氮掺杂石墨烯负载锰催化分解高湿臭氧性能
陈冬斌丁静亚张国林范 兰孙 林程 峰*,刘永龙陈依柯许 琦*,
(1盐城工学院化学化工学院,盐城224051)
(2盐城工学院机械工程学院,盐城224051)
(3江苏兰丰环保科技有限公司,盐城224051)
(4盐城工学院江苏省新型环保重点实验室,盐城224051)
0 Introduction
Ground-level ozone is hazardous to human health and damages ecosystems.In recent years,it has become a major seasonal environmental pollutant,and the study of ozone catalytic decomposition technology has become one of the hotspots in the“Battle of the Blue Sky”[1-5].Among the many catalysts that catalyze ozone decomposition,manganese oxide catalysts exhibit excellent catalytic activity at room temperature[6-10].However,under high relative humidity(RH)conditions,water competes with ozone for adsorption,reducing the opportunity for ozone to bind to active sites,resulting in a decrease in the performance of manganese oxide catalysts for catalyzing ozone decomposition[11-15].
To address these problems,tremendous efforts have been devoted to increasing the concentration of oxygen vacancies on the surface of manganese oxide catalysts,but little work has focused on the effect of the electronic structure of the carrier on the performance of the catalyst[16-21].In this paper,nitrogen-doped graphene(NG)was prepared,and the lone pair of electrons on the nitrogen atoms in graphene can be transferred to the manganese catalyst,which can not only improve the ozone decomposition ability of the manganese catalysts,but also increase the charge density of oxygen vacancies,weaken the adsorption of water molecules,and improve the moisture resistance of the catalyst.
1 Experimental
1.1 Main reagents
Reagents include graphene(95%,Tianjin Baima Technology Co.,Ltd.),Mn(NO3)2·4H2O(analytically pure,Sinopharm Chemical Reagent Co.,Ltd.),sodium nitrate(NaNO3,analytically pure,Yangzhou Hubao Chemical Reagent Co.,Ltd.),sodium borohydride(NaBH4,analytically pure,Sinopharm Chemical Reagent Co.,Ltd.),concentrated sulfuric acid(H2SO4,98%,Jiangsu Jiangsu Tongsheng Chemical Reagent Co.,Ltd.),melamine(C3H6N6,analytically pure,Sinopharm Group Chemical Reagent Co.,Ltd.),potassium permanganate(KMnO4,analytically pure,Sinopharm Group Chemical Reagent Co.,Ltd.),hydrogen peroxide(H2O2,30%,Sinopharm Group Chemical Reagent Co.,Ltd.),argon(Ar,99.99%,Yancheng Lintai Gas Co.,Ltd.).Deionized water was self-made.
1.2 Preparation of catalysts
Preparation of graphene oxide(GO)[22]:First,3 g NaNO3and 3 g graphite powder were added to 144 mL of concentrated sulfuric acid under stirring in an icewater bath.Then,18 g of potassium permanganate was added in batches(about 0.5 g each time with a time interval of 30 s),stirred for 90 min,and then warmed up to 35℃for 2 h.Finally,120 mL of deionized water was added to the turbid solution,and 15 mL of 30%H2O2was added and stirred for 10 min after cooling,and the graphene oxide was obtained by centrifugation,washed with water to neutral,and ultrasonic exfoliation for 2 h,and the solid GO was obtained by freeze-drying.
Preparation of NG[23]:Firstly,GO powder and melamine with a mass ratio of 1∶5 were mixed,ground,and sieved,and then the mixture was heated to 700℃at a rate of 5℃·min-1in an argon atmosphere and maintained for 2 h and then cooled to room temperature.The product was removed from the soluble residue with deionized water,and freeze-dried to obtain a black powder.
Preparation of NG-supported manganese oxides(nMnOx)/NG[24]:First,five portions of 100 mL of deionized water containing 0.16 g of NG powder were sonicated for 0.5 h.Manganese nitrate solutions of 0.50,1.00,2.00,and 5.00 mL with a concentration of 0.1 mol·L-1were added,and the mixture was stirred for 18 h.Then,0.10,0.20,0.40,and 1.00 g of sodium borohydride were added,and stirring was continued for 16 h.Then,the slurry was separated by centrifugation and washed with deionized water to neutralize,and the samplesnMnOx/NG(n=0.5,1,2,5,ndenotes the volume of manganese nitrate solution)powders were obtained by vacuum drying at 80℃.
Preparation of manganese oxide-loaded GO(nMnOx/GO):40 mL of GO dispersion(the GO concentration was about 40 mg·mL-1)was added 100 mL of deionized water,and after being sonicated for 0.5 h,a certain volume of manganese nitrate solution(the volumes of manganese nitrate were 0.50,1.00,2.00 and 5.00 mL with a concentration of 0.1 mol·L-1)was added.After the mixture was stirred for 18 h,1.0 g of sodium borohydride was added respectively,and the stirring was continued for 16 h.The slurry was centrifuged,and the obtained solid was washed with deionized water until neutral and dried at 80℃to obtainnMnOx/GO powder(n=0.5,1,2,5,ndenotes the volume of manganese nitrate solution).
1.3 Characterization instruments of catalysts
X-ray diffraction(XRD,PANalytical,Netherlands X′Pert3Powder)was tested by CuKαradiation(λ=0.154 nm),operating voltage and current were 40 kV and 100 mA,respectively,and the 2θrange was 10°-90°.X-ray photoelectric spectroscopy(XPS,ESCALAB,250Xi)was utilized on AlKαradiation(1 486.6 eV)as the X-ray origin.Scanning electron microscope(SEM,Japan HITACHI company)images were measured on 450 fields(Nova Nano SEM)emission,operating voltage and current were 5 kV and 10 mA,respectively.Transmission electron microscope(TEM)images were characterized by Hitachi 200 kV field(JEOL JEM-2100F)emission transmission electron microscope.Raman spectroscopy was characterized by LabRamHR Evolution,the excitation wavelength was 514 nm.Automatic specific surface and porosity analyzer were measured on Micromeritics 3Flex(degas at 300℃for 6 h,degassing environment was a vacuum,77 K).Electron spin-resonance spectroscopy(ESR)was characterized by Bruker EMXplus,the center field was 3 502.00 G,and the sweep width was 100.0 or 6 000.0 G.
1.4 Performance evaluation of catalytic decomposition of ozone
The flow chart of the catalytic decomposition ozone device was shown in Fig.1.The catalytic decomposition performance of the catalyst was tested in a fixed-bed continuous flow reactor.The ozone decomposition performance of the catalysts was tested under atmospheric pressure conditions at 25℃and different RH(20%,40%,60%,and 80%)with 0.90 g of quartz sand mixed with 0.10 g of catalyst(40-50 mesh)filled in a quartz tube reactor(Φ=10 mm).Ozone was generated from O2by a high voltage discharge from an ozone generator.The RH of the gas stream was measured using a humidity probe(Benetech,GM1361+).The total gas flow through the quartz reactor was controlled at 1 500 mL·min-1and the ozone concentration was blended through the nitrogen carrier gas at 85.7 mg·m-3.The inlet and outlet ozone concentrations were measured using a Model 106-L ozone online analyzer(2B Technologies,Boulder,Co.,United States).The ozone decomposition rate(X)was calculated using the following equation:
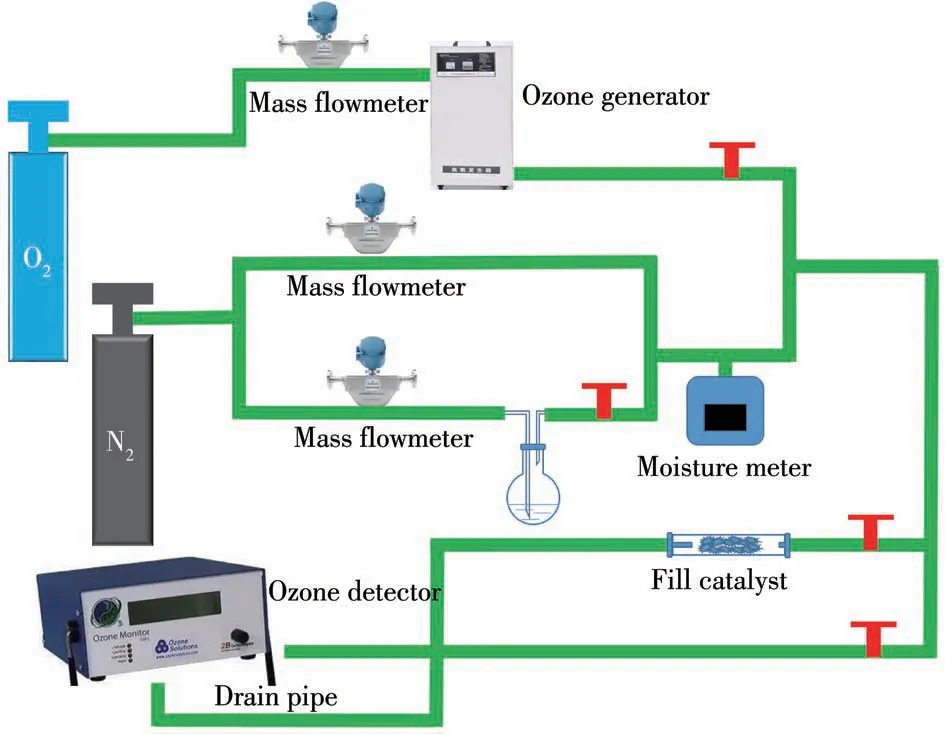
Fig.1 Flow chart of catalytic decomposition of ozone experiment
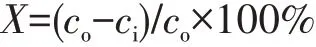
Where thecoandciare the outlet and inlet concentrations of ozone.
2 Results and discussion
2.1 Structural analysis and characterization
The XRD patterns of NG andnMnOx/NG with different manganese loadings are shown in Fig.2.A broad diffraction peak at 2θof 26.0° was present in all samples.The strong diffraction peak of graphene was located at 25.8°,which is due to the effect of thermal annealing and nitrogen doping,GO restores the graphite crystal structure[25],resulting in the shift of the characteristic peak of NG.nMnOx/NG also had a characteristic peak near 26.0°,and the loading of Mn does not change the structure of NG,but the peak intensity gradually decreased with the increase of the loading.
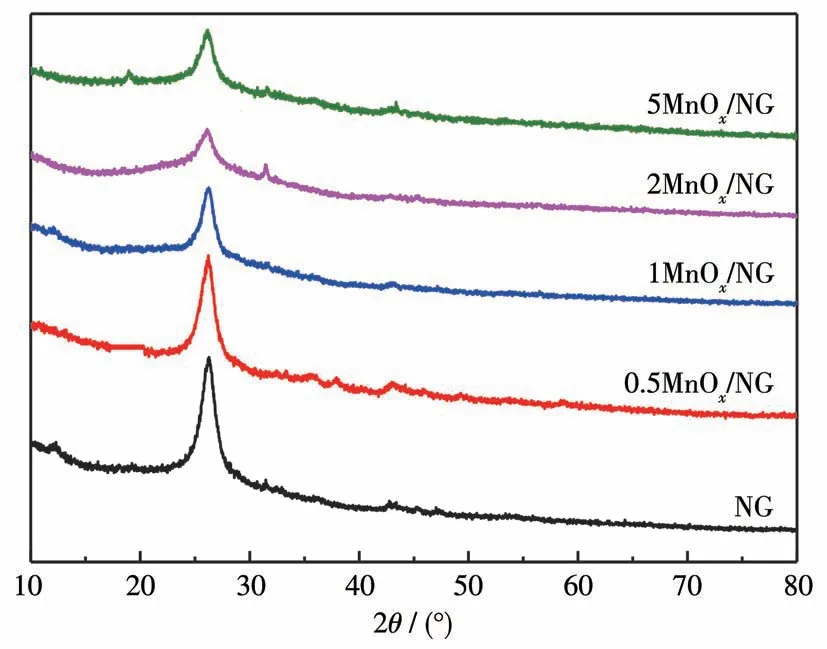
Fig.2 XRD patterns of NG and nMnOx/NG with different Mn loadings
The nitrogen adsorption-desorption isotherms and pore size distributions of NG andnMnOx/NG catalysts are shown in Fig.3 and Fig.S1(Supporting information).Nitrogen adsorption-desorption isotherms showed typical hysteresis loops in all samples,belonging to typeⅣisotherms,indicating that the samples had mesoporous structures[26].The specific surface area,pore size,and pore volume of all samples are shown in Table S1.The specific surface area,pore size,and pore volume of NG were 635 m2·g-1,10.07 nm,and 2.74 cm3·g-1,respectively.The specific surface areas ofnMnOx/NG were in a range of 81 to 146 m2·g-1,the pore diameters ofnMnOx/NG were between 4.82 and 7.58 nm,and the pore volumes ofnMnOx/NG were in the range of 0.23 to 0.33 cm3·g-1.The results indicate that the manganese oxide has been loaded on the graphene.
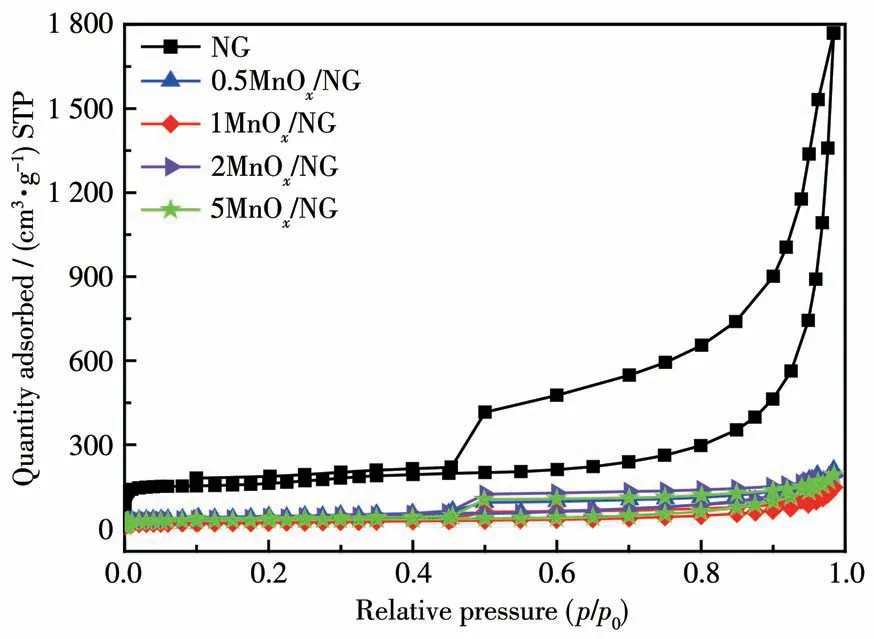
Fig.3 Nitrogen adsorption-desorption isotherms of NG and nMnOx/NG
The TEM images of 5MnOx/NG catalysts are shown in Fig.4a-4f.5MnOx/NG catalysts were thin sheetlike structures with folds,and the NG nanosheets were randomly stacked together,showing a uniform laminar flow morphology similar to pristine graphene.The loaded Mn does not disrupt the layered structure of the catalysts.At 5 nm size,its crystalline surface spacing was 0.257 nm,which is consistent with the(111)surface of MnO[27].
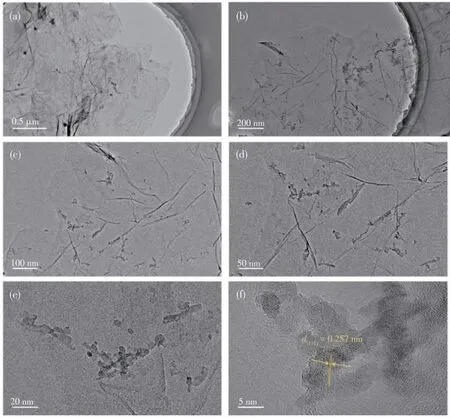
Fig.4 TEM images of 5MnOx/NG
The energy dispersive spectroscopy(EDS)images of the 5MnOx/NG catalyst are shown in Fig.5.It can be seen from the figure that there were C,N,O,and Mn elements in the material,and N and Mn were uniformly dispersed on the carrier,which increases the active site of the catalyst and improves the utilization rate of Mn.It is beneficial to improve the performance of the catalytic decomposition of ozone.
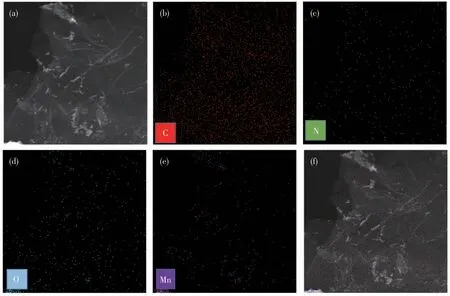
Fig.5 EDS images of 5MnOx/NG
2.2 Ozone decomposition performance
The performance ofnMnOx/NG catalytic decomposition of ozone was tested using a controlled temperature fixed bed with an initial ozone concentration of 85.7 mg·m-3,a temperature of 25℃,and an air velocity of 30 000 mL·gcat-1·h-1.As shown in Fig.6a,all the catalysts with different loadings,including NG,could decompose ozone.5MnOx/NG showed the best activity for ozone decomposition,maintaining a 100% decomposition rate for ozone within 24 h.2MnOx/NG catalyst could maintain a 100% decomposition rate for the first 5 h,the decomposition efficiency gradually decreased after 5 h,and the decomposition rate remained above 80% after 24 h.The catalytic efficiency of 1MnOx/NG and 0.5MnOx/NG catalysts decreased gradually with the increase of reaction time,the decomposition rate was 75% and 60% after 24 h,respectively.The above results indicated that the loading of manganese and the ozone decomposition rate is proportional.It is noteworthy that NG can still maintain more than 55% ozone decomposition rate after catalyzing ozone for 24 h.In addition to the adsorption effect of graphene nitride,the introduction of nitrogen creates more surface defects,which is beneficial to the decomposition of ozone.
For comparison,the catalytic decomposition performance of different loadings ofnMnOx/GO catalysts for ozone is shown in Fig.6b.Except for the GO,all thenMnOx/GO have catalytic decomposition activity for ozone.With the increase in the amount of MnOx,the catalytic performance gradually increased,and 5MnOx/GO had the best catalytic performance in 24 h.However,the catalytic activity ofnMnOx/GO rapidly decreased in 10 h under 60% RH,the detailed results of 5MnOx/GO under different RH are listed in Fig.S2.
Humidity resistance is one of the important indicators of ozone decomposition catalysts.To explore the ozone decomposition performance ofnMnOx/NG under high RH,we selected 5MnOx/NG,which had the best ozone decomposition effect at RH=20%,and tested the catalytic ozone decomposition effect under high RH conditions.The catalytic ozone decomposition performances of 5MnOx/NG at different RH levels(20%,40%,60%,and 80%)are shown in Fig.6c.The results showed that the catalyst decomposition activity of ozone varied greatly at different RH levels.At 20%RH,the catalyst maintained a 100% decomposition rate of ozone,and even when it increased to 40%,it was completely removed within 22 h,but it still maintained a 97% decomposition rate after 22 h.When the RH increased to 60%,the ozone decomposition rate was 100% within 19 h,the decomposition rate gradually decreased to 94%.When the RH continued to increase to 80%,the catalyst can maintain a 100% decomposition rate of ozone within 8 h,and in the following 3 h,the decomposition rate decreased to 85%,and after 11 h,the decomposition rate gradually decreased,and by 24 h,it maintained at about 80% of ozone decomposition rate.
The above experiments showed that the 5MnOx/NG catalyst had high ozonolysis activity and exhibited good stability and moisture resistance at high RH.To test the effect of RH on the structure and performance of the catalyst,alternating experiments with a time interval of 2 h were done at RH levels of 80% and 20%.The results are shown in Fig.6d.5MnOx/NG still had a good ozone decomposition rate,with a 100%decomposition rate for ozone within 20 h,and after 20 h,the ozone decomposition rate decreased to 95%.When the RH was adjusted back to 20%,the ozone decomposition rate increased to 100%,and when the RH was adjusted back to 80%after 2 h,the decomposition rate decreased to 90%,and when the RH was adjusted back to 20%,the ozone decomposition rate increased to 100% again.The experimental results show that the catalyst structure was not damaged at high RH,and the increase of humidity does not lead to the change of active site structure on the catalyst surface.
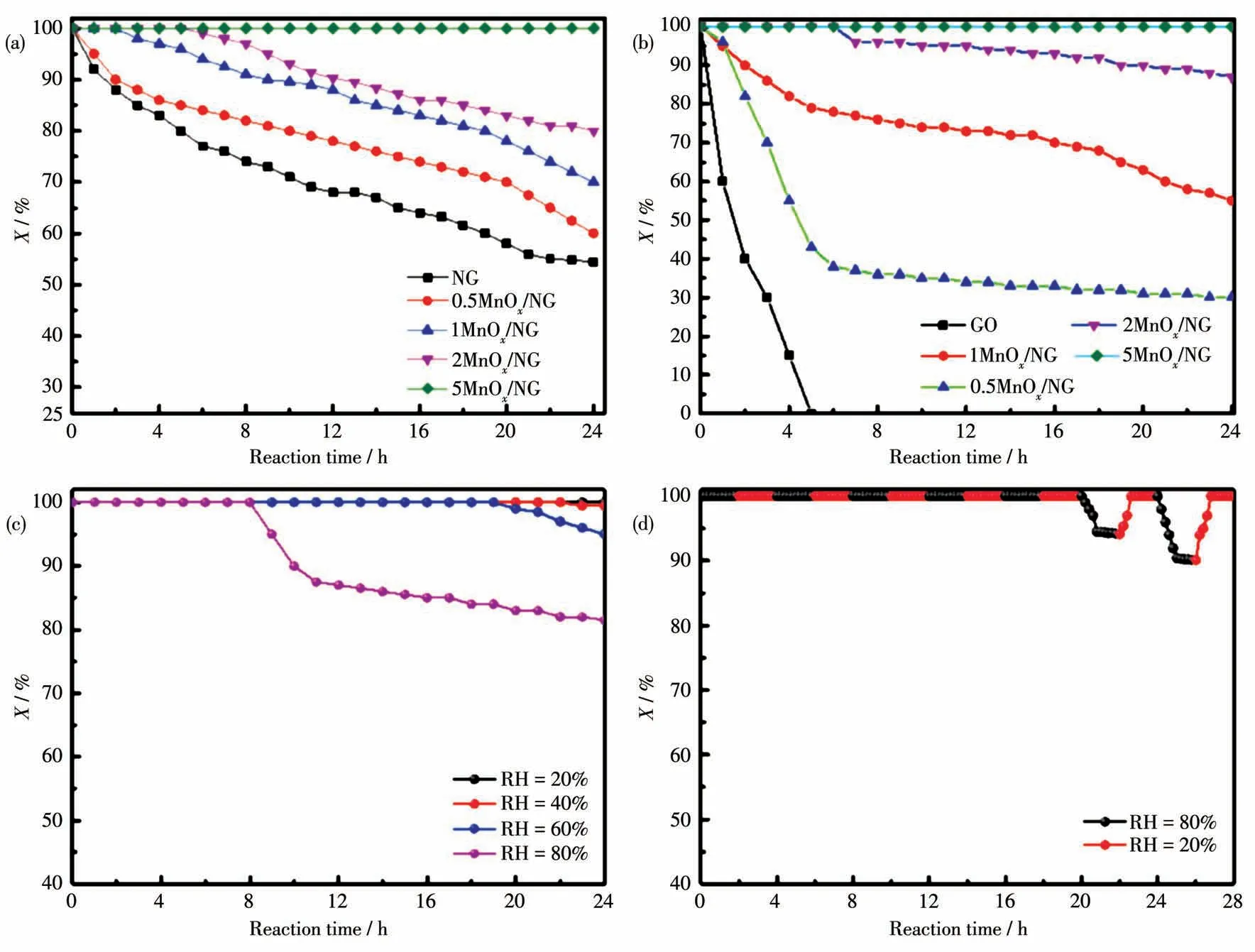
Fig.6 Ozone decomposition rate on(a)nMnOx/NG and(b)nMnOx/GO;(c)Ozone decomposition rate on 5MnOx/NG under different humidities;(d)Ozone decomposition rate on 5MnOx/NG at 20%and 80%RH alternately
2.3 Catalyst surface properties
XPS tests were performed on the samples to analyze the composition and state of the elements in the samples.Fig.7a shows that the elements C,N,Mn,and O are present in the 5MnOx/NG catalyst,and the elemental contents of Mn,O,and N in the 5MnOx/NG catalyst were 1.87%,6.43%,and 10.27%,respectively.The C1sspectrum of NG is shown in Fig.7b,the sharp peaks are attributed tosp2hybridized carbon atoms in graphite,and the two small peaks obtained by peak fitting at 285.8 and 287.5 eV indicate that the carbon atoms bonded to the doped nitrogen atoms aresp2andsp3hybridized carbon atoms,respectively[28],indicating the formation of C—N bond in the NG prepared by thermal annealing.The N1sspectrum is shown in Fig.7c,four peaks can be fitted at 398.2,399.5,400.8,and 402.6 eV.In NG,the total doping amount of N was 10.27%,and the proportions of pyridine nitrogen,pyrrole nitrogen,and graphite nitrogen were 40.47%,31.52%,and 22.62%,respectively.The two peaks at 398.2 and 399.5 eV correspond to pyridine and pyrrole nitrogen,which contribute to the formation ofπconjugated systems with a pair ofp-electrons in the graphene layer.Pyridine nitrogen and pyrrole nitrogen have unpaired electrons,which can interact with thedvirtual orbital of Mn and are beneficial to the stability and dispersion of manganese oxides and the formation of more active sites.When the carbon atoms in the graphene layer are replaced by nitrogen atoms in the form of graphitic nitrogen,the corresponding peak in the high-resolution N1sspectrum is located at 400.8 eV,and the high-energy peak at 402.6 eV is usually identified as nitrogen oxide[29].The N1sspectrum shows that pyridine nitrogen is the main component in the prepared NG.
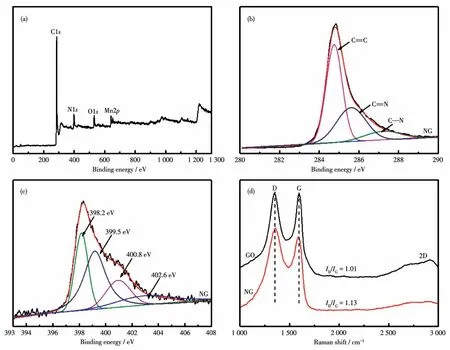
Fig.7(a)XPS survey spectra of 5MnOx/NG;(b,c)C1s and N1s XPS spectra of NG;(d)Raman spectra of GO and NG
The Raman spectra of GO and NG are shown in Fig.7d.The D-band and G-band are corresponding to the peaks of 1 350 and 1 580 cm-1.D-band refers to a k-point phonon pattern withA1gsymmetry,which is a common feature ofsp3defects and disorder in carbon,while G-band is usually anE2gphonon pattern withsp2C=C,which can be used to explain the degree of graphitization[30].The relative peak strength ratio ofID/IGcan be used to characterize the defect characteristics of carbon materials.The strength ratio of NG(1.13)was higher than that of GO(1.01),indicating that moresp3defects are formed in the carbon in NG,which will improve the catalytic decomposition of ozone.The 2D peak is the most significant feature of graphene in the Raman spectra,and its position and shape can clearly distinguish monolayer,bilayer,and less layered graphene.For NG prepared by thermal annealing,the 2D peak appeared at about 2 700 cm-1.Raman spectra showed a wider peak,which indicates that fewer layers of graphene can be obtained from NG prepared by thermal annealing.
The Mn2pXPS results of the fresh and used 5MnOx/NG catalysts are shown in Fig.8,where the catalysts contain three manganese species:Mn2+,Mn3+,and Mn4+species corresponding to three fitted peaks at 641.6,643.1,and 644.4 eV,respectively[31].The mixing of Mn2+,Mn3+,and Mn4+facilitates the ozone decomposition rate[32].The proportions of Mn2+,Mn3+,and Mn4+in the fresh and used catalyst are shown in Table 1,the proportion of Mn2+decreased by 11.3%,the proportion of Mn3+increased by 7.6%,and the proportion of Mn4+increased by 3.7%,indicating that during the catalytic decomposition of ozone,the low-valent Mn2+and Mn3+species are oxidized to produce Mn3+or Mn4+.The strong oxidation of ozone resulted in the decrease of the low-valent manganese and the increase of the high-valent manganese.
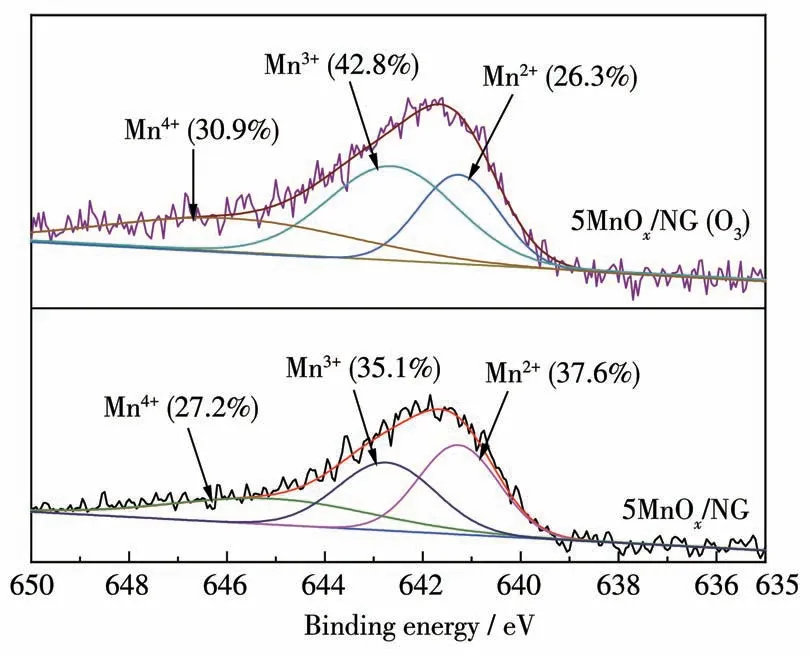
Fig.8 Mn2p XPS spectra of 5MnOx/NG before and after catalytic decomposition of ozone
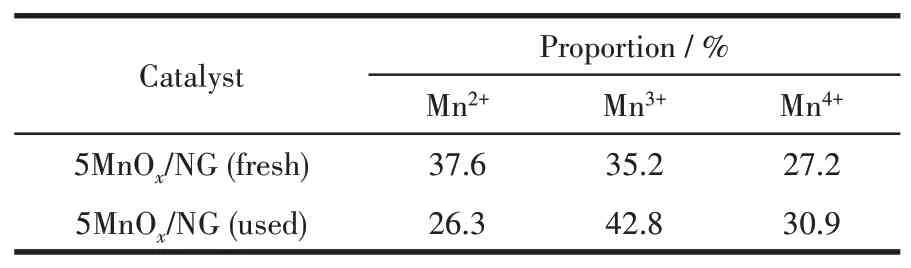
Table 1 Proportions of Mn2+,Mn3+,and Mn4+in the fresh and used 5MnOx/NG catalysts
The O1sXPS results of the fresh and used 5MnOx/NG catalysts are shown in Fig.9.The fitted peaks located at 529.8,531.3,and 533.5 eV belong to the oxygen from lattice oxygen(OL),surface oxygen vacancies(OV),and surface hydroxyl species(OA)[33],respectively.The proportions of OL,OV,and OAin the fresh catalyst were 26.8%,28.7%,and 44.5%,respectively.The proportions of OL,OV,and OAafter treatment with ozone were 19.1%,29.6%,and 51.3%,respectively.The decrease of OLafter treatment with ozone indicates that the low-valent manganese in the catalyst is oxidized to high-valent manganese.Since the high-valent manganese requires more oxygen atoms to be coordinated,it leads to the increase of the content of OVand OAwhen water is adsorbed on the surface.
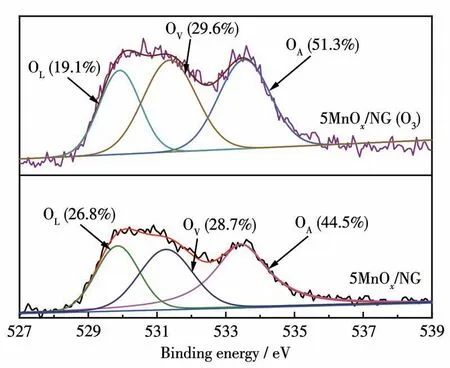
Fig.9 O1s spectra of 5MnOx/NG before and after decomposing ozone
To further confirm the existence of oxygen vacancies,the ESR spectrum of the 5MnOx/NG catalyst was shown in Fig.10.A pair of symmetrical peaks appeared in the magnetic field around 3 500 G,indicating the existence of oxygen vacancies in the catalyst.Through quantitative analysis,the concentration of oxygen vacancies was 1.296×1017spins per mg.
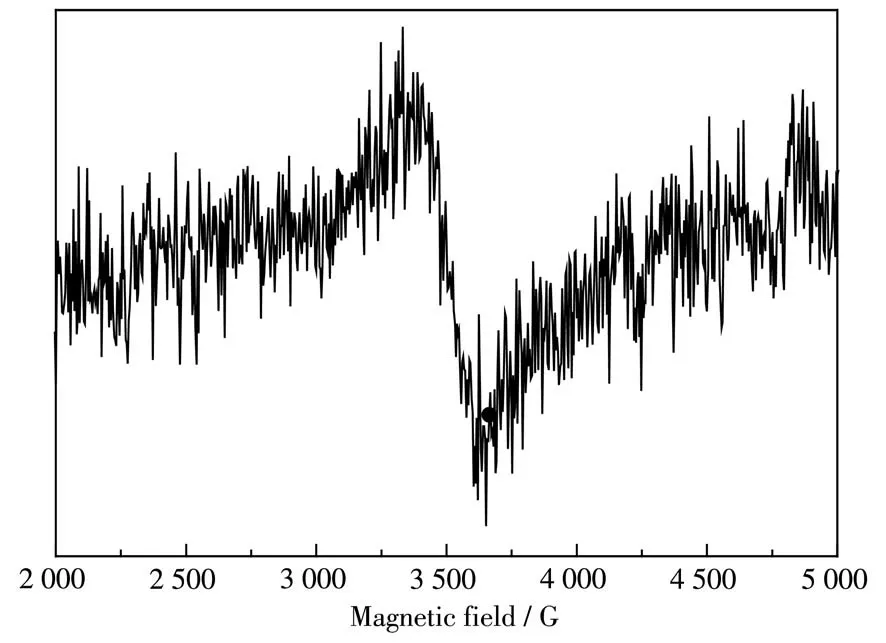
Fig.10 ESR spectrum of 5MnOx/NG
2.4 Ozone decomposition mechanism and moisture resistance analysis
The possible process of catalyst decomposition of ozone is as follows:Firstly,ozone adsorbs on oxygen vacancies and then gets electrons from the Mn2+or Mn3+,which are oxidized to Mn3+or Mn4+,ozone decomposes and releases O2.Secondly,the ozone continues to interact with the oxygen at the reaction site to produce O—O adsorbate and release another molecule of O2.Finally,the O—O adsorbate is desorbed with O2.In the ozone decomposition reaction,the first step reaction belongs to Langmuir-Hinshelwood(L-H)reaction[34-35],i.e.,ozone is adsorbed on the active site.The second step reaction belongs to the Eley-Rideal(E-R)reaction[34-35],where the ozone molecules are not in direct contact with the original oxygen atoms,and the principle is shown in Fig.11.
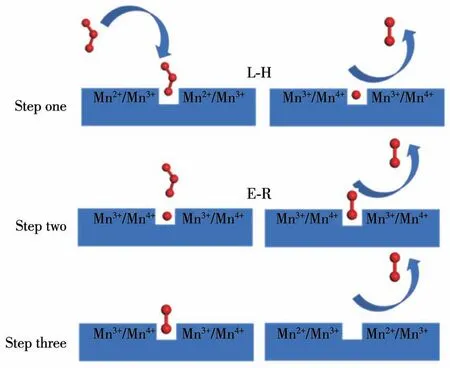
Fig.11 Possible decomposition mechanism of ozone on 5MnOx/NG
The schematic diagram of electron transfer in the catalyst is shown in Fig.12.Firstly,there are a lot of N atoms in NG in the form of pyridine nitrogen,pyrrole nitrogen,and graphitic nitrogen.The lone pair electrons in the N atoms can be transferred to the manganese oxides and accumulate in the active sites,resulting in the low-valent manganese atoms in the catalyst will lose electrons more easily,which promotes the decomposition of ozone.Secondly,the increase of electron density of oxygen vacancies will also be unfavorable to the adsorption of water molecules,thus improving the moisture resistance of the catalyst.
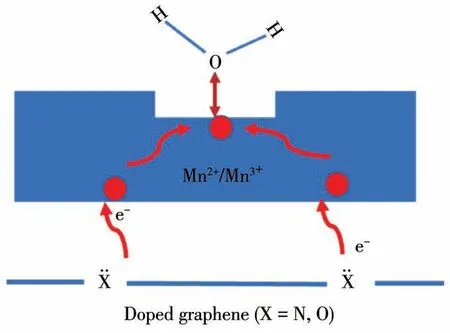
Fig.12 Schematic diagram of electron transfer in nMnOx/NG
3 Conclusions
Nitrogen-doped graphene was prepared by thermal annealing of melamine and graphene oxide.nMnOx/NG was prepared using the impregnation reduction method,where manganese oxide was present as an amorphous oxide.The decomposition of 5MnOx/NG reached 100% for dry ozone and 80% for high RH ozone within 20 h.The lone pair electrons of the nitrogen atoms in nitrogen-doped graphene can be transferred to MnOx,which increases the charge density of the active site,reduces the adsorption energy of water molecules,and enhances the moisture resistance of the catalytic material.
Supporting information is available at http://www.wjhxxb.cn

