镉基杂化晶体的介电相变和蓝白光致发光双重特性
吴 婷 丁 坤 伦蒙蒙 张 铁 张 毅 付大伟
(东南大学,分子铁电科学与应用江苏省重点实验室,有序物质科学研究中心,南京211189)
Multifunctional materials with both photoluminescence and phase transition properties,as a new type of photoelectric materials,possess great potential applications in solar cells,sensors,optoelectronic components,etc[1-5].Organic-inorganic hybrid metal halides have been studied as candidates for light-emitting materials due to their structural tunability,superior stability,easy preparation,etc[6-13].Meanwhile,much attempt has been made to achieve bistable dielectric switch materials through organic-inorganic hybridization[14-20].It is worthwhile to combine reversible phase transitions and luminescence within organic-inorganic hybrids,which will both realize the dielectric switch and achieve tunable luminescence properties[21-23].The phase transitions of crystalline materials may be related to the internal structure of organic cations for their diversity and tunability[24-25].A large amount of organic-inorganic hybrids,such as(pyrrolidinium)MnCl3[26],(R)-and(S)-3-(fluoropyrrolidinium)MnCl3[27],and(3-pyrrolinium)MnCl3
[28],have been proved to be excellent perovskitetype phase-transition dielectrics and ferroelectrics with photoluminescence.More importantly,it was considered to be effective to modify organic cations by introducing halogen,which may consciously tune the dielectric properties of materials[29-34].The course of designing and synthesizing new phase-transition materials by introducing different halogens would open up wireless possibilities.
Therefore,the organic-inorganic hybrid metal halides,as significant functional carriers,offer possibilities for materials with both luminescent and electrical stimuli-responsive properties[35].On the one hand,phase transitions caused by the motion of organic cations would be implemented via tuning organic cation frameworks[5,36-39].On the other hand,it is meaningful to select different inorganic coordination metal ions and coordination modes to design ideal photoluminescence properties[40-42].Low-electronegativity transition metals have usually been reported to exhibit remarkable photoluminescence properties owing to their unfilled valencedorbitals[43],such as manganese-based hybrid materials,based on various metal-ion coordination geometry,often emit different colors with green,red,etc[44-46].Polynucleard10metal spatial cadmium has also attracted much attention for its photoluminescence,optical,and electrical properties,exhibiting blue or blue-white emissions at different excitation wavelengths[47].As we all know,white light emission shows great significance,and single-component white lightemitting materials would become promising candidates for the replacement of traditional solid-state lighting[7].It is fortunate that metal ion withd10configuration,Cd2+,was able to function as a luminescent center to obtain highly efficient broadband white-light emission.Previous studies on Cd halides with white-light emission and dielectric phase change,however,have rarely been reported[48].
Inspired by the regulation of organic cation and the introduction ofd10configuration metal ion Cd2+,we have successfully synthesized two photoelectric materials,(bempy)Br(1)and(bempy)2CdBr4(2)(bempy=1-bromoethyl-1-methylpyrrolidinium).Surprisingly,compound2displayed a reversible phase transition behavior at around 357 K,on the contrary,compound1showed no intention.Furthermore,although both1and2exhibited blue-white emission under UV light,it is worth noting that2showed two emission peaks by introducing the Cd2+ion.It can be speculated that the regulation of the inorganic framework is of first importance for the difference in phase transition behavior and optical properties within such two compounds.Consequently,we have successfully designed a novel multifunctional material that associates reversible dielectric phase transitions with outstanding photoluminescence properties,developing new chances for potential applications in integrated optoelectronic devices.
1 Experimental
1.1 Synthesis
All chemicals and reagents are analytically pure and can be used without further purification.To synthesize compound1,400 mmol ofN-methylpyrrolidine was dissolved in 100 mL of acetonitrile,and then 400 mmol of 1,2-dibromoethane was added dropwise.The mixed solution was stirred at 70℃for 48 h.The reacted mixture was distilled under reduced pressure and washed three times with ethyl acetate to obtain a brown solid,which was the product.
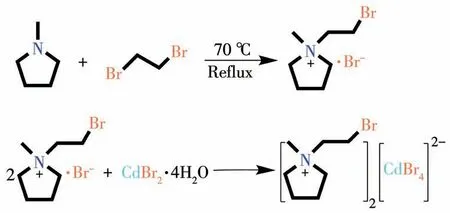
Scheme 1 Synthesis of compounds 1 and 2
Compound1and CdBr2·4H2O were dissolved in the mixed solution of water,methanol,and acetonitrile(1∶1∶1,V/V).The off-white crystals of compound2were obtained by slow evaporation of the mixed solution.The phase purity of the crystal was verified by powder X-ray diffraction(PXRD)and IR analysis(Fig.S1 and S2,Supporting information).
1.2 Physical measurements
Differential scanning calorimetry(DSC),PXRD measurement,dielectric measurements,and photoluminescence spectroscopy measurements are illustrated in the Supporting information.Variable-temperature single-crystal X-ray diffraction data for compounds1and2were collected using a Rigaku Syn007 diffractometer with MoKα(λ=0.071 073 nm)radiation in theωscan mode.
2 Results and discussion
2.1 Thermal properties of 1 and 2
Thermal analysis is an effective means to detect temperature-induced phase transitions.The crystalline powders of both compounds were measured by DSC to confirm the reversible phase transition in the heating/cooling circle.As shown in Fig.1a,no apparent thermal anomaly was observed in compound1.After the smaller anion Br-was replaced by the larger anion group[CdBr4]2-,a pair of endothermic and exothermic peaks appeared at 357.8 K/336.5 K in the heating/cooling cycle,indicating a reversible change(Fig.1b).The comparison of Fig.1a and 1b shows that the strategy of introducing Cd2+to substitute larger metal framework anions for smaller individual inorganic anions to conduct phase transitions works well.As illustrated in Fig.1b,the wide hysteresis of 21.3 K and relative sharp peaks reveal that it is a first-order phase transition.Therefore,compound2undergoes a reversible first-order phase transition at around 357 K.According to the DSC curve,theΔSfor compound2was 34.44 J·mol·K-1.Based on the Boltzmann equation,ΔS=RlnN,in whichRis the gas constant andNis the number of possible orientations of the disordered system,the calculatedNvalue of2was significantly greater than that of1,revealing the order-disorder characteristics of the phase transition.
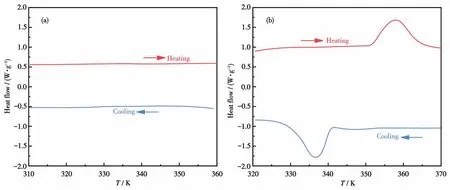
Fig.1 DSC curves of compounds 1(a)and 2(b)
2.2 Crystal structure analysis of 1 and 2
The single-crystal structure of1was obtained by X-ray diffraction measurements.It crystallizes in the orthorhombic non-central space groupPna21witha=1.280 29(16)nm,b=0.667 95(8)nm,c=1.190 9(2)nm,Z=4,andV=1.018 4(2)nm3(Table S1).The asymmetric unit of1consists of a bempy cation and a Br-anion(Fig.2a).All ions are in the ordered normal state.
For compound2,it crystallizes in the monoclinic centrosymmetric space groupP21/cat the low temperature(253 K),witha=1.552 21(7)nm,b=0.967 55(3)nm,c=1.726 45(7)nm,β=107.355(4)°,Z=4,andV=2.474 81(18)nm3(Table S2).The asymmetric unit of2is composed of two bempy cations and one monomeric[CdBr4]2-anion(Fig.2b)that adopts a twisted tetrahedral structure with the Cd—Br bond lengths in the normal range of 0.256 50(8)-0.261 46(8)nm,and the Br—Cd—Br bond angles varying from 105.19(3)° to 111.99(3)°(Table S3).As illustrated in Fig.S3,compound2exhibits a 0D stacking structure at 253 K.As the temperature increased,the thermal motion of the molecules became more intense,bringing about the deterioration of the diffraction data,thus,we cannot obtain the specific high-temperature structure of compound2.

Fig.2 Asymmetric unit of compounds 1(a)and 2(b)
2.3 Variable-temperature PXRD analysis of 2
Variable-temperature PXRD experiments were fulfilled on compound2to further investigate more about the structural phase transition.As represented in Fig.3,the difference in diffraction peaks was almost negligible below the phase transition point.However,the quantity and location of diffraction peaks changed more obviously when the temperature reached the phase transition point,especially in the 2θdegree range from 18°to 30°.The peak at 18.2°remained,but the peak at 19.1° disappeared.Simultaneously,diffraction peaks at 20.3°,21.5°,and 22.7° were merged into two diffraction peaks at 21.2° and 22.3°.Besides,the diffraction peaks below 18° and over 30° have also shown diminutive distinction.Noticeable changes in diffraction peaks from low temperature to high temperature can be a piece of strong evidence to prove the occurrence of structural phase transition.
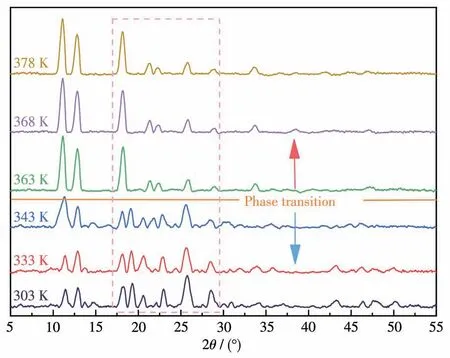
Fig.3 Variable-temperature PXRD patterns of 2
2.4 Dielectric properties of 1 and 2
The temperature-dependent dielectric measurement is a reliable method to identify phase transitions as the dielectric constant,correlated with the nature of the phase transition,shows obvious abnormalities around the phase transition temperature.Compound1had no obvious dielectric anomalies in the heatingcooling curves(Fig.S4),conversely,compound2displayed a prominent step-like dielectric anomaly during the heating-cooling process,revealing a reversible phase transition(Fig.4a),which matched the DSC analysis well.
For compound2,a remarkable stepped dielectric anomaly was exhibited at 354 K after being heated.During the cooling cycle,the ε′of2decreased gradually to around 20 at high temperatures and then decreased rapidly at around 351 K,continuing to decrease slowly from 346 K,which manifested a clear slope in the curve.The abrupt change in the reversible dielectric constant in the heating and cooling curves confirms the existence of the reversible phase transition.To further detective the relationship between the dielectric constant and frequencies during the phase transition,the dielectric constant of2was measured at frequencies of 5,10,100 kHz,and 1 MHz.It illustrates that the phase transition temperature was independent of frequencies but the dielectric constant value decreased with the frequencies increasing(Fig.4b).
2.5 Photoluminescence properties of 1 and 2
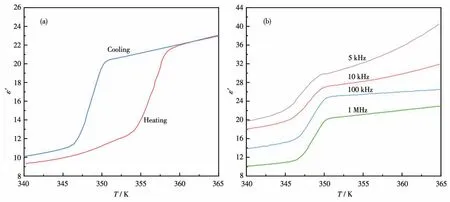
Fig.4(a)Real part(ε′)of the dielectric constant of 2 at 1 MHz in the heating and cooling curves;(b)Real part(ε′)of the dielectric constant of 2 at 5,10,100 kHz,and 1 MHz in the cooling curves
The photoluminescence properties and intrinsic mechanisms of compounds1and2were investigated at room temperature.The excitation spectra with a peak of around 300 nm for both compounds are shown in Fig.S5.The emission spectra of compounds1and2were obtained at the excitation wavelength of 307 nm(Fig.5a and 5b).It can be seen that1exhibited an emission maximum at 538 nm with a Stokes shift of 231 nm.For compound2,in addition to an emission peak at 547 nm,another emission peak appeared at 750 nm,which may be caused by[CdBr4]2-anion.Both compounds exhibited broadband emissions that are attributed to blue-white light.The CIE chromaticity coordinates of the light emission were calculated as(0.271 1,0.406 2)for1and(0.280 0,0.417 9)for2,respectively(Fig.6a).The images in Fig.6b and 6c represent the luminescence of1and2under UV irradiation,respectively,both of which show blue-white light emission.Compound1emitted very weak fluorescence under UV light,which may be caused by intermolecular interactions,while the fluorescence of compound2was slightly stronger,but not enough to be visible under natural light.
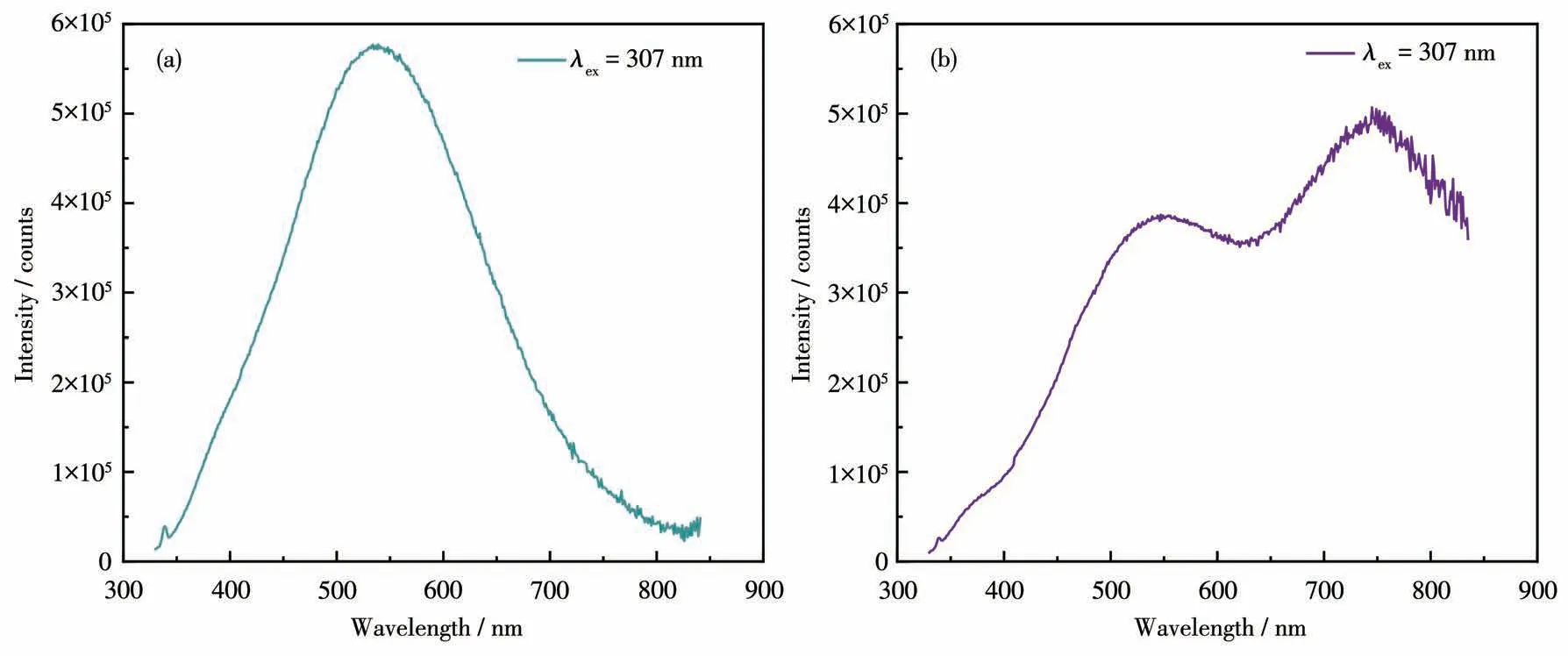
Fig.5 Emission spectra of compounds 1(a)and 2(b)
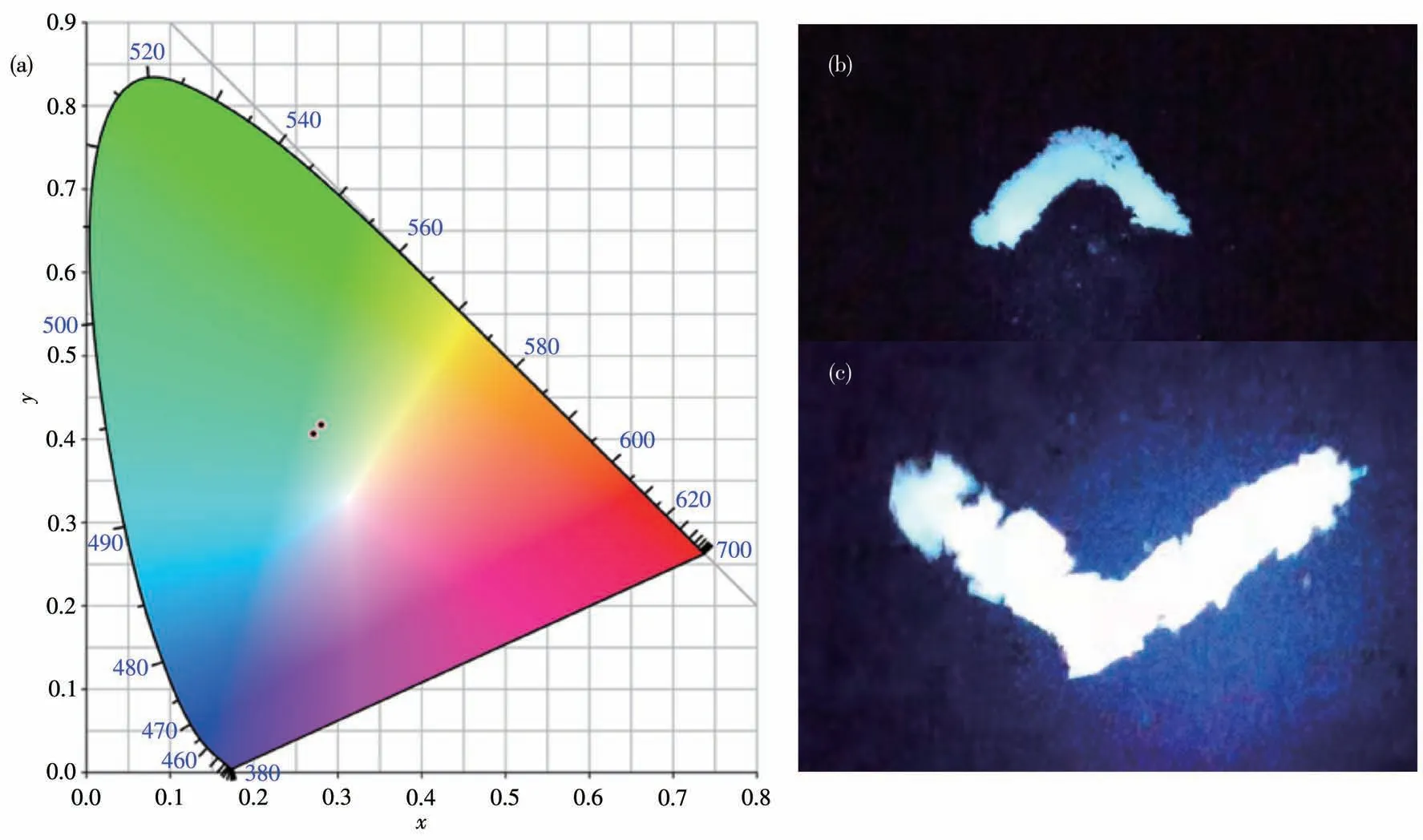
Fig.6(a)CIE chromaticity coordinates of 1(left)and 2(right);Photographs of powder of(b)1 and crystals of(c)2 under UV light irradiation
Structure determines properties.To explore the essential reasons for photoluminescence,the structural compositions of compounds1and2were determined.Compared with the emission spectra of1and2,at the same excitation wavelength,the emission peaks at around 540 nm were roughly the same location,but with different intensities.The emission peak of2was weaker than that of1.It is speculated that the photoluminescence properties at around 540 nm are mainly derived from the organic cation bempy+.Theoretically,the position of the fluorescence peak is influenced by compound structures and has nothing to do with the excitation wavelength.When Cd2+was introduced into the inorganic framework,the anions-cations interaction might reduce the photoluminescence intensity and produce a new emission peak at 750 nm.For2,the emission intensity at 750 nm was significantly stronger than that at 547 nm.Moreover,the broadband blue-white light emission is ascribed as a combination of free exciton emission and self-trapped exciton emission[48].The enlargement of the anion leads to a change in the emission peaks.Therefore,it is an effective strategy to change the position of the emission peaks through the regulation of the inorganic framework to obtain desired fluorescence properties.Regrettably,the disadvantage is that the quantum yields of1and2were not higher enough,and future research needs to focus on improving the quantum yield.
3 Conclusions
In summary,we have successfully prepared two crystalline compounds,in which the organic-inorganic hybrid halide(bempy)2CdBr4(2)showed a reversible phase transition and efficient blue-white emission by introducing Cd2+.In addition,the mechanism of its photoluminescence behavior may be originated from the synergistic effect of organic and inorganic components,and the intrinsic factor of broadband blue-white light emission probably benefits from the combination of free exciton emission and self-trapped exciton emission.Our work provides a design option for exploring multifunctional materials with more remarkable reversible phase transitions and tunable luminescence properties.
Supporting information is available at http://www.wjhxxb.cn

