三(三甲基硅烷)类添加剂助力5 V镍锰酸锂电池实用化
张立恒顾海涛罗 英董庆雨沈炎宾*,解晶莹*,,
(1哈尔滨工业大学化工与化学学院,哈尔滨150001)
(2上海空间电源研究所,空间电源技术国家重点实验室,上海200245)
(3中国科学苏州纳米技术与纳米仿生研究所国际实验室卓越纳米科学中心,苏州215123)
0 Introduction
High energy density,better safety,and low price of the next generation lithium-ion battery(LIB)are expected for power energy storage systems by continually optimizing these existing battery chemistries[1-5].Cathode materials with higher operating voltage can further increase their energy density than the ones with high discharge capacity alone[6-7].So far,numbers of cathode materials with high discharge platforms have been reported[8-11].One of the representatives is spinel LiNi0.5Mn1.5O4owing to its high lithium-ion utility ratio,safety,superior rate capability,and high discharge platform of 4.7 V(vs Li/Li+)[12-13].However,its practical application in commercial cells is still confronting several challenges,such as low Coulombic efficiency(CE)and poor cyclability[14-18],which is essentially caused by catalytic and oxidative decomposition of electrolyte components on the surface of charged LiNi0.5Mn1.5O4electrodes[19].Nowadays,numerous studies have shown that functional additives can improve interface stability and cycle performance[20].Among those additives,siloxanes-based additives are widely investigated to relieve the decomposition of electrolyte components and suppress the self-discharge behavior of LiNi0.5Mn1.5O4electrodes by building a protective layer,such as tris(trimethylsilyl)borate(TMSB)and tris(trimethylsilyl)phosphite(TMSPi)[21-27].Their trimethylsilyl groups display high HF-scavenging ability,contributing to enhancing the stability of interface layers and inhibiting the dissolution of transition metal ions from the bulk cathodes.However,their comparative evaluations in practical LiNi0.5Mn1.5O4-based cells and the difference of effect mechanism in LiPF6-based electrolyte have not been reported systematically.It is also unclear how the central atom of tris(trimethylsilyl)-based additive affects the interface properties of highvoltage cathode and graphite anode in practical application.
This work provides a comparative study on TMSB and TMSPi alone as electrolyte additives for LiNi0.5Mn1.5O4electrodes.Combining density functional theory(DFT)computation and electrochemical characterization,the possible mechanism of TMSB and TMSPi in ethylene carbonate(EC)-LiPF6-based electrolytes was systematically explored.Meanwhile,their effect on graphite anode and the pouch cells was also concerned in this work.
1 Experimental
1.1 Electrolyte and electrode preparation
The baseline electrolyte(BE)composed of 1.2 mol·L-1LiPF6and EC/ethyl methyl carbonate(EC/EMC,3∶7,V/V)was purchased from DoDoChem(China,H2O content:ca.8.4 mg·L-1).1%TMSB and 1%TMSPi(TCI chemicals,99%)were introduced to the BE and stored for 24 h in an argon-filled glovebox(PRS226/S10-0963,H2O content<0.1 mg·L-1)to follow-up measurement.
To evaluate the electrochemical performance of the BE and additives-containing electrolytes,2025-type coin cells and 0.5 Ah Graphite||LiNi0.5Mn1.5O4pouch cells were fabricated.The cathode was prepared by coating the mixture LiNi0.5Mn1.5O4powders(XingNeng New Materials Co.,Ltd.),Super P(Amerys),and polyvinylidene fluoride(PVDF,HSV900,Solvay)(mass ratio of 90∶5∶5)onto Al current collector with mass loading of 22.0 mg·cm-2.For coin cells,the areal mass loading was 4.0 mg·cm-2.The anode was prepared by coating the mixture of graphite(G25,Shanshan Tech Co.,Ltd.),Super P,sodium carboxymethyl cellulose(CMC,Walocel CRT2000 PPA12,Dow Wolff Cellulosics®),and styrene butadiene rubber(SBR,Lipaton SB5521,Synthomer)(mass ratio of 93∶2∶2∶3)on the Cu foil.For coin cells and pouch cells,the areal mass loading of the graphite electrode was 9.0 mg·cm-2.The 0.5 Ah pouch cells were designed and assembled with the negative/positive area-capacity ratio(N/P)ofca.1.18(according to the calculation with 130 mAh·g-1for cathodes and 330 mAh·g-1for graphite anode)in the drying room.The Celgard 2325 separator was used in all these cells.
1.2 Electrochemical measurement
Charge-discharge tests were performed on the LAND test system(CT 2001A)with a voltage range of 3.5-5.0 V for Li||LiNi0.5Mn1.5O4coin cells and 3.5-4.9 V for Graphite||LiNi0.5Mn1.5O4pouch cells.Electrochemical impedance spectra(EIS)were obtained on VMP-300 electrochemical workstation(Bio-Logic)with a voltage amplitude of 10 mV and a frequency range of 5 MHz-0.1 Hz.The electrochemical behaviors of electrolytes were also examined by linear sweep voltammetry(LSV)test using Li||Al coin cell at a scan rate of 0.1 mV·s-1.For cyclic voltammetry(CV)measurements,the experiments were also conducted on VMP-300 using Li||LiNi0.5Mn1.5O4coin cells with a potential range from the open circuit voltage(OCV)to 6.0 V at a scan rate of 0.1 mV·s-1.
1.3 Characterization
The ionic conductivities of the electrolytes were measured by a METTLER TOLEDO Seven Direct SD30(Inlab 710 electrode).The electrode morphology was collected by scanning electron microscope(SEM,S4800,Hitachi,Japan)operated at 5 kV.The interface layers were observed by transmission electron microscope(TEM,JEOL JEM-2100F)operated at 200 kV.The X-ray diffraction(XRD)patterns of fresh and cycled electrodes were collected on a Bruker D8 Advance(Germany)diffractometer at a scan rate of 5(°)·min-1and the range of 10°-80° using CuKαradiation withλ=0.151 48 nm under 40 kV and 40 mA.The X-ray photoelectron spectroscopy(XPS)was carried out on AXIS-ULTRA DLD-600W(SHIMADZU-KRATOS.Co.,Ltd.)to assess the interface composition of cycled electrodes,and the pass energy was fixed at 30.0 eV.Inductively coupled plasma(ICP)tests were conducted by the Thermofisher iCAP-7200 Duo instrument.Importantly,before the SEM,TEM,XRD,ICP,and XPS analysis,cycled electrodes were rinsed with pure dimethyl carbonate(DMC)to remove superfluous electrolyte and dried for 12 h at 50℃in an argon-filled glove box,then stored in the bottles for the subsequent tests.
1.4 Theoretical calculations
Geometry optimization of molecules was executed by the Dmol3module in Material Studio Software.A frequency analysis was obtained at DFT.Moreover,the relative Gibbs free energy was calculated at 298.15 K.
2 Results and discussion
2.1 Reactivity of selected additives in EC-LiPF6-based electrolyte
HOMO energy level based on the molecular orbital theory has been widely adopted to predict the oxidation tendency of organic molecules[28].According to DFT calculation results,as shown in Fig.1a,TMSB and TMSPi are prone to lose electrons for their high HOMO(-7.70 and-6.26 eV,respectively)relative to EC(-8.31 eV)and EMC(-7.96 eV).Therefore,they can be preferentially oxidized and contribute to the formation of protective film on the charged LiNi0.5Mn1.5O4electrodes.LSV tests were adopted with Li||Al cells from OCV to 5.5 V(vs Li/Li+)at a scan rate of 0.1 mV·s-1to evaluate the oxidation potential and electrochemical reactivity of additives.As shown in Fig.1b,the LSV curves showed that TMSB began to be oxidized over 3.7 V(vs Li/Li+)with relatively high oxidation current as the potential increase,displaying higher reactivity under high voltage,which may affect the initial CE of cells.By contrast,TMSPi appeared to be oxidized at a lower potential though the oxidation current was relatively small,displaying a higher electrochemical window,indicating that TMSPi had a faster filmpassivating ability at comparatively low potential than TMSB.The CV result in Fig.S1(Supporting information)is consistent with the above conclusions.
Moreover,TMSB and TMSPi,especially for TMSPi,display high reactivity with HF molecule via the P—O and O—Si cleavage reaction,as shown in Fig.2,which is in good harmony with the report about the HF-scavenging ability of TMSPi in EC-LiPF6based electrolyte[29-30].Similarly,the reactions of TMSB or TMSPi with LiF via the O—Si bond cleavage are thermodynamically favorable.Moreover,electron deficiency of B in TMSB also displays a high affinity with F-,as shown in Fig.S2,which can reasonably accelerate the dissolution of LiF in interface layers,resulting in reduced interface impendence among cycling.
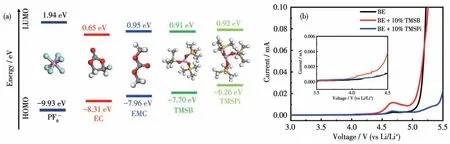
Fig.1(a)Chemical structure and its HOMO/LUMO energy level ofPF6-andorganic molecules;(b)LSV curves from OCVto5.5V(vsLi/Li+)at a scanrate of 0.1 mV·s-1 indifferentelectrolytes
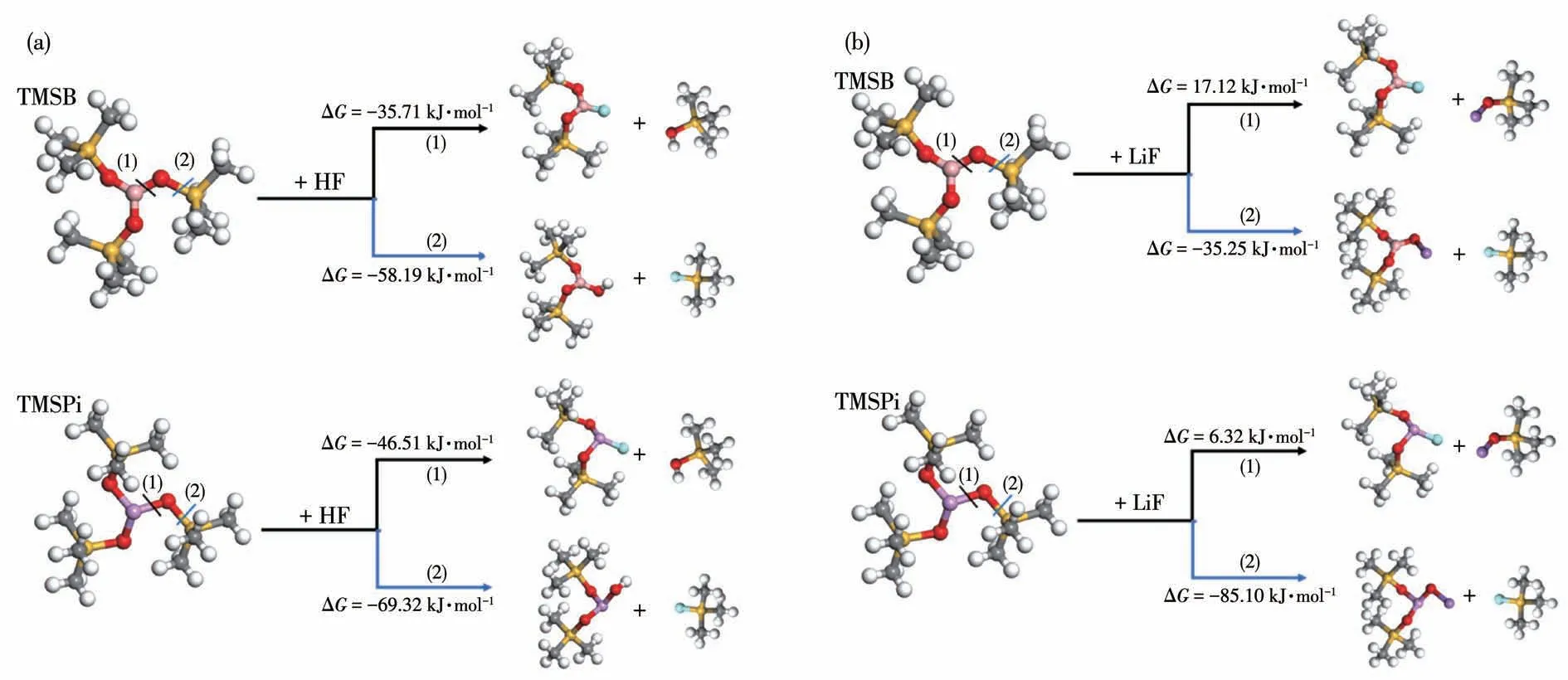
Fig.2 Reaction energies(ΔG in kJ·mol-1)of TMSB and TMSPi with HF(a)or LiF(b)molecule by Dmol3 module
2.2 Electrochemical performance of Li||LiNi0.5Mn1.5O4 electrode
Fig.3 shows the cycling stability of Li||LiNi0.5Mn1.5O4coin cells in BE and additive-containing electrolytes at 1C and 25℃after precycling at 0.2C for three cycles.The 1% addition(mass fraction)was decided by the reported literature[22].LiNi0.5Mn1.5O4electrodes in additive-containing electrolytes showed excellent cycling stability,without noticeable capacity fading over 200 cycles.Its capacity-retention was improved from 85.9%(BE,average CE of 99.2%)to 92.3%(TMSBcontaining electrolyte)and 95.1%(TMSPi-containing electrolyte)along with an enhanced CE of 99.4%.It is worth noting that there exists sharp capacity fading in BE after 150 cycles,mainly due to the massive consumption of electrolytes on the surface of LiNi0.5Mn1.5O4and the breakdown of the electrically conductive network[31].Significantly,both TMSB and TMSPi can hinder the capacity fading of LiNi0.5Mn1.5O4,suggesting that two selected additives can effectively stabilize the cathode/electrolyte interface and relieve the decomposition of electrolyte components.Moreover,as shown in Fig.3a and 3f,LiNi0.5Mn1.5O4electrode in TMSPicontaining electrolyte exhibited a higher discharge platform and specific capacity with lower polarization(Fig.3c)than that in BE or TMSB-containing electrolyte,suggesting that TMSPi-derived cathode interface layer(CEI)exhibits faster kinetics of the charge transfer on the surface of the LiNi0.5Mn1.5O4electrodes[27,29].The medium-voltage of the LiNi0.5Mn1.5O4electrode during cycling is displayed in Fig.3b.Clearly,its discharge platform kept dropping during cycling in BE,mainly ascribing to the increased cell impedance[31].Surprisingly,both two selected additives can effectively hinder the platform voltage dropping,especially for TMSPi-containing electrolytes,as shown in Fig.3b and 3d-3f.The results show that LiNi0.5Mn1.5O4in TMSPicontaining electrolyte can maintain a higher discharge platform than others during cycles,which may mainly be due to its low impedance and fast Li+transport in this phosphate-containing protective layers.
The resistance changes of Li||LiNi0.5Mn1.5O4cells before and after cycling in different electrolytes are shown in Fig.4.EIS curves of LiNi0.5Mn1.5O4mainly comprise electrolyte resistance(Rs),interfacial resistance(RSEI),charge transfer resistance(Rct),and Li+diffusion(Wf,a slop line at low frequency)in electrode[32].Table 1 shows the corresponding fitting data ofRSEIandRct.The result indicates thatRSEIof LiNi0.5Mn1.5O4electrode after 200 cycles in BE was higher than that of additive-containing electrolytes,resulting from the oxidative decomposition of electrolyte components.Above all,RSEIandRctbefore and after 200 cycles in additivecontaining electrolytes had no obvious change,revealing the electrically conductive network and interface layers of LiNi0.5Mn1.5O4electrodes remain stable during cycling.Significantly,RSEIof LiNi0.5Mn1.5O4electrode in TMSB-containing electrolyte after 200 cycles was slightly decreased,mainly owing to the strong affinity of B with F-and PF6-,leading to the dissolution of LiF and stabilization of electrolytes.While LiNi0.5Mn1.5O4electrodes in TMSPi-containing electrolyte show the lowest initial cell impedance,conducive to its high discharge platform.
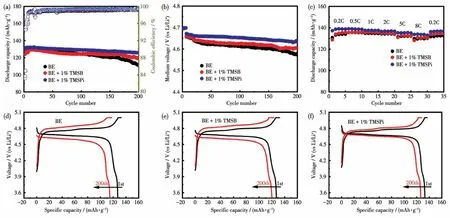
Fig.3(a)Cyclic performance and(b)medium voltages of Li||LiNi0.5Mn1.5O4 half cells in different electrolytes at 1C;(c)Rate capability of Li||LiNi0.5Mn1.5O4 half cells in different electrolytes;(d-f)Charge-discharge curves of Li||LiNi0.5Mn1.5O4 half cells at 1C before and after 200 cycles in BE and additive-containing electrolytes

Fig.4 EIS spectra and fitting results of the Li||LiNi0.5Mn1.5O4 half cells before and after 200 cycles in BE(a),TMSB-containing(b),and TMSPi-containing(c)electrolytes at 1C and 25℃
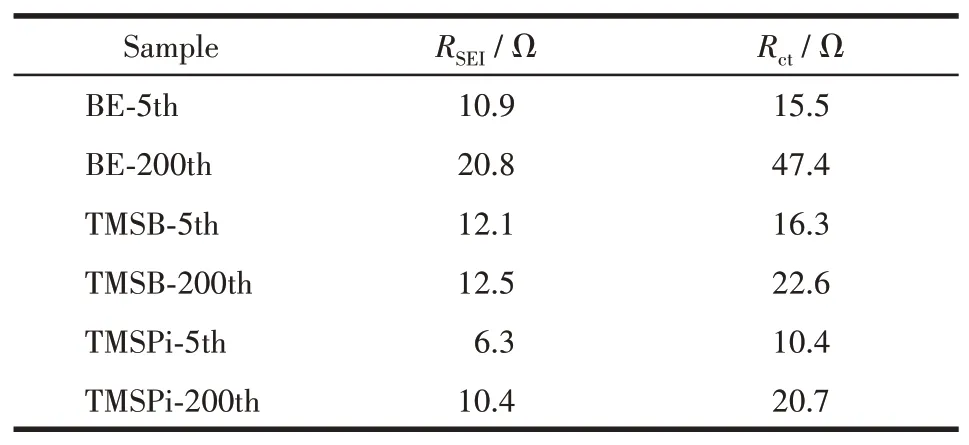
Table 1 Fitting resistances of the cycled Li||LiNi0.5Mn1.5O4 cells in different electrolytes
Though the above work has suggested that low mass loading(4.0 mg·cm-2)and excessive electrolyte can support the Li||LiNi0.5Mn1.5O4cells to achieve acceptable cycle performance in BE or additivecontaining electrolytes,it has no clear distinction between the passivating ability of additives on the surface of LiNi0.5Mn1.5O4electrodes.Complex factors include oxidative decomposition of electrolyte components,the breakdown of electrically conductive networks,and others,all of which can cause the fading of battery performance.Conversely,with high mass loading and short cycles,the capacity degradation of Li||LiNi0.5Mn1.5O4cells is mainly derived from the oxidative decomposition of electrolyte components.As shown in Fig.S3,the LiNi0.5Mn1.5O4electrodes(22.0 mg·cm-2)in BE suffered severe capacity fading after 50 cycles at 0.5C with a low CE of 98.7%,resulting from the massive decomposition of electrolyte components.Significantly with selected additives,the cycling stability of Li||LiNi0.5Mn1.5O4was dramatically enhanced,especially for TMSPi with a high CE of 99.4%.It can be inferred that the ability of TMSPi on passivating the LiNi0.5Mn1.5O4electrode interface is superior to TMSB.
2.3 Morphology and interface layer of the LiNi0.5Mn1.5O4 electrode
Fig.5 shows the morphology and interface layer of the fresh and cycled LiNi0.5Mn1.5O4electrode.Different from the fresh electrode,the surface of LiNi0.5Mn1.5O4particles after cycling in BE is covered with amorphous products,mainly resulting from the oxidative decomposition products of electrolyte components and binders[20,33].Few amorphous products were found on the surface of LiNi0.5Mn1.5O4electrodes after cycling in additive-containing electrolytes,as shown in Fig.5c-5d,indicating both TMSB and TMSPi can effectively relieve this decomposition reaction.Moreover,TEM results showed a similar thickness of interface layer on the surface of LiNi0.5Mn1.5O4particle after cycling in additive-containing electrolytes(2-3 nm),which is thinner and more uniform than that in BE(15-20 nm).Despite,the surface of LiNi0.5Mn1.5O4electrode after cycling in TMSPi-containing electrolyte(Fig.5d and 5h)was not smooth as that in TMSB-containing electrolyte,existing a few discernible amorphous products,mainly due to the trace deposition of by-product from the high chemical reactivity of TMSPi with LiPF6.
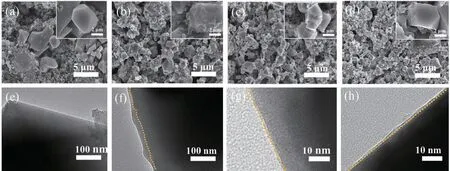
Fig.5 SEM and TEM images of the fresh LiNi0.5Mn1.5O4(a,e)and LiNi0.5Mn1.5O4 after 200 cycles in BE(b,f),TMSB-containing(c,g),and TMSPi-containing(d,h)electrolytes
2.4 Change of crystal structure for the LiNi0.5Mn1.5O4 electrode
The crystal structure changes of the pristine and cycled LiNi0.5Mn1.5O4are shown in Fig.S4.Compared with the fresh electrodes,the main diffraction peaks of LiNi0.5Mn1.5O4electrodes at(311)and(400)were weakened after 200 cycles in BE.Surprisingly,the intensities of main diffraction peaks of LiNi0.5Mn1.5O4electrodes after cycling in additive-containing electrolytes keep strong,indicating that the selected additives can protect the crystal structure from disintegrating for longterm cycling.Especially for TMSPi-containing electrolytes,the XRD patterns of the cycled LiNi0.5Mn1.5O4electrode showed higher intensity among them,indicating TMSPi is more beneficial to the structural stability of LiNi0.5Mn1.5O4.
Elemental analysis of Li||LiNi0.5Mn1.5O4cells after 200 cycles was performed by ICP tests on the Li metal anode,as shown in Fig.6.Significant Mn was deposited on the lithium metal anode after cycling in BE.However,with the selected additives,the Mn concentration was greatly reduced,especially for TMSPi-containing electrolytes.Therefore,both two additives can effectively prevent the dissolution of transition metal from the bulk LiNi0.5Mn1.5O4and stabilize its crystal structure.Significantly,TMSPi does much better than TMSB,which is in line with XRD data.
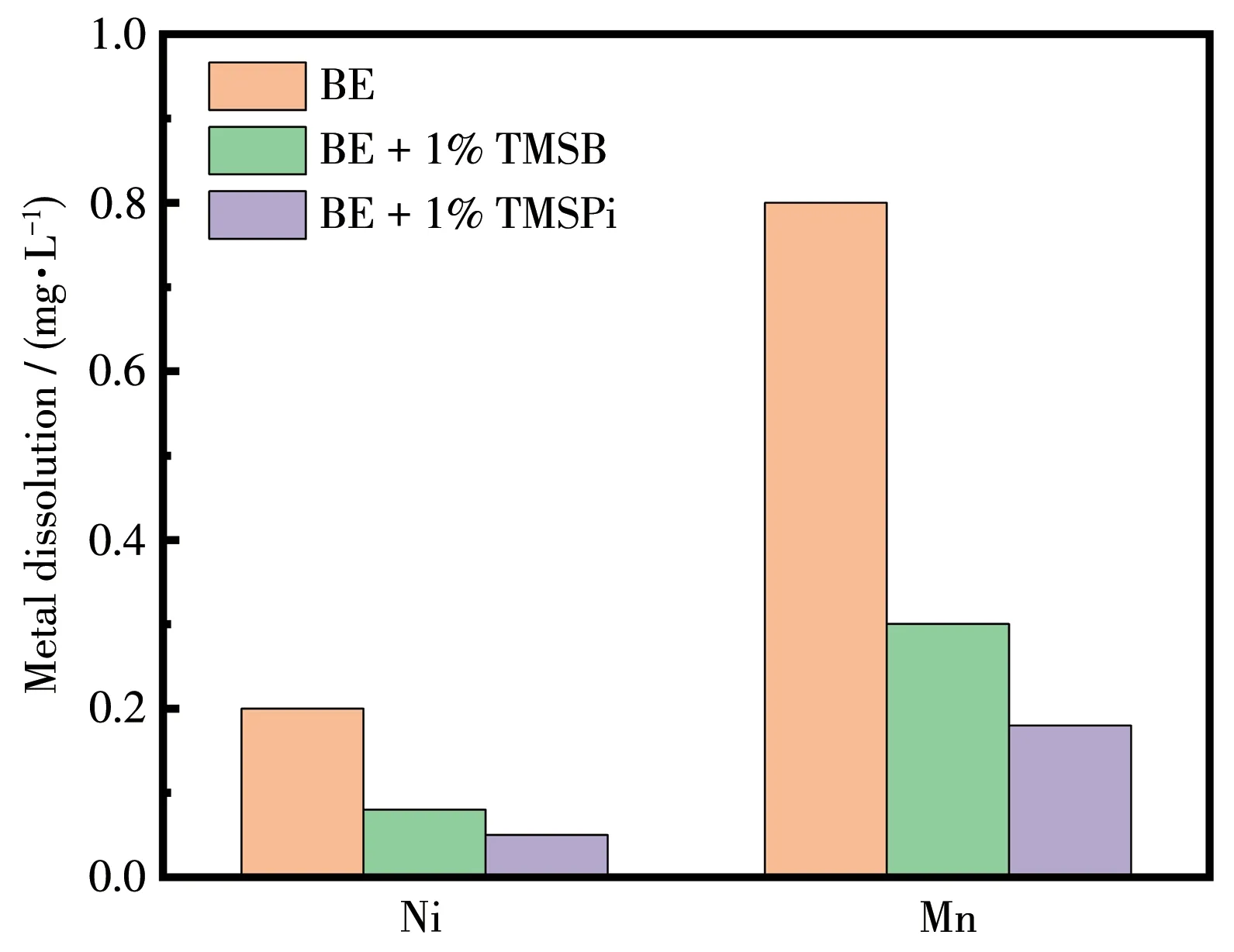
Fig.6 ICP results about Mn and Ni concentration on Li metal anode after 200 cycles
2.5 Surface compositions of the LiNi0.5Mn1.5O4 electrode
The analysis of surface compositions was identified by XPS.As shown in Fig.7,the P2p,F1s,O1s,and C1sspectra of the LiN0.5Mn1.5O4electrodes after 200 cycles in different electrolytes.In the P2pspectra,the peaks of LixPFy(136.7 eV)and LixPOyFz(134.1 eV)represent the decomposition products of LiPF6and they were reduced in TMSB-containing electrolyte.This result is consistent with F1sspectra(LixPFy),indicating the addition of TMSB can suppress the decomposition of the LiPF6.It may owe to the electron-withdrawing of boron acting as a stabilizer for LiPF6.Moreover,the trimethylsilyl group can react with HF and LiF to form a strong Si—F bond,reducing the content of high resistance of LiF.The peak at about 529.6 eV in O1sspectra is attributed to metal—O(M—O)in LiNi0.5Mn1.5O4.For the LiNi0.5Mn1.5O4electrodes after cycling in additivecontaining electrolytes,the intensity of M—O is considerably stronger than that in BE,indicating a thinner surface film is formed in additive-containing electrolytes,which is in line with TEM data.Besides that,the peak corresponding to C—O(533.6 eV)and C=O(531.7 eV)in O1sspectra correspond with the C1s spectra(Fig.7d).The C—O bond,mainly caused by the decomposition of organic carbonate solvents,is slightly weaken in the additive-containing electrolytes,suggesting the decomposition of the electrolyte can be relieved by adding additives.Moreover,the C—Si bond was detected in the C1sspectra on the LiNi0.5Mn1.5O4surface with TMSB-containing electrolyte,which is a benefit to forming a more stable Si-rich protective film[34].
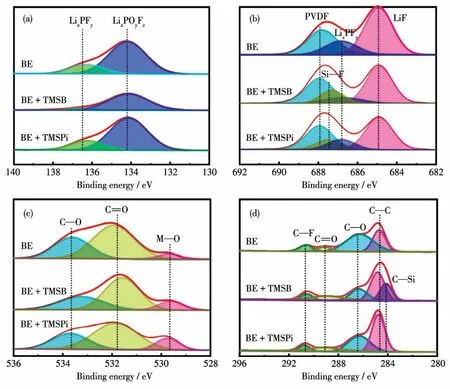
Fig.7 XPS spectra of the CEI layer of the LiNi0.5Mn1.5O4 cathodes in different electrolytes after 200 cycles at 1C;(a)P2p,(b)F1s,(c)O1s,and(d)C1s
2.6 Effect of the selected additives on graphite electrode
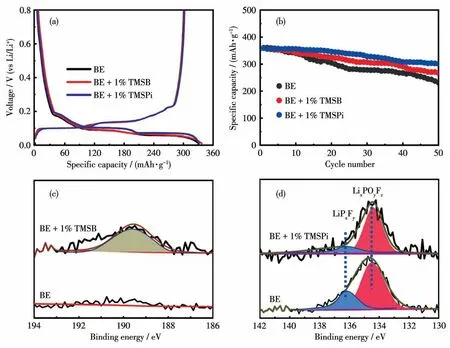
Fig.8(a)Initial charge and discharge curves and(b)cycle performance of the graphite anodes in BE and additive-containing electrolytes;(c)B1s XPS spectra of the graphite anodes after 50 cycles in BE and TMSB-containing electrolytes;(d)P2p XPS spectra of the graphite anodes after 10 cycles in BE and TMSPi-containing electrolytes
For its practical application of additive-containing electrolytes in full cells,the effect of additives on the anode should not be overlooked.The Li||Graphite cells were assembled with BE and additive-containing electrolytes.Fig.8a shows the initial discharge/charge curves of Li||Graphite cells at 0.1C.Obviously,the discharge curve of the graphite electrodes in TMSBcontaining electrolytes almost coincided with that in BE.When cycling in TMSPi-containing electrolyte,the Li||Graphite cells showed a highest discharge platform and a lowest onset charge potential(0.043 V(vs Li/Li+))than BE(0.061 V(vs Li/Li+))and TMSB-containing electrolyte(0.051 V(vs Li/Li+)).It reveals that the selected additives are more conduce to decreasing the cell polarization of graphite anode,especially for TMSPi.Moreover,the TMSB/TMSPi-containing electrolytes,especially for TMSPi,can efficiently improve the electrochemical performance of Li||Graphite cells,as shown in Fig.8b.More borate or phosphate-containing species were also detected from the surface of graphite anode in the additives-containing electrolyte(Fig.8c,8d),which is beneficial to Li+transportation.However,theoretical calculation showed that TMSB(0.91 eV)and TMSPi(0.92 eV)had higher LUMO values than EC(0.65 eV),indicating that they are difficult to be reduced in presence of EC via the electrochemical reaction on a graphite surface.Yim et al.has proved that the spontaneous decomposition reactions for neutral or cation state TMSPi and TMSB are thermodynamically forbidden[35].However,it is highly likely to undergo attack by nucleophiles(CH3O-)on the graphite anode surface to participate in the SEI layer when TMSPi and EC coexist.The calculation results in Fig.S5 showed that the Li+affinity was greater for TMSPi than for EC,indicating TMSPi is likely to form an association with Li+in the presence of EC.It is thermodynamically favorable for Li+-TMSPI and CH3O-to react via O—Si bond cleavage,leading to the formation of((CH3)3SiO)2POLi and(CH3)3SiOCH3,participating in the formation of SEI layer on a graphite electrode.
2.7 Cycling stability of Graphite||LiNi0.5Mn1.5O4 pouch cells
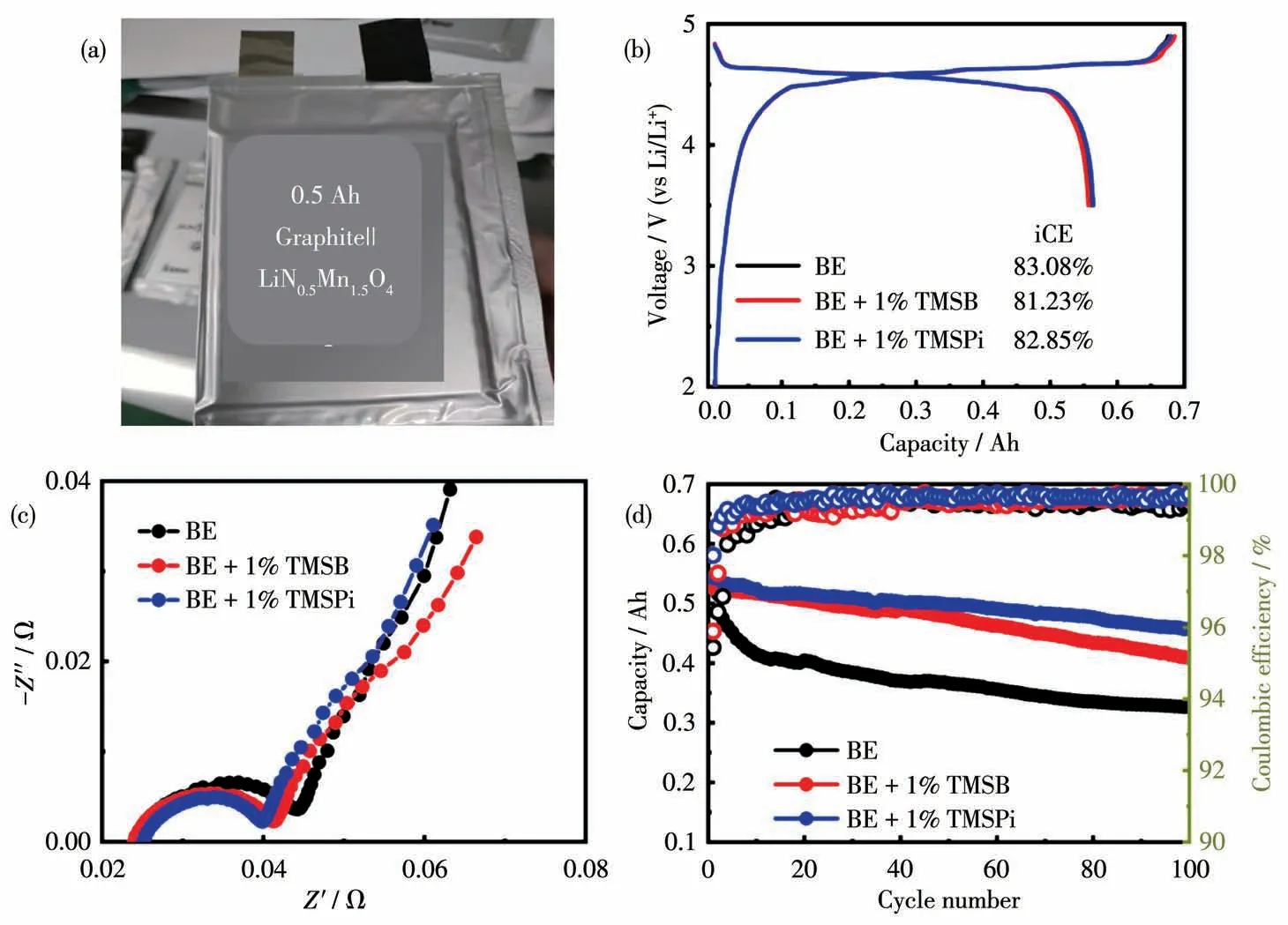
Fig.9(a)Image of 0.5 Ah Graphite||LiNi0.5Mn1.5O4 pouch cells,(b)initial charge-discharge curves at 0.1C,(c)cell impedance,and(d)cycle performance at 1C in BE and additive-containing electrolytes
0.5 Ah Graphite||LiNi0.5Mn1.5O4pouch cells were fabricated to evaluate the comprehensive effects of selected additives on practical application,as shown in Fig.9.In the first precycling process at 0.1C,the cells delivered an initial CE of 83.08%(BE),81.23%(TMSB),and 82.85%(TMSPi)with a discharge capacity of about 0.55,0.54,and 0.55 Ah respectively,indicating that TMSPi is more helpful to improve its energy density of pouch cells along with low initial cell impedance(Fig.9c).Upon cycling at 1C(500 mA)and 25℃,the pouch cells in BE suffer from rapid capacity fading,with only 60.5% of capacity retention after 100 cycles,as shown in Fig.9d.Encouragingly,the cells with selected additives displayed relatively high retention(88.9% for TMSPi,77.4% for TMSB,respectively).Hence,the capability of TMSPi on enhancing cycle performance for LiNi0.5Mn1.5O4was superior to TMSB.Besides that,the CE during cycling is another important indicator for LiNi0.5Mn1.5O4electrode-based cells[36].The main fading mechanism and low CE of LiNi0.5Mn1.5O4electrode are the oxidation decomposition reactions of electrolyte components[19].A relatively high CE of cells with functional additives indicates that these parasitic reactions have been effectively suppressed.Compared to the cells in BE(an average CE of 99.3%),the CE of pouch cells with TMSB and TMSPi can reach 99.5% and 99.6% in the first 30 cycles and remain stable in the following cycles.Consequently,TMSPi is a promising and better-selected additive than TMSB for LiNi0.5Mn1.5O4-based cells in practical application.However,the aged TMSPi-containing electrolyte is unable to provide a protective effect on LiNi0.5Mn1.5O4due to the consumption of LiPF6and TMSPi accompanied by side reaction,resulting in inferior cyclic stability than cells in TMSB-containing electrolyte,as shown in Fig.S8.Therefore,the practitioner must offer a comprehensive evaluation from multiple perspectives in selecting suitable additives for practical application.
However,the high chemical reactivity of the selected additive may affect the thermal stability of LiPF6-based electrolytes.Storage experiments are conducted with accelerated aging at 40℃for four weeks.BE and TMSB-containing electrolytes can remain stable during storage.However,the TMSPicontaining electrolyte became cloudy after aging for weeks(Fig.10a)and its ion conductivity decreased from 8.34 to 7.75 mS·cm-1(Table S1).It may mainly ascribe to its spontaneous reaction with LiPF6,generating the insoluble of(Me3SiO)PF2and(Me3Si)POF2and increasing Li+diffusion obstruction with the consumption of LiPF6and TMSPi accompanied by this side reaction[23,37].As shown in Fig.10a,the reactions between TMSPi and LiPF6can release more energy(-314.33 kJ·mol-1)than TMSB(-265.69 kJ·mol-1),suggesting TMSPi had a high chemical reactivity in LiPF6-based electrolyte.Based on the aged TMSPicontaining electrolyte after storage at 40℃,the cells display relatively inferior cycling stability than that in TMSB-containing electrolyte,indicating that the consumption of TMSPi cannot support enough protection for high voltage cathode.Therefore,TMSPi displayed a lifetime limit as the additive in LiPF6-based electrolyte for high voltage electrodes.It is necessary to keep the TMSPi-containing electrolyte fresh for considerable evaluation and application.
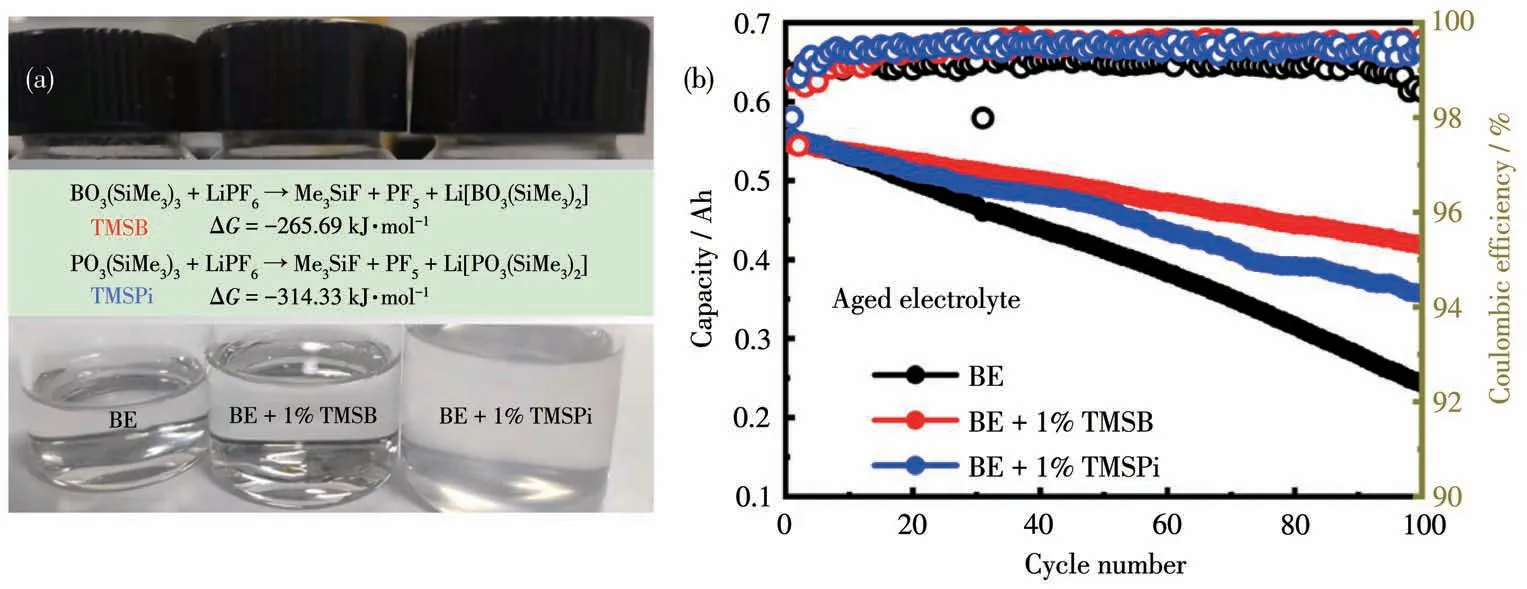
Fig.10(a)Change of electrolytes after being stored at 40℃for four weeks and reactivity;(b)Cycle performance of 0.5 Ah pouch cells at 1C in aged BE and additive-containing electrolytes for two weeks at 40℃
3 Conclusions
TMSB or TMSPi as the selected additive was devoted to supporting LiNi0.5Mn1.5O4for practical application.Their effects on high voltage cathode and graphite anode were systematically studied.Both two selected additives can be preferentially oxidized,participating in the formation of an interface layer on high voltage cathodes,resulting in enhanced Columbic efficiency and cyclic stability of LiNi0.5Mn1.5O4.Moreover,the theoretical calculation suggests that the Si—O groups in TMSB or TMSPi,especially for TMSPi,can act as the HF scavenger in LiPF6-based electrolytes.Significantly for TMSB,its electron-withdrawing atoms of B can act as the stabilizer of LiPF6.TMSPi with higher HOMO value can rapidly passivate the surface of LiNi0.5Mn1.5O4and reduce the initial cell polarization,leading to superior electrochemical performance for LiNi0.5Mn1.5O4based cells.Moreover,TMSPi is likely to form an association with Li+in the presence of EC and participate in the formation of the SEI layer on graphite electrodes via O—Si band cleavage.As a result,Graphite||LiNi0.5Mn1.5O4pouch cells with TMSPi-containing electrolyte displayed capacity retention of 88.9% after 100 cycles at 1C.However,TMSPi displayed a certain lifetime limit in LiPF6-based electrolytes for its high chemical reactivity.
Acknowledgments:This work was financially supported by the National Natural Science Foundation of China(Grants No.21805186,21625304,21733012),the Shanghai Rising-Star Program(Grant No.20QB1401700),and the Technology Commission of Shanghai Municipality(Grant No.19160760700).
Supporting information is available at http://www.wjhxxb.cn

