Different levels of MUC5AC and MUC5B genes expression in severe allergic versus non-allergic asthma
Bita Mohammadi , Mohammadreza Saghaf , Amirhossein Mohajeri Khorasani , Mohammad Reza Khakzad
Abstract Asthma affects about 330 million individuals worldwide while 10% of asthmatic patients develop the severe type.The two main phenotypes of severe asthma are allergic and non-allergic.Notably, remodeling and mucus overexpression are hallmarks of severe asthma upon dysregulation of MUC5AC and MUC5B.In severe allergic asthma due to the initiation of the allergic cascade, immune cells are recruited and a large number of inflammatory mediators will be produced leading to the overexpression of MUC5AC and MUC5B in the airways.Moreover, the production of mediators including tumor necrosis factor α (TNF-α) and interleukin 13 (IL-13) will cause airways’ muscle proliferation.Both overproductions of mucin and muscle proliferation will lead to remodeling progression.On the other hand, in severe non-allergic asthma, fewer immune cells are involved but still, the expression of MUC5AC is enhanced.However, MUC5B might increase less than the amount of its expression in allergic phenotype due to the lower number of involved immune cells and mediators.In the non-allergic phenotype mediators such as interleukin 17 (IL-17) and transforming growth factor β (TGF-β)are responsible for muscle proliferation.The result of mucus overexpression and muscle proliferation is remodeling progression in the non-allergic severe asthma.Therefore, we hypothesize that MUC5AC and MUC5B overexpression in severe allergic asthma is greater than in severe non-allergic asthma.Hence, remodeling progression is more intensive in severe allergic asthma.In conclusion, given the central roles of MUC5AC and MUC5B in mediating the asthma severity, they can be treated as potential therapeutic targets in severe asthma.
Keywords: MUC5AC, MUC5B, remodeling, severe asthma, allergy*Corresponding to: Mohammad Reza Khakzad.Shahinfar faculty of Medicine, Azadi St.Mashhad, Iran.Department of Immunology, Islamic Azad University, Mashhad, Iran.Email: kzdmrkh@gmail.com
Introduction
Globally, 334 million people suffer from asthma, with an annual mortality rate of 180,000 [1].10% of asthmatic patients develop severe asthma among whom [2], perpetual symptoms and extensive exacerbations are reported [3].Thus, a high dose of Inhaled corticosteroid (ICS) with a controller like Long-acting beta-agonists(LABA) is recommended as a treatment.Remodeling and mucus hypersecretion are the main complications associated with severe asthma, which may lead to incomplete medication techniques or poor medication adherence.Remodeling is diagnosed with alternations in the airways and is affected by airway inflammation.The symptoms of severe asthma are relapsing-remitting, which include coughing, dyspnea, wheezing, and chest pain [3].Multi detector-row CT (MDCT) scans have revealed thicker airway walls in severe asthmatic patients compared to mild cases and healthy individuals, illustrating the degree of remodeling and the amount of airflow obstruction [4].Allergic asthma is a subtype of asthma that is activated by allergens like pollen.On the other side, nonallergic asthma has no specific trigger and it is less common among the asthmatic patients.This subtype is more frequent in women and has a later onset [5].Notably, more than 50% of the severe asthmatic patients have allergic asthma with increased levels of interleukin 13 (IL-13), interleukin 4 (IL-4), and interleukin 5 (IL-5) as well as recruitment of T helper 2 (TH2) cells and eosinophils as the inflammatory cells.These patients could be resistant to highdose treatments.The allergic phenotype may be shown with the following characteristics: allergen-driven asthma, Forced Expiratory Volume in 1 Second (FEV1) < 60%, blood eosinophil > 150 per μL,sputum eosinophil ≥ 2%, fractional exhaled nitric oxide (FeNO) ≥20 ppb [3].Another phenotype is severe non-allergic asthma, which has remained elucidated.Thus far, the diagnosis practices heavily rely on several factors such as patient’s history and environmental determinants, sputum neutrophils > 65%, sputum eosinophils < 2%,FeNO < 20 ppb, and recruitment of neutrophils, T helper 17 (TH17)cells, and T helper 1 (TH1) cells [6].Airway remodeling, characterized by a series of changes in form,content, and configuration of airway epithelium [7], is a common complication in asthmatic patients [8,9].Histological observations enumerate an array of significant disruptions upon remodeling including thickening of the lamina reticularis, inflammatory cell infiltration, subepithelial fibrosis, epithelial spilling, goblet cell hyperplasia [10], metaplasia [11], angiogenesis in the airway wall,smooth muscle hyperplasia and hypertrophy.Asthma is accompanied by airway changes including cell proliferation, massive dropping of epithelial cells, and goblet cell hyperplasia which triggers mucus hypersecretion in the airway [12].Goblet cells, derived from Clara cells and Ciliated cells, increase mucin production in response to many signaling pathways.Several reports have indicated that TH2 cytokines, such as interleukin 9 (IL-9) and IL-13 affect mucins release and goblet cell metaplasia.Mucus hypersecretion is associated with chronic upper respiratory disease as well as airway narrowing and poor asthma management [13].
Mucus is a mixture of water, ions, and mucins [14].MUC5B,MUC5AC,MUC2, andMUC19are expressed in the airway [15]whereasMUC5ACandMUC5Bare the dominant mucins in the respiratory tract [16].MUC5ACandMUC5Bare expressed in bronchial goblet cells and submucosal glands, respectively [17].MUC5Bprimarily impacts the mucociliary clearance (MCC), where its deficiency may cause airway narrowing, inaccurate cleaning,high infection risk, inflammation with ineffective macrophage phagocytosis, and impaired immune responses [18].On the plus side, the exact role ofMUC5ACin the respiratory tract of asthmatic patients has remained elusive.However, preliminary studies on asthmatic patients indicate overexpression ofMUC5AC[12,17,19-22] compared to dysregulation ofMUC5B[17,19,22].The main factors affectingMUC5ACoverexpression include (1)proinflammatory cytokines (IL-4, IL-13, interleukin 1b (IL-1b),Tumor necrosis factor α (TNF-α), interleukin 17 (IL-17)), (2) growth factors (TGF-β, epidermal growth factor (EGF)), (3) viral and bacterial infections (4), and airways pollutant particles (cigarettes,diesel exhaust particles (DEP), ozone).On the other hand,MUC5Bdysregulation is affected by increased IL-13, inducing inflammation with TH2 recruitment whereby the allergic pathway is initiated.The allergic mediators cause mucus hypersecretion which makes alterations in its biochemical properties and stimulates mucus plug formation [23].Notably, remodeling might advance by the formation of mucus plugs [24].
Our observations on the conducted research in the literature investigating the role of MUC5 genes dysregulation in severe asthma indicate a serious inconsistency between the findings of different research groups.As of now, there is no large-scale data on expression patterns ofMUC5BandMUC5ACin severe allergic and non-allergic asthmatic patients.This is even more critical in the case ofMUC5Bwhere there is no precise consensus on severe asthma,as well as if bothMUC5ACandMUC5Bsimultaneously increase or not.Moreover, there is no concrete data on the correlation betweenMUC5BandMUC5ACexpression and the intensity of remodeling in these two phenotypes of severe asthma.Therefore, in this article,we aim to review the correlations between the dysregulation ofMUC5ACandMUC5Band remodeling in patients with severe allergic and non-allergic asthma followed by hypothesizing the characteristics of the differences.
Hypothesis
Severe asthma is closely associated with remodeling and mucin overexpression, and also, there might be a reciprocal correlation betweenMUC5BandMUC5ACoverexpression and remodeling intensity.Meanwhile, there are significant differences in severe asthma phenotypes.In severe allergic asthma overexpression ofMUC5ACandMUC5Bmight be greater.WhileMUC5ACandMUC5Bin severe non-allergic asthma are not as highly expressed in severe allergic asthma.In severe allergic asthma (Figure 1),DC presents captured allergen to T helper 0 (TH0).Epithelial cells, which are induced by allergen, release mediators including interleukin 33 (IL-33), interleukin 25 (IL-25), and Thymic stromal lymphopoietin (TSLP) leading to the differentiation of TH0 to TH2.Activated TH2 produces cytokines (IL-4, IL-13, IL-5, IL-9, and TNF-α).Particularly, IL-4, IL-13, and TNF-α triggerMUC5AChypersecretion by affecting the goblet cells.Alternatively, IL-13 stimulatesMUC5Bproduction from the submucosal gland.In addition, IL-4 leads to isotype switching of B cells’ antibodies to produce immunoglobulin E (IgE) which binds to mast cell’s (MC)FcɛRI.In the second contact with the allergen, degranulation of mast cell granules (vascular endothelial growth factor (VEGF),prostaglandin D2 (PGD2), leukotriene D₄ (LTD4), and IL-13) takes place.Moreover, the released IL-13 from mast cells stimulates submucosal glands for producingMUC5B.VEGF and IL-13 contribute to smooth muscle proliferation.Additionally, innate lymphoid cells type 2 (ILC2) are activated by epithelial cytokines and produce IL-13, leading toMUC5ACandMUC5Bhypersecretion,and recruit eosinophils by producing IL-5.Recalled eosinophils emit cytokines (IL-13 and TNF-α) which are accompanied by mucus overexpression.Finally,MUC5ACandMUC5Boverexpression, and muscle proliferation lead to remodeling development.
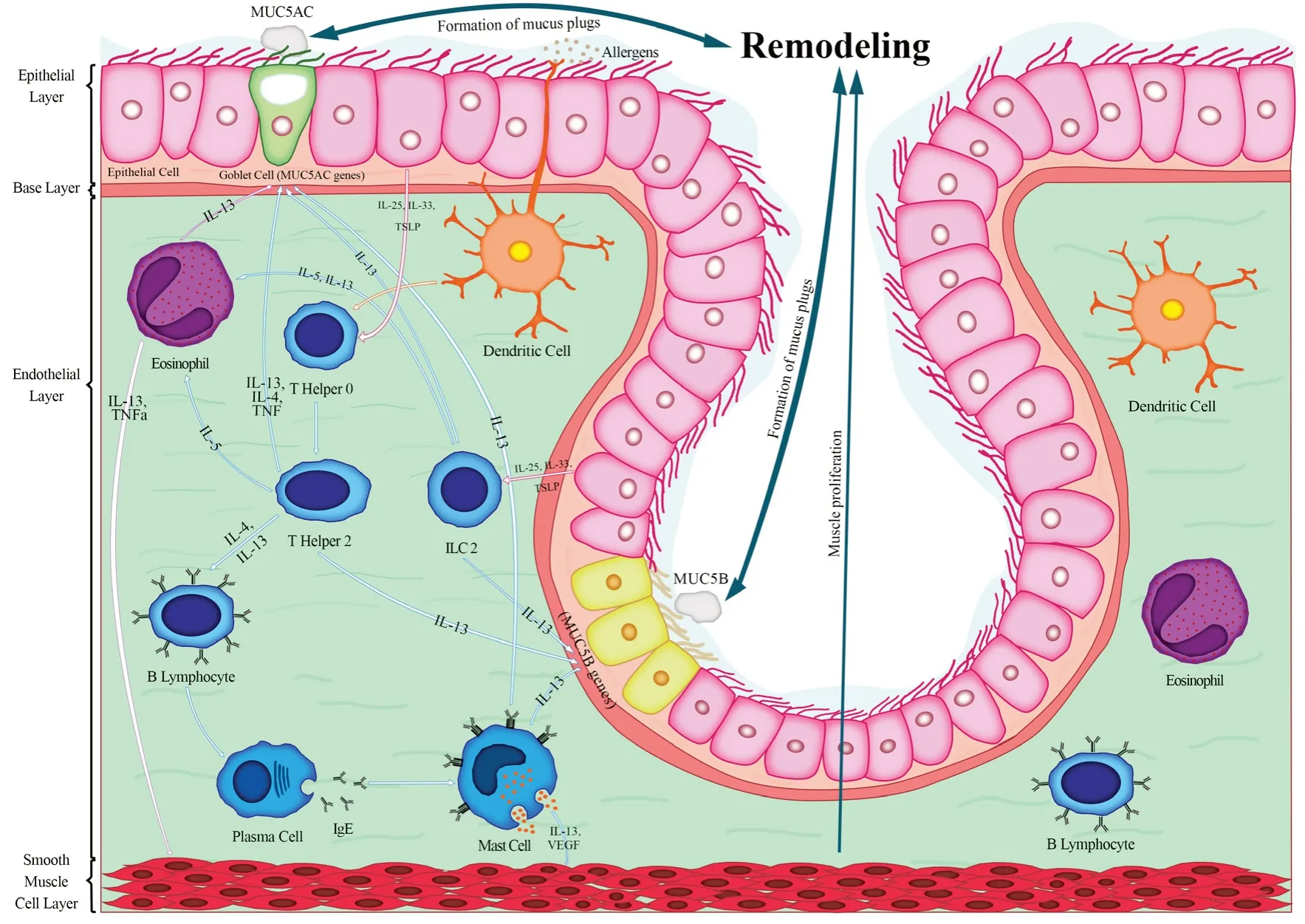
Figure 1.Severe allergic asthma.In the allergic pathway, several immune cells are involved, while TH2 and eosinophils play crucial roles in asthma pathogenesis.Activated TH2 and eosinophils release IL-13 and TNF-α leading to the goblet cells and submucosal glands hyperplasia,which causes excessive production of MUC5AC and MUC5B and will accelerate the formation of mucus plugs.Also, these mediators lead to airwall muscle hypertrophy which causes more airflow obstruction.Pre-existing remodeling is aggravated through the formation of mucus plugs and muscle hypertrophy in severe allergic asthmatic patients.
In severe non-allergic asthma (Figure 2), allergens are presented to TH0 by DC resulting in its differentiation to TH17.interleukin 17A (IL-17A), interleukin 17F (IL-17F), interleukin 25 (IL- 25),and interleukin 22 (IL-22) are released by TH17.IL-17A not only makes neutrophils activated, to produce VEGF and TGF-β but also stimulates submucosal glands to produceMUC5B.TGF is directly associated with goblet cells hyperplasia leading to hypersecretion ofMUC5AC.Notably, VEGF, TGF-β, and interleukin 6 (IL-6) produced by neutrophils lead to smooth muscle proliferation.Smooth muscle proliferation and mucus overexpression imply remodeling progression.
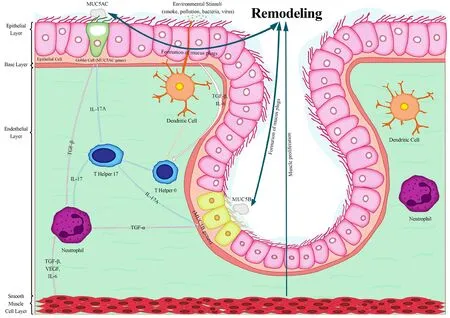
Figure 2.Non-allergic asthma.In the non-allergy pathway, fewer immune cells predicate in mucus overproduction.TH17 and neutrophils release IL-17A and TGF-β leading to the up-regulation of MUC5AC and MUC5B and also muscle proliferation.However, the formation of mucus plugs, muscle hypertrophy, and the progression of pre-existing remodeling is prolonged in severe non-allergic asthma due to the lower amount of produced inflammatory mediators.
As mentioned above, remodeling progression with allergic responses is more intensive since the allergic cascade is initiated and more cytokines are secreted from the mast cells, eosinophils,ILC2, and TH2.Moreover, in allergic asthma, interleukin 1β (IL-1β) which is initially released from epithelial cells and macrophages will activate T cells for initiating the inflammation.Then activated T cells, TH2, and macrophages produce TNF-α which is responsible for the activation of Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB), by phosphorylation of Inhibitory Kappa B Kinase α (IKKα) and IKKβ.On the plus side, IL-4 and IL-13 are produced by TH2 and ILC2, which induce the Mitogen-Activated Protein Kinase (MAPK) pathway.These mechanisms lead toMUC5ACenhancement [25].On the other hand, in nonallergic asthma, in response to stimulators, epithelial cells produce TGF-β which is responsible for the activation of TH17 by the MAPK cascade.Then TH17 releases IL-17, which causes the phosphorylation of IKKα and IKKβ and motivates the production of NF-κB which is a key factor inMUC5ACoverexpression.
On the opposite side, there is a substantial contradiction aboutMUC5Bexpression in severe asthma.As reported by Bonser et al.[17],MUC5Bis reduced by overproduction of IL-13 in severe allergic asthma.However, a study on a murine model by Park et al.[26] demonstrates high levels of IL-13 decrease Forkhead Box A2(FOXA2), which is a down-regulator ofMUC5BandMUC5AC,so the expression of bothMUC5BandMUC5ACmight increase.Overall, we suggest in severe allergic asthma due to the high production of IL-13, which inhibits the function ofFOXA2,MUC5Bmay alter [27].Nonetheless, severe non-allergic asthma is more complicated because dominant mediators like IL-17A activate NFκB and TGF-α to stimulateMUC5Bexpression.However, in the absence of IL-13, FOXA2 may inhibit theMUC5Bpromoter.But we propose the enhancement ofMUC5Bin severe asthmatic patients is related to the activation of NF-κB versus healthy individuals.
The process of tissue remodeling may be exacerbated by the dysregulation ofMUC5BandMUC5AC.Therefore, severe allergic asthmatic patients, in contrast to non-allergic ones, are more susceptible to remodeling progression with augmented mucus dysregulation and smooth muscle proliferation.This hypothesis is critical because it represents the differences in the expression of these two genes and also their correlation with remodeling, and provides evidence to treat them as potential therapeutic targets in severe asthmatic patients who are suffering from frequent exacerbations and intensive symptoms.
Evaluation of hypothesis
There is almost no clear evidence of dysregulation of airway mucins,speciallyMUC5ACandMUC5B, in human samples with severe asthma.However, we will review some related research relevant to ours.In Martinez-Rivera’s study [13, 14], 2 asthmatic patients were assessed, 54.2% had severe persistent asthma also 22.7%had an asthma control test (ACT) score ≤ 15, and 48% had FEV1/forced vital capacity (FVC) ratio < 70%.Their results indicate that mucus hypersecretion is related to shortness of breath and severe exacerbation.Moreover, mucus hypersecretion is associated with airway narrowing and the inability to manage asthma symptoms.In addition, they revealed that the elderly with protracted asthma have mucins overexpression [13].Evans et al.[9] observed murine models to checkMUC5AC’s effects on allergic airway hyperactivity.In their study, mice exposed to an allergen, showed upregulation ofMUC5ACwhileMUC5Bexpression was consistent.In addition,MUC5ACinterferes in mucus plugging while its removal did not dwindle the allergic inflammation9.In another study, Shahzeidi et al.[28] revealed that allergen-exposed asthmatic mice had goblet cells metaplasia on the first three days of the exposure which had led toMUC5ACoverexpression.Additionally, the recruitment of inflammatory cells, especially eosinophils were reported [28].
For evaluating our hypotheses, we suggest a study consisting of three groups: 1.Severe allergic asthmatic patients; 2.Severe nonallergic asthmatic patients; 3.Control group which consists of mild to moderate asthmatic patients.Patients should be categorized in severe allergic and non-allergic asthma based on GINA guideline classification and advice of respiratory physicians, then the patients should undergo the spirometry and FeNO tests to place in their right groups.Meanwhile, Chest computerized tomography(CT) scans from asthmatic patients are required for assessing bronchial wall thickness, the intensity of airway remodeling, and also observing for mucus plugs for probable airway obstruction.Additionally, blood samples are required for counting the blood eosinophils with blood smears which are fixed by methanol and stained with Giemsa.Besides, measuring the level of specific IgE for aeroallergen in the patients’ serum through an ELISA procedure is needed.The next step is to collect the sputum from the patients after nebulizing with hypertonic saline.The collected sputum should be purified to obtain the epithelial cells of the airway by adding the Dithiothreitol (DTT), then filtering the mixture by cell strainer to purify the cells, ultimately centrifuge for getting the cell pellet.After this step, a bit of the pellet should be used for microscopic cell smear investigations which should be fixed by the methyl alcohol 95%, stained by hematoxylin and eosin (H&E),and finally, count the cell smears’ eosinophils by microscope.The remained pellet is used for RNA extraction following the cDNA synthesis which will apply for real-time PCR with theMUC5ACandMUC5Bspecific primers to measure the expression of these genes.Finally, the patients’ gene expression and their CT scans should be assessed to find out about remodeling progression and if there is an up-regulation inMUC5BandMUC5ACgene expression in severe allergic and non-allergic asthmatic patients.Consequently,we propose that the bronchial wall (BW) is thicker, and additive mucus plugs from MUC5 overexpression is seen in severe asthmatic patients in contrast to mild to moderate ones, mainly the airflow obstruction and remodeling is greater in patients with higher sputum eosinophils and serum IgE levels which demonstrates the effect of the allergic pathway in severe asthma as a potential cause of serious exacerbations and poor asthma management.However, in severe non-allergic asthma, BW should be thicker than the control group while thinner than severe allergic asthma, also the amount of overproduced MUC5 and mucus plugs should be higher than the controls and lower than allergic asthma because of the less infiltrated immune cells.Hence, these findings could suggest better medical adherence and mildness of the asthma severity in severe non-allergic in contrast to severe allergic asthma.
Conclusion
Although more than 100 genes have been identified to be associated with asthma [29], some appear to be more involved in intensifying tissue remodeling, resulting in worsening the clinical conditions of the patients.Increased mucin secretion in the airway is associated with chronic upper airway disease, more exacerbation, and poor asthma control.MUC5ACis upregulated in both allergic and nonallergic, but its expression is more intensive in the allergic pathway.MUC5Bmay be enhanced in allergic phenotype by IL-13 which activates theMUC5Bpromoter.On the other hand, severe nonallergic is more complicated because, in the absence of IL-13,an inhibitor of theMUC5Bpromoter might be activated while other released mediators might increaseMUC5B.As mentioned,remodeling is a perpetual phenomenon in severe asthmatic patients and is responsible for mucus overexpression leading to the formation of mucus plugs.Notably, in severe allergic asthma formation of mucus plugs might be accelerated.These plugs will intensify remodeling.Thus, there would be reciprocal relation betweenMUC5BandMUC5AChypersecretion and remodeling.Assessing the intervening techniques to change the gene expression in severer asthmatic patients is of high importance for further investigation.Also, given that there are no concrete precision treatments for severe asthmatic patients in both phenotypes, these genes can be nominated as potential therapeutic targets.
- Life Research的其它文章
- Exploring the practice of common oral diseases among the patients visiting in the selected dental college and hospital in Bangladesh
- Research progress on iron homeostasis regulation and heart-related diseases
- The regulatory variant rs17612742 confers the risk of large artery atherosclerotic stroke by increasing the expression of endothelin receptor type A
- The establishment of pancreatic islet isolation in India – an update on human pancreatic islet transplantation
- Physiological signal processing in heart rate variability measurement: A focus on spectral analysis
- Research status of persistent vegetative state in recent 20 years: A bibliometric analysis

