微型Y型撞击流混合器强化快速沉淀反应可控制备碳酸锶微球
姚瀚植 游 攀 张俊杰 骆培成
(东南大学化学化工学院,南京211189)
0 Introduction
As an important inorganic chemical raw material,strontium carbonate(SrCO3)is widely used in the production of electronics,ceramics,and coating[1].In recent years,the rapid development of the electronics industry has brought new opportunities and challenges to the development and utilization of SrCO3products.Among them,using various additives to synthesize functionalized SrCO3products with special forms,thereby giving them better application performance,has become an attractive research topic[1].The morphologies of SrCO3prepared so far include spherical,needle,spindle,flake,dumbbell,olive,peanut,bunch,rod,etc.,among which spherical SrCO3has attracted more and more attention due to its wide applications in electronic ceramics,catalytic support,and other fields.
In the past few years,many scholars have successfully prepared spherical SrCO3particles using a variety of additives as morphology control reagents.For example,Yu et al.[2]used poly-(stryrene-alt-maleic acid)(PSMA)as an additive to prepare spherical SrCO3with good dispersion.Zou et al.[3]prepared spherical SrCO3by gas-liquid precipitation method.Liu et al.[4]synthesized micron-sized SrCO3microspheres using phthalic acid and isophthalic acid as regulators.Du et al.[5]synthesized SrCO3microspheres with a particle size of 200-400 nm,using ionic liquid(1,1,3,3-tetramylguanidylate)as a solvent and SrCl2·6H2O,NaOH,and CO2as raw materials.To avoid using expensive ionic liquid solvents,Cao et al.[6]prepared spherical SrCO3particles by hydrothermal method using cetyltrimethylammonium bromide(CTAB)as a soft template.These pioneering works have strongly promoted the development of spherical SrCO3preparation technologies.However,developing a high-efficiency and low-cost technology that can be used for large-scale industrial production remains a major goal.
Co-precipitation reaction of cationic salt solution and carbonate solution is a popular method to prepare micron-sized or nano-sized particles,in which the mixing efficiency of the reactants plays a vital role in the regulation of supersaturation level and its distribution in the crystallizer.Thus,many studies have focused on improving the mixing efficiency of the precipitation equipment to prepare particles with specific particle size,particle size distribution(PSD),and morphology.The crystallizers with mixing intensification studied are T-jet mixer[7-9],Y-jet mixer[10-12],impinging stream jet reactor[13-14],vortex reactor[15-16],multi-entry vortex reactor[17-18],rotating disc reactor[19-20],rotating liquid membrane reactor[21],multi-orifice impinging transverse(MOIT)jet mixer[22],among which the Y-jet mixer is a preferred device,due to not only its simple structure but also a rapid generation of high supersaturation,thereby producing nano-sized or micron-sized particles with a narrow PSD[11].
In this study,a miniature Y-jet mixer was used as the crystallizer to prepare micron-sized SrCO3particles by co-precipitation reaction of aqueous solutions of strontium chloride(SrCl2)and sodium carbonate(Na2CO3).EDTA was used as the additive and its effect on the morphology of the prepared particles was discussed.We intend to study the effects of EDTA concentration(cEDTA),and reactant concentrations on the morphology and PSD of the prepared SrCO3particles and explore how EDTA regulates the morphology of SrCO3particles in the miniature Y-jet mixer.
1 Experimental
1.1 Materials
Analytical grade SrCl2(99%,Shanghai D&B Biological Science and Technology Company),Na2CO3(99.8%,Shanghai MERYER Company),EDTA(99%,Sinopharm Chemical Reagent Company),and distilled water were used to prepare SrCO3particles.
1.2 Apparatus and methods
The experiment was carried out at 25℃.In the experiment,an aqueous solution of SrCl2with(or without)EDTA was used as solution A and an aqueous solution of Na2CO3as solution B.The schematic diagram of the experimental setup is shown in Fig.1a.Two reactant fluids enter the Y-jet mixer at the same molar concentration and flow rate by gear pumps.Fig.1b shows the configuration of the miniature Y-jet mixer adopted in this work.The inner diameters of the two inlet pipes and the mixing pipe were all 2 mm and the length of the mixing pipe was 10 mm.The angle of collision between two inlet streams was 60°.The flow rates of the two streams were all fixed at 100 mL·min-1.The Reynolds number of the mixed stream in the mixing pipe,was 1 056,indicating that the flow belongs to the laminar regime.The precipitation process was carried out at room temperature.The concentrations of SrCl2(cs)and Na2CO3(cc)remained the same,whereas the molar concentration ratio of EDTA to SrCl2(RE=cEDTA/cs)was changed.SrCO3suspension was collected and rinsed several times with distilled water and pure ethanol,respectively.Wet particles were then dried in a vacuum drying oven at 80℃for 24 h.
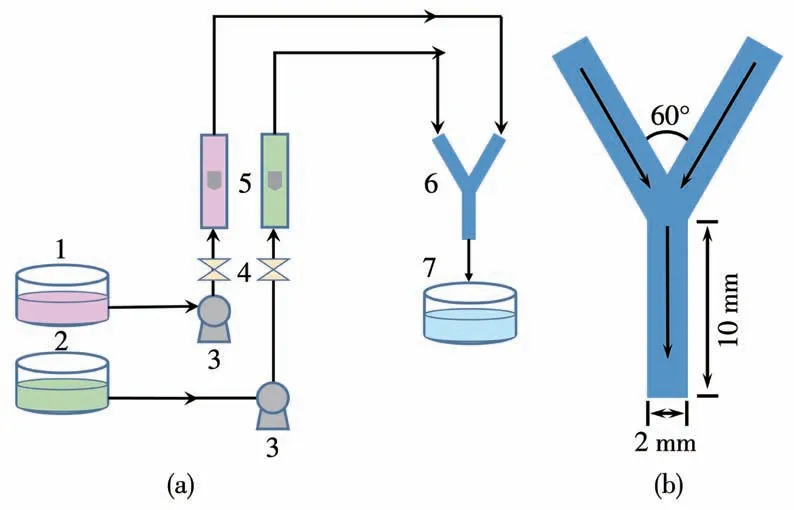
Fig.1(a)Experimental setup of the co-precipitation process in(b)the miniature Y-jet mixer
1.3 Characterization of SrCO3 particles
The multifunctional energy-level X-ray powder diffractometer(XRD,UltimaⅣ)was used to identify the phase structure and purity of the SrCO3particles,with CuKα(λ=0.154 06 nm)radiation at a scan rate of 0.02(°)·s-1.The accelerating voltage and applied current were 36 kV and 20 mA,respectively.The scanning electron microscope(SEM,Nova NaNoSEM450)was used to measure the morphology of the SrCO3particles,with an acceleration of voltage at 15 kV.PSD and volume-weighted average particle size(d43)were measured by the Malvern Mastersizer 3000 particle size analyzer(MAN0475-06-EN-00).
2 Results and discussion
2.1 Morphology and crystal form
The effect of the amount of EDTA added on the morphology of the prepared particles was first studied.It is seen that the prepared SrCO3particles are bundlelike structures with a particle size of about 5 μm when EDTA was not added,as shown in Fig.2a.When the reactant concentrations were kept constant(cs=cc=0.1 mol·L-1)and a certain amount of EDTA was added,spherical SrCO3particles were obtained,as shown in Fig.2b-2d.Moreover,no obvious aggregation between the primary particles was observed,implying that the prepared particles are expected to have good dispersion in the liquid in practical applications.WhenREwas increased,it can be seen intuitively from the images that the size of SrCO3particles decreased first and then increased.WhencEDTAexceeded 0.04 mol·L-1,the precipitation reaction process slows down significantly,i.e.particulate matter in the suspension collected from the outlet of the Y-jet mixer was significantly reduced.In addition,serious aggregation between SrCO3particles was observed by SEM(Fig.2e).
The concentration of the reactants is another important factor affecting the precipitation process.At a lower concentration of the reactants,almost no solid particles were formed due to low supersaturation levels.Thus,csandccwere first increased to 0.2 mol·L-1with a lowerREof 0.1.SEM image in Fig.2f shows that the particle size is similar to that in the case ofcs=0.1 mol·L-1andRE=0.1(Fig.2b).This indicates that theREof EDTA to SrCl2has a greater effect on the particle size than the reactant concentrations.To have a further check of this,REwas fixed at 0.2,andcswere changed from 0.1 to 0.25 mol·L-1.From Fig.2g to 2i,the morphology and particle size of SrCO3particles obtained were very similar.This confirms that the molar concentration ratio of EDTA to SrCl2is the key factor affecting the particle morphology and size.
XRD measurements were then carried out to determine the crystal form of the prepared SrCO3particles.Fig.3 shows that the diffraction peaks of the prepared particles under different conditions are all consistent with those(110),(111),(221),(113),(132),and(002)crystal faces,which are characteristic peaks of orthorhombic SrCO3crystal(PDF No.05-0418).Moreover,there was no impurity peak,implying that the prepared SrCO3particles are very pure.
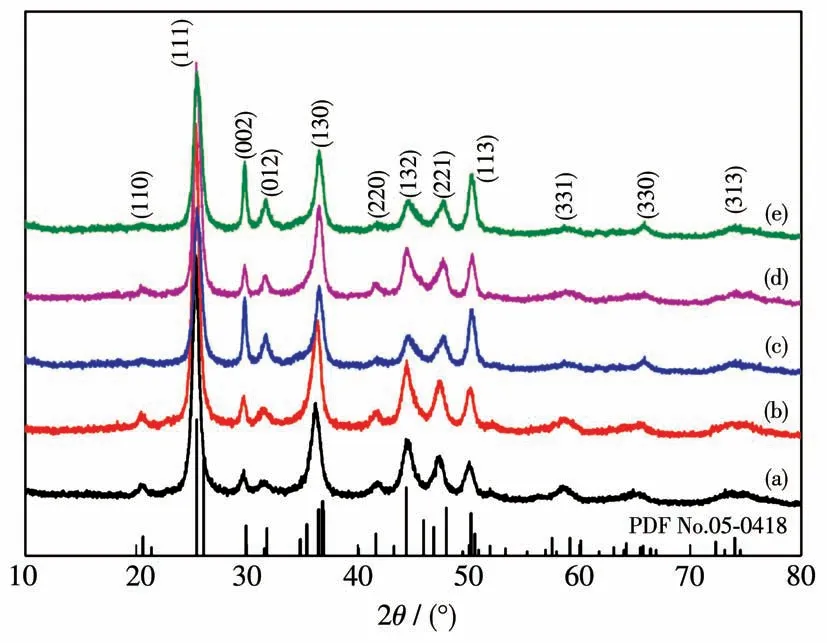
Fig.3 XRD patterns of SrCO3 particles
2.2 Particle size distribution
From Fig.2,it is also seen that the amount of EDTA added has a significant effect on the size of the prepared particles.To have a quantitative analysis,the PSD profiles from experiments by using Malvern Mastersizer 3000 are compared in Fig.4.When EDTA was not added or at a low concentration(RE=0 or 0.1),the PSD profiles exhibited a bimodal distribution.Compared with SEM images,it can be seen that the bundle-like particles were of different sizes when EDTA was not added,and there were some irregular small SrCO3particles whenRE=0.1.They are the reasons for bimodal distributions in the PSD profiles.Whereas the profiles were unimodal distributions when the amount of EDTA added increased.However,whenREcontinued to increase to 0.4,the particle aggregation became more serious,and the spherical morphology deteriorated,resulting in a wider PSD with the multimodal distribution.In addition,when the molar concentration ratio was fixed atRE=0.2 whereas the reactant concentrations were changed,particle size distribution curves almost coincided with each other,as shown in Fig.4b.
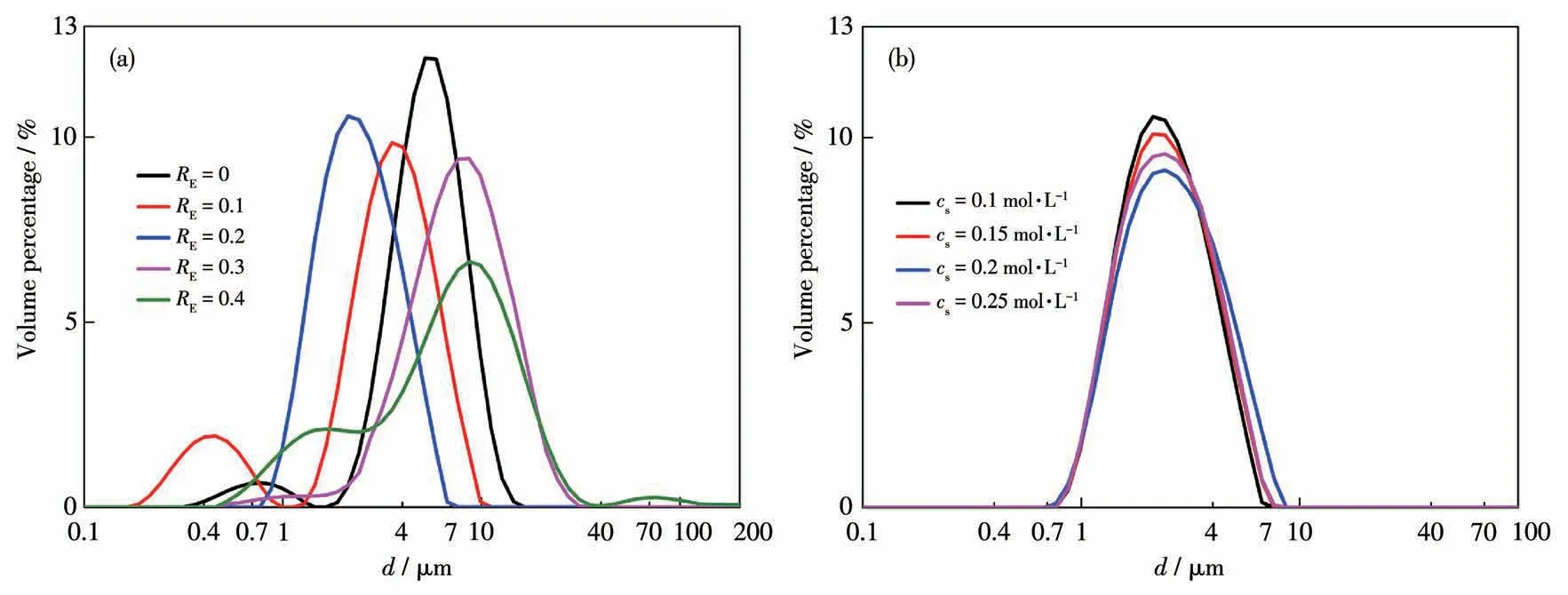
Fig.4 PSD of SrCO3 particles prepared under different conditions of(a)cs=0.1 mol·L-1 and(b)RE=0.2
To have a quantitative analysis,d43,and the uniformity,σ,were calculated and plotted againstREandcsin Fig.5.Here,σis the degree to which the PSD deviates from the middle.The larger the value of σ,the wider the particle size distribution.Whencswas fixed at 0.1 mol·L-1,increasing EDTA amount results first in a slight decrease followed by a rapid increase ind43.This indicates there exists an optimized amount of EDTA added to regulate the particle size.Whileσhad a slight increase when the morphology changed from bundle to sphere after adding EDTA.A further increase inREled to a first slight decrease and then a sharp increase in σ.It is seen that the case ofRE=0.2 had the smallest size ofd43=2.77 μm and the most uniform size distribution ofσ=0.372.When the molar concentration ratio was fixed atRE=0.2 andcswere changed,d43andσdid not change significantly with the increase ofcs(Fig.5b).This indicates that the molar concentration ratio of EDTA to SrCO3has an important influence on the particle size and its distribution uniformity.There are relative maximum values ofd43andσwhencs=0.2 mol·L-1.
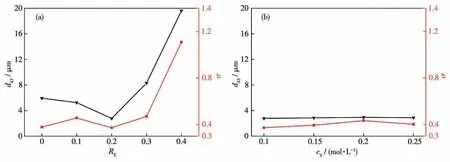
Fig.5 Effect of the molar concentration ratio of EDTA to SrCO3 on the particle size and the distribution uniformity
We also compare the spherical SrCO3particles prepared in this work with those in the published works by different methods,as listed in Table 1.EDTA has also been used to control the morphology of SrCO3particles by a carbonization reaction of Sr(OH)2with CO2

Table 1 Comparison of different methods for preparing spherical SrCO3 particles
[3].Spherical SrCO3particles of 200-800 nm were obtained whenRe=0.2,which is very similar to this work.When PSMA was used as the additive by using the co-precipitation method in a stirring flask[2],changing the amount of PSMA results in different particle morphologies,such as bundles,dumbbells,irregular aggregates,and spheres.The obtained spherical SrCO3particles were about 5-10 μm.Ultrasonic has also been used to intensify the co-precipitation process[23].In the absence of additives,when the solvent was water,the obtained SrCO3particles were fusiform structures with a particle size of about 3-5 μm.When the solvent was changed into ethylene glycol,it can be observed that the shape of the particles becames spherical and the particle size was about 0.5-1 μm.When the hydrothermal method was used[24],urea was added to the solvent as an additive.After 2 h of reaction at 120℃and 24 h of etching by HCl,spherical SrCO3particles with a size of 300-500 nm were obtained.When the hydrothermal reaction time increased to 8 h,there were flower-like SrCO3particles with a particle size of about 10 μm,which are very similar to the particle prepared without EDTA additive in this work.In the microemulsionmediated solvothermal method[6],the autoclave was used as a crystallizer with CTAB as an additive.Rodshaped particles with a length of 1-2 μm can be prepared at a lower molar ratio of H2O to CTAB,while the spherical SrCO3particles with a size of 0.5-1 μm were obtained at a higher molar ratio.This work uses the miniature Y-jet mixer as the crystallizer to intensify the co-precipitation process,with EDTA as additive to prepare spherical SrCO3.The prepared particles had an average particle size of 2-3 μm and a narrow PSD.The method does not require complicated reaction devices,and the subsequent drying conditions are very convenient,making it easier for industrial applications.
2.3 Mechanisms of morphology regulation by EDTA
The above experiments have proved that the precipitation process can be well regulated by adding EDTA and micron-sized SrCO3microspheres were successfully prepared.This should be attributed to the carboxyl groups on the EDTA molecule,which makes EDTA a strong chelating agent to form stable complexes with many metal ions[25].In the precipitation process of SrCO3,when EDTA is not added,the short rodshaped strontium carbonate grows along the axial direction and forms a bundle structure.When EDTA is added,a stable coordination compound of EDTA-Sr is formed,which is very easily adsorbed on the surface of SrCO3particles.EDTA-Sr formed a coating layer around SrCO3particles,leading to growth inhibition in the axial direction[26].Thus,all crystal faces grow at the same rate,resulting in a decrease in the slenderness ratio and gradually growing into spherical particles.Fig.6 shows a schematic mechanism of how EDTA regulates the morphology of SrCO3from rod-like to spherical particles.
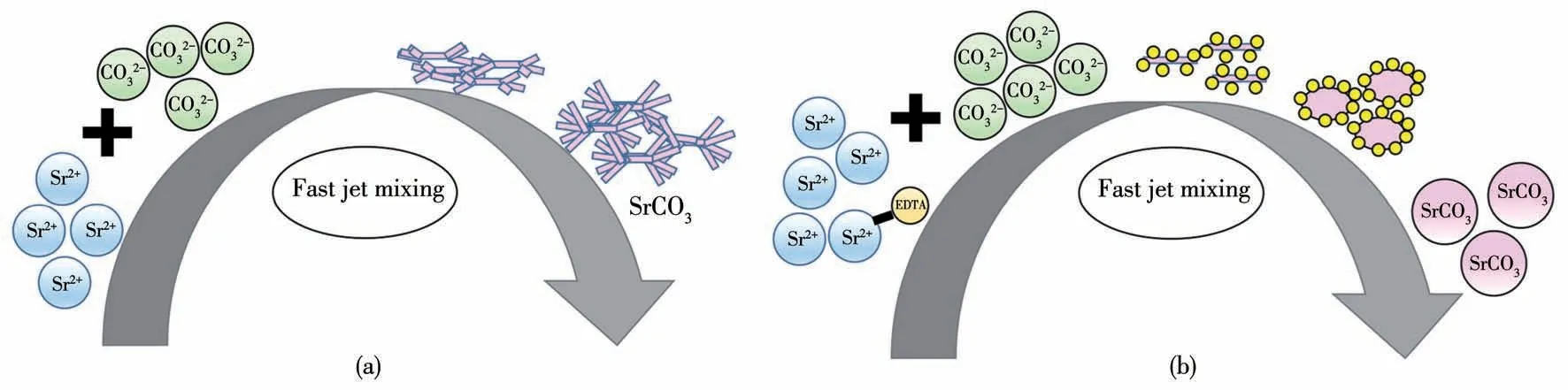
Fig.6 Mechanism diagrams of SrCO3 formation in the miniature Y-jet mixer(a)without or(b)with adding EDTA
In addition,the obvious separation of nucleation and growth stages is the basic requirement for the formation of homogeneous particles[27].It has been reported that adding EDTA to the system can delay the precipitation of SrCO3and prolong the nucleation induction period of crystals[28],which is beneficial to the separation of two stages.From Fig.2,4,and 5,one can see that the size of SrCO3particles was very uniform when enough EDTA was added to the system.Moreover,the miniature Y-jet mixer can mix two solutions evenly within several milliseconds,leading to uniform distribution of the supersaturation level[29],which is also favorable to prepare SrCO3particles with a narrow PSD.With a further increase in the amount of EDTA added,more strontium ions are consumed to form the coordination compound of EDTA-Sr,leading to a further decrease in the supersaturation level and nucleation rate.This is conducive to the growth into large particles,which has been proven by the above experimental results.Nevertheless,a more mechanistic approach such as molecular simulation should be used in the future studies to further prove the morphology regulation mechanism by EDTA.
3 Conclusions
In this work,the co-precipitation method was used to prepare SrCO3particles.A miniature Y-jet mixer was used to intensify the mixing efficiency of the reactant solutions and EDTA was used as the additive to control the particle morphology.The particles were characterized by SEM,XRD,and PSD measurements.
In the absence of EDTA,SrCO3particles with bundle-like morphology could be obtained.When EDTA was added,uniform spherical SrCO3particles were prepared continuously for several milliseconds.The prepared particles are all orthorhombic phase crystals with very narrow PSD.The molar concentration ratio of EDTA to SrCl2,RE,is the key factor affecting the particle morphology and size.WhenREis fixed,the reactant concentration of SrCl2,cs,has a slight effect on the particle size and its uniformity.In the case ofRE=0.2 andcs=0.1 mol·L-1,the prepared particles have the smallest size ofd43=2.77 μm and the most uniform size distribution ofσ=0.372.The mechanism has been proposed that EDTA-Sr is adsorbed on the surface of SrCO3particles,which inhibits the axial growth rate of crystals,making all crystal faces have the same growth rate and gradually grow into spherical particles.At the same time,the addition of EDTA facilitates the separation of nucleation and growth stages,and the use of a miniature Y-jet mixer redistributes supersaturation,all of which make the SrCO3particle size more uniform.Compared with the methods in the published works,the preparation process in this work can be accomplished in a continuous mode within several milliseconds,thus,providing a high-efficiency and low-cost route to prepare micron-sized SrCO3spheres.
Acknowledgments:We acknowledge the financial support from the National Natural Science Foundation of China(Grant No.22078058).

