accelerates reaction kinetics and suppresses the“shuttle effect”[20-21].
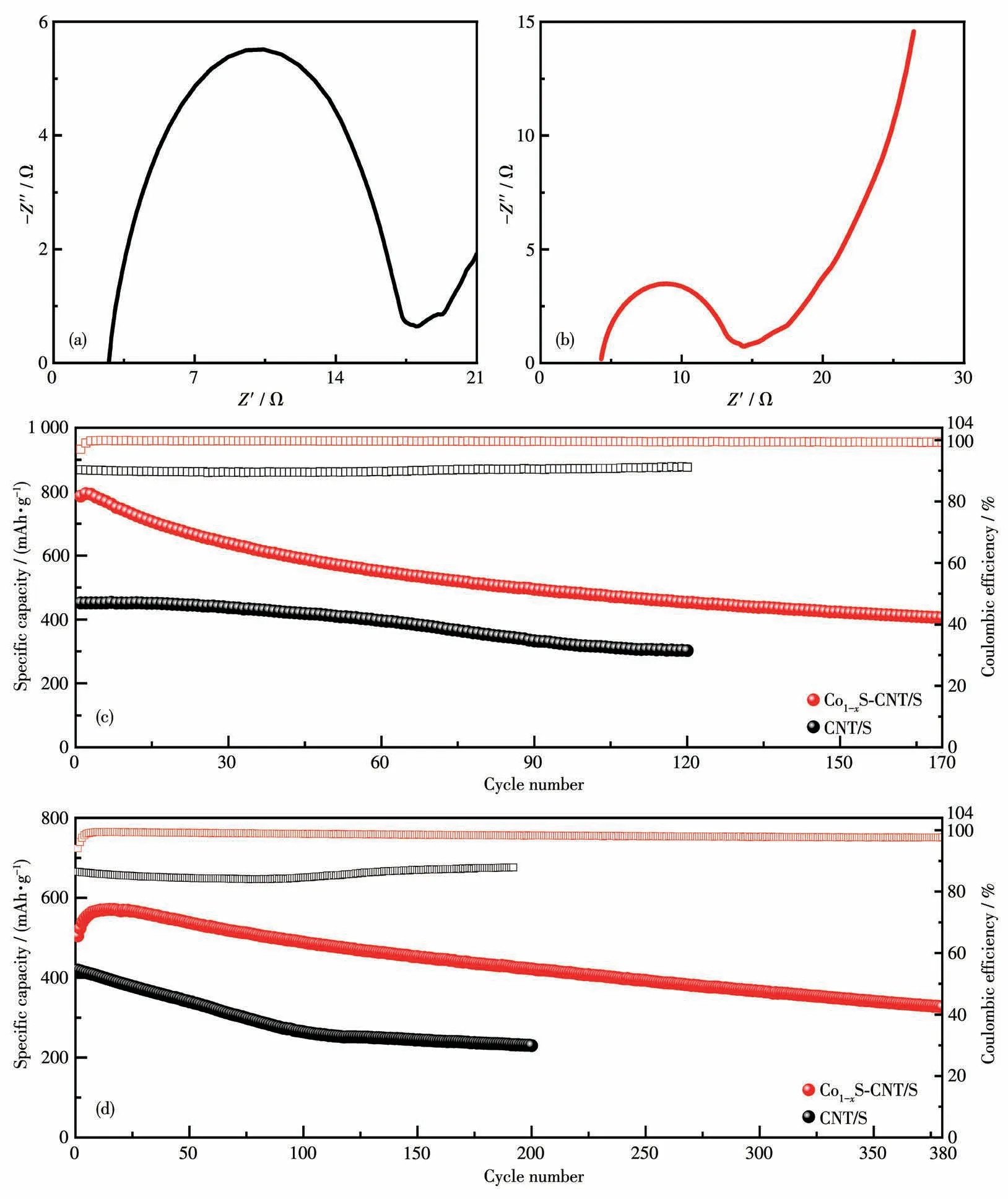
Fig.4 Nyquist plots of the(a)Co1-xS-CNT/S and(b)CNT/S electrodes;Cycle-performance of the Co1-xS-CNT/S and CNT/S electrodes at(c)0.5C and(d)1C
The initial charge-discharge profiles of the Co1-xSCNT/S and CNT/S electrodes are shown in Fig.5a and 5b.Comparing the charge-discharge profiles of the 1st,200th,and 380th cycles,the Co1-xS-CNT/S electrode showed a lower attenuation rate.Furthermore,the two charge-discharge platforms correspond to the multistep redox reactions of sulfur,and the voltage difference between the discharge plateau and discharge plateau of the Co1-xS-CNT/S electrode(261.6 mV)was lower than that of the CNT/S electrode(315.6 mV),reflecting the fast reaction kinetics and excellent reversibility of the Co1-xS-CNT/S electrode[19].The short voltage jumps during charging curves corresponding to the overpotential of Li2S activation are displayed in Fig.5c and 5d.The lower overpotential suggests that the activation ability of Li2S is more stable,therefore the activation ability of Li2S in the Co1-xS-CNT/S electrode is stronger.In addition,the overpotential difference change rate of the Co1-xS-CNT/S electrode(8.2%)before and after cycling was much smaller than that of the CNT/S electrode(72.6%),indicating a more stable electrochemical performance of the Co1-xS-CNT/S electrode[22-23].
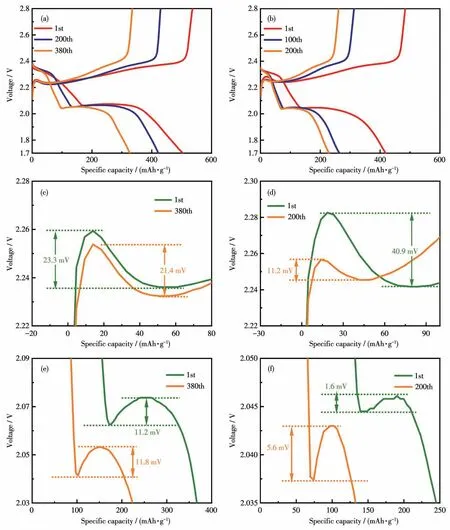
Fig.5 Galvanostatic charge-discharge curves of the(a)Co1-xS-CNT/S and(b)CNT/S electrodes;Charge voltage profiles of the(c)Co1-xS-CNT/S and(d)CNT/S electrodes in the first and last cycles at 1C;Voltage dip at Li2S nucleation point of the(e)Co1-xS-CNT/S and(f)CNT/S electrodes in the first and last cycle at 1C
And the nucleation kinetics of Li2S can be reflected using the voltage dip between the nucleation site of Li2S and the tangent line in the discharge profile(Fig.5e and 5f).The voltage drop difference indicates the conversion rate of S8and the nucleation rate of Li2S.The voltage dip change rate of the Co1-xS-CNT/S electrode(5.3%)before and after cycling was much smaller than that of the CNT/S electrode(250%),indicating that the Co1-xS-CNT/S electrode had better stability during cycling[22-23].All the results indicate that the activation ability of Li2S and reaction kinetics have been enhanced by the introduction of Co1-xS.
3 Conclusions
In summary,a composite of the hexagonal Co1-xS decorating hierarchical porous carbon was successfully synthesized and used as a sulfur host for LSBs.In the composite host,the CNT constructed with hexagonal Co1-xS and CNT demonstrates the ultrahigh specific surface area and pore volume,which are beneficial to the wetting of electrolytes and the physical anchoring of LiPSs.And at the same time,the CNT improves the electronic conductivity and stabilizes the structure of the electrode.The Co1-xS provides chemical adsorption of LiPSs,and accelerates the redox process of the LiPSs to Li2S.The synergistic action of Co1-xS and CNT confer satisfactory electrochemical performance especially the cycle stability and rate performance of the Co1-xS-CNT/S electrode.The Co1-xS-CNT/S electrode delivered a reversible capacity of 405.6 mAh·g-1after 170 cycles at 0.5C.Besides,it also obtained stable Coulombic efficiencies(over 99.2%).

