Chx10+V2a interneurons in spinal motor regulation and spinal cord injury
Wen-Yuan Li, Ling-Xiao Deng, Feng-Guo Zhai, Xiao-Yu Wang, Zhi-Gang Li , Ying Wang,
Abstract Chx10-expressing V2a (Chx10+V2a) spinal interneurons play a large role in the excitatory drive of motoneurons.Chemogenetic ablation studies have demonstrated the essential nature of Chx10+V2a interneurons in the regulation of locomotor initiation, maintenance, alternation, speed, and rhythmicity.The role of Chx10+V2a interneurons in locomotion and autonomic nervous system regulation is thought to be robust, but their precise role in spinal motor regulation and spinal cord injury have not been fully explored.The present paper reviews the origin, characteristics,and functional roles of Chx10+V2a interneurons with an emphasis on their involvement in the pathogenesis of spinal cord injury.The diverse functional properties of these cells have only been substantiated by and are due in large part to their integration in a variety of diverse spinal circuits.Chx10+V2a interneurons play an integral role in conferring locomotion, which integrates various corticospinal, mechanosensory, and interneuron pathways.Moreover, accumulating evidence suggests that Chx10+V2a interneurons also play an important role in rhythmic patterning maintenance, leftright alternation of central pattern generation, and locomotor pattern generation in higher order mammals, likely conferring complex locomotion.Consequently, the latest research has focused on postinjury transplantation and noninvasive stimulation of Chx10+V2a interneurons as a therapeutic strategy, particularly in spinal cord injury.Finally, we review the latest preclinical study advances in laboratory derivation and stimulation/transplantation of these cells as a strategy for the treatment of spinal cord injury.The evidence supports that the Chx10+V2a interneurons act as a new therapeutic target for spinal cord injury.Future optimization strategies should focus on the viability, maturity, and functional integration of Chx10+V2a interneurons transplanted in spinal cord injury foci.
Key Words: axons; central nervous system; central pattern generator; Chx10; differentiation;interneurons; locomotion; motor neurons; propriospinal; spinal cord injuries; therapy; transcription factor; transplantation; V2a neuron
Introduction
Spinal cord injury (SCI) is a serious disabling neurological disease, which can lead to movement disorders, pain, and autonomic nerve dysfunction.As a consequence, SCI brings devastating physical and economic burdens to patients and families.The latest research shows that the incidence rate of SCI in the United States is 502–583 cases per 100,000 people each year (GBD 2017 US Neurological Disorders Collaborators et al., 2021).
The spinal cord houses networks of interneurons that work together to coordinate movement.The introduction of spinal interneurons in spinal cord networks is beneficial to SCI regeneration.These distinct neuron subclasses play specific functional roles in timing, rhythmicity, intensity, and coordination of locomotion.The spinal locomotor networks integrate diverse inputs that ultimately direct motor neuron innervation of skeletal muscle.The V2a subclass has been identified as an ipsilateral, excitatory premotor interneuron,which has been implicated in many processes from left-right limb alternation to fast swimming in zebrafish (Menelaou et al., 2014).Of note, ablation of the Chx10 transcription factor, which is critical for V2a fate specification, leads to motor deficits in skilled reaching and diaphragmatic activity in mammals(Jensen et al., 2019).Furthermore, diseases, such as SCI, amyotrophic lateral sclerosis, and traumatic brain injury (TBI), feature varying degrees of Chx10+V2a interneuron loss, corroborating their functional importance(Zholudeva et al., 2017; Salamatina et al., 2020; Zavvarian et al., 2020).Thus,the role of Chx10+V2a interneurons in locomotion and autonomic nervous system regulation is thought to be robust, but their precise role in spinal motor regulation has not been fully explored.
Accordingly, this article summarizes the developmental origins of Chx10+spinal interneurons and provides background regarding the molecular characteristics of V2a interneurons, including their transcriptome and functional roles with particular emphasis on the emerging science surrounding the role of V2a interneurons in SCI as potential therapeutic targets.This review will aid in better understanding of Chx10+V2a interneuron regulation mechanisms involved in producing therapeutic strategies for SCI.
Search Strategy
The articles used in the present Chx10 interneuron review were retrieved using search terms previously reported by Dougherty et al.(2017).The studies cited in the present review were retrieved by an electronic search of Web of Science, Google Scholar and PubMed databases.Searches were performed from January 2000 to March 2022.The time of literature search was April 2022.The search strategy and selection criteria utilized the following keywords and terms: V2a neurons (MeSH Terms), Chx10 (MeSH Terms), spinal cord injuries (MeSH Terms), spinal interneurons (MeSH Terms),central pattern generator (MeSH Terms), differentiation (MeSH Terms), and transcription factor (MeSH Terms).We also used various combinations of the above search terms to access the literature more specifically.No limit was placed on the year of publication or authorship, and the present review includes 71 references.The seminal literature is illustrated in Figure 1.

Figure 1|Timeline showing the role of Chx10+V2a interneurons in spinal motor regulation and diseases in the literature.
Early V2a Progenitor Signals
The ventral spinal cord is segregated off into a progenitor domain and sites of future mature neurons, which is determined by the unique combination of transcription factors and gradient of signaling molecules.V2a interneurons originate from p2 domain progenitors.One of the earliest molecular indicators of a fate differentiation of V2 progenitors is the expression of Lhx3 marked by the absence ofIsl1
.This profile differentiates the earliest V2 cells from progenitor motoneurons (MNs) in mouse (Wu et al., 2006).Typically,Onecut transcription factors expressed in nascent motor neurons are tasked with maintaining the expression of Isl1; however, a recent study has identified thatNkx6.2
, occurring downstream of Onecut transcription factors in the spinal cord, also contributes to the generation of V2a interneurons (Sander et al., 2000) and is critical for their proper migration as demonstrated by a transgenic mouse model (Toch et al., 2020).Another set of early V2a drivers include the E3 ubiquitin ligase,RNF220
, and the zinc finger protein,ZC4H2
,which cooperate to degradeDbx1/2
andNkx2.2
, which are ventral progenitor factors directing the p0, p1, and p3 domains (Kim et al., 2018).Moreover,coexpression ofRNF220
andZC4H2
induces the expression of Nkx6.1, an important player in the patterning of the p2 and pMN domains (Kim et al., 2018).Approximately 50% of V2 cells expressing these early molecular characteristics will become V2a interneurons, and the remainder V2 cells will become ipsilateral, inhibitory V2b interneurons in the adult mouse spinal cord(Al-Mosawie et al., 2007).V2a/V2b Differentiation
Notch signaling is a subsequent driver of V2 fate in V2 progenitors.Specifically,a high presence of Notch induces the expression of the Hes5 homolog,her15.1
, to drive the V2b lineage in zebrafish (Mizoguchi et al., 2020).After the specification of V2b immature interneurons, a reduction in Notch signaling is necessary to further drive the mature V2b fate and expression of the V2b markers,Tal1
andGata3
(Mizoguchi et al., 2020).Additional evidence has demonstrated that the Foxn4 transcription factor cooperates with Mash1 to specify V2b in the early stages in transgenic mice (Li et al., 2005).Incidentally,the reduction in Notch signaling relieves the V2a program from inhibition,inducing the Chx10 (Vsx1/2)-driven transcriptional program, which includesSox14
,Shox2
, andVglut2
, genes integral to maturation of the V2a interneuron subtype in mice and chicks (Dougherty et al., 2013; Katsuyama et al., 2021).In addition to Notch signaling, transforming growth factor beta (TGF-β) signaling induces V2a fate through the activation ofSmad3
(Lundfald et al., 2007).Figure 2 provides a consolidated visual summary of the V2a interneuron differentiation program.The differentiation of V2a/V2b interneurons ensures different aspects of the stereotypic pattern of locomotor activity, including left-right alternation (V2a), flexor-extensor alternation (V2b), and robustness and rhythmicity (V2d).The production of the appropriate balance of the differentiation of V2a/V2b cells on a precise developmental schedule is critical for neural development (Francius et al., 2016).
Figure 2|Developmental signals and transcription factors specifying V2a interneurons.
Chx10 Repressive Elements and Coregulation of V2a and Motoneuron Fates
The Chx10 homeobox protein is present in V2a interneurons (Clovis et al.,2016).While Lhx3 is sufficient to induce the formation of V2a interneurons in spinal neural progenitors, Chx10 is required to trigger the expression of subsequent V2a specification genes, such asSox14
,Shox2
, andVglut2
.UnlikeLhx3
, ectopic expression of Chx10 is sufficient to initiate Sox14+V2a interneurons in MN areas as demonstrated in chicks.Interestingly, Chx10 simultaneously induces V2a identity and suppresses MN fate specification by suppressing the MN genes,Hb9
,Isl1
,Isl2
, choline acetyltransferase (ChAT
),andVAChT
.Chx10 also suppresses the expression of non-V2 genes, such asDbx1
andOlig3
(Clovis et al., 2016).Constitutively activated/repressed forms of Chx10 have been generated by fusing the C-terminal half of Chx10, containing the DNA-binding homeobox domain, to the VP16 transcriptional activation domain or the Engrailed transcriptional repression domain.Experiments using these constructs have demonstrated that Chx10 represses MN genes through two independent mechanisms.First, the transcriptional repressor drive of Chx10 is responsible for promoting V2a fate by and competitively binding to the response element normally occupied by the Isl1-Lhx3-NLI cofactor complex.This is interchangeably termed the “hexamer response element (HxRE)” due to its bond with the self-dimerizing NLI cofactor.Chx10 secures cell fate in V2a interneurons by binding to and preventing the activation of HxREs, thereby inhibiting ectopic activation of the MN differentiation program and enabling the expression of interneuron determinants (Debrulle et al., 2020).Second,RNA-seq analysis has identified 12 genes coregulated by either Chx10 or Isl1-Lhx3, all of which have been implicated in MN development or neural functions, and at least two of which interact with Chx10 or Isl1-Lhx3 through shared response elements in mice (Clovis et al., 2016).Both Vsx1 (paralog that developmentally predates Chx10) and Chx10 inhibit the Isl1-Lhx3-NLI hexamer from binding to the response element in mice and in chick neural tubes(Debrulle et al., 2020).Although Vsx1 promotes V2a identity and inhibits MN differentiation, it is not necessary for V2 fate establishment in mouse (Debrulle et al., 2020).Even though Isl1 is expressed in all MN subtypes in mice and chicks, resulting in the suppression of Chx10 expression and V2a interneuron specification, Isl1 does not demonstrate V2a fate suppression in human MNs,suggesting the existence of a distinct suppressive mechanism (Qu et al.,2014).
Similar to Chx10 competitively binding and repressing the hexamer response element, HB9 (a MN-specific homeodomain transcription factor)competitively binds the Lhx3-NLI complex (interchangeably termed the“tetramer response element”), which inhibits the binding of Lhx3/NLI,preventing the activation of Chx10 and the V2 program in chick neural tubes(Lee et al., 2008).Hb9 inhibits Vsx1 and Chx10 expression in early MNs.For example, Hb9 binds to theChx10
-TeRE complex and prevents its activation by the MN hexameric complex.Hb9 further secures MN fate by preventing Vsx1 and Chx10 activation, actively promoting MN development while suppressing V2a interneuron fate (Debrulle et al., 2020).Role of V2a Interneurons in Central Pattern Generation
Previous studies have implicated the presence of interneuron components in the central pattern generation (CPG) control of hindlimb locomotion in rodents (Crone et al., 2008; Rybak et al., 2015; Ziskind-Conhaim and Hochman, 2017).For example, two models by which the locomotor CPG may integrate first and last order interneurons include interconnected classical half-center oscillators, resulting in a unit burst generator (UBG) that generates an excitatory MN drive and coordinates locomotor rhythms (Grillner et al.,2011) or a two-level architecture model comprised of a half-center rhythmgenerator (RG) and a pattern formation (PF) network with reciprocal inhibitory interactions between antagonist neural populations at each level (McCrea and Rybak, 2008).Despite the available evidence, the role of interneurons in CPG remains debated (McCrea and Rybak, 2008) as a majority of available data is derived from computational models or molecularly defined (rather than functionally defined) interneuron populations in the locomotor circuitry (Rybak et al., 2015).
A model presented by Crone et al.(2008) places the V2a interneurons in a key position within the mammalian CPG, implicating the interneuron type with the integration of sensory, supraspinal, and rhythm-generating inputs as well as influencing both ipsilateral and contralateral circuitries to achieve coordinated locomotion (Crone et al., 2008; Grillner and Kozlov, 2021).The inability of sensory inputs to produce locomotor rhythms suggests that V2a interneurons may serve as a sensory gateway into the CPG, while the inability to evoke locomotion from brainstem stimulation suggests either that V2a interneurons mediate supraspinal inputs to the CPG or that V2a interneurons expressed in the lower brainstem generate locomotor signals to the spinal CPG.Additionally (as we will detail in the subsequent section), V2a interneurons receive rhythmic synaptic drive, which increases with locomotor frequency, prompting the recruitment of additional V2a interneurons at higher locomotor frequencies (Rybak et al., 2015).
The available data suggest significant differences in the CPG organization of swimming versus walking animals (Rybak et al., 2015).For instance, V2a interneurons are known to provide excitation of MNs in mammals but are not capable of initiating rhythmic burst activity as they do in zebrafish.Moreover,mammalian CPG is more complex than zebrafish CPG because it is required to coordinate all the muscles from different limbs to enable walking, trotting,and galloping.In the next section, we will discuss the precise roles of V2a interneurons in locomotor regulation with an emphasis on structural and morphological underpinnings.
Role of V2a Interneurons in Initiation,Frequency, Amplitude, and Timing Control of Locomotion
Lundfald et al.investigated the projection pattern and transmitter phenotype of the V2 population as well as the overlap of EphA4 expression with V2 interneuron markers (Lundfald et al., 2007), and they indicated that all V2 subtypes project ipsilaterally and that V2a interneurons provide ipsilateral glutamatergic excitatory input to MNs and V0 commissural interneurons.Ipsilateral projecting V2a interneurons additionally provide excitatory input to inhibitory glycinergic interneurons (Lundfald et al., 2007).These researchers also reported that the EphA4 axon guidance molecule is an important regulator of V2 interneuron projection during embryogenesis and in neonates but indicated that it is not essential for maintaining ipsilateral projections in adult mice (Lundfald et al., 2007).
Early born V2a interneurons exhibit monosynaptic inputs on local MNs and are activated during strong movements, such as escape and fast swimming.In contrast, later born interneurons are active upon weaker, sustained movements, such as weak swimming (Kimura et al., 2006), suggesting two distinct subpopulations of V2a interneurons.In a subsequent profile of V2a interneurons in zebrafish, two types of V2a interneurons have been identified as follows: one class that forms synapses with other excitatory V2a interneurons and inhibitory V0d interneurons to provide timing control,and the second type forms synapses exclusively with local MNs to provide amplitude control (Menelaou and McLean, 2019).In addition, dorsal V2a interneurons project longer distances than ventral V2a interneurons, and they achieve this in a dorsal-biased manner.Dorsal V2a interneurons have higher synapse densities proximally, likely allowing for increases in swimming frequency when more output is required by the less excitable, dorsal-most motoneurons as demonstrated in mice (Menelaou and McLean, 2013).
Other researchers have clarified that distal targeting descending(unidirectional) V2a interneurons predominantly target slow MNs in“bursting” potentiation, while bidirectional V2a interneurons preferentially target fast motor neurons in a weaker, “non-bursting” potentiation pattern in adult zebrafish (Song et al., 2018; Sternberg et al., 2016; Wiggin et al.,2012).The case for frequency-dependent V2a regulation of MNs is further demonstrated by silencing of the excitatory drive in larval zebrafish, in which fast escape responses, diminished spontaneous slow locomotion, and lower locomotor frequencies have been observed in a model of Chx10+ ablation(Sternberg et al., 2016).Calcium imaging has demonstrated that suppression of fast locomotion is mainly driven by diminished recruitment of dorsal motoneurons but that slow locomotion is likely inhibited by silenced ventral V2a interneurons.However, slow locomotion is not fully abolished due to overlapping glutamatergic drive of ventral V0 neurons at those frequencies.This evidence corroborates the dorsal-biased density distribution of V2a-MN synapses.
Sternberg et al.(2016) demonstrated that V2a interneurons are necessary for the initiation of fast swimming, agreeing with the earlier findings of Kimura et al.(2013), which showed that channelrhodopson-forced excitation of hindbrain reticulospinal V2a interneurons is sufficient to evoke a swimming response in zebrafish (Kimura et al., 2013; Sternberg et al., 2016).In addition to fast/slow regulation, a recent study has indicated that V2a interneurons form three excitatory circuit modules activated at 3 Hz (slow), 3–8 Hz(intermediate), or > 8 Hz (fast), thereby eliciting subsequent excitatory postsynaptic potentials (EPSPs) in zebrafish (Song et al., 2020).Song et al.(2020) reported that these circuits obey a hierarchy (fast < intermediate <slow) such that the fastest recruited module controls the rhythm generated,instructing the preferred speed range of the slower modules (Song et al.,2020).The transformation of tonic excitation into rhythm-like bursts is intrinsic, persisting even in the presence of synaptic inhibition.Moreover,the minimum burst frequency matches the recruitment frequency observed during active swimming.Importantly, stimulation of descending command axons is sufficient to initiate intrinsic bursting prior to swimming activity,substantiating the previously implicated role of V2a interneurons in initiating locomotion (Song et al., 2020).
While studies have previously implicated V2a interneurons in locomotor vigor determination, Wiggins et al.(2012) demonstrated through ablation techniques that V3 excitatory interneurons display a more exclusive regulatory capacity of locomotor vigor as demonstrated in swimming larval zebrafish.Collectively, the evidence supports a diversity of V2a distribution, projection,and electrophysiological signatures that endow a range of locomotor operations (Ampatzis et al., 2014).Perhaps none was more evident than the incremental recruitment of slow, intermediate, and fast V2a-MN modules,which endow an intrinsic “gearshift” (Ampatzis et al., 2014) for the regulation of locomotor speed.
V2a Interneuron Modulation
In addition to the unique features that confer V2a output, a range of modulating inputs has been elucidated.For example, the frequency regulation of V2a interneurons in zebrafish is regulated by inhibitory V1 inputs to V2a-MN circuits during slow or fast swimming (Kimura and Higashijima, 2019).In neonatal mice, perforated patch clamp recordings have demonstrated a potent depolarizing effect of serotonin (5HT) on V2a interneurons, increasing their mean firing rate (Dietz et al., 2012), which agrees with the well characterized effects of serotonin on locomotion.To date, the majority of literature presented has focused on controlled study of the V2a interneuron frameworks based on fictive locomotion or simulated excitation.
Knafo et al.(2017) advocated the importance of studying the of role of V2a interneurons in locomotion within the context of an intact mechanosensory feedback system; they report that during active locomotion, fast circuits are enhanced, while slower circuits are repressed, ultimately resulting in the increased recruitment of spinal motor neurons for prolonged fast swimming in zebrafish.When Rohon-Beard neurons (glutamatergic mechanosensory neurons) are silenced, locomotor speeds decrease.Electrophysiological observation has confirmed that Rohon-Beard neurons form a monosynaptic connection with dorsal (“fast-type”) V2a interneurons, likely driving enhanced speeds during active locomotion in zebrafish (Knafo et al., 2017).Rohon-Beard neurons offer a glimpse into the potential mechanosensory feedback regulation of V2a interneurons by other cell types as Rohon-Beard neurons exist transiently and primarily in zebrafish (Reyes et al., 2004).Finally,developmental deficits can alter the proper distribution and maturation of spinal interneuron subtypes.In addition to the cascades of transcription factors previously described, deletions, such as the loss of Wt1 in dl6 spinal neuron progenitors, have been shown to decrease V2a interneurons in embryonic mice and are correlated with slower and uncoordinated locomotion in mouse (Schnerwitzki et al., 2018).A visual summary of the V2a spinal network is presented in Figure 3.
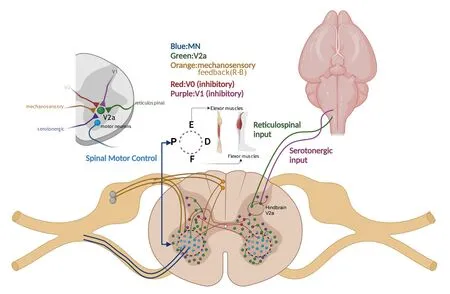
Figure 3|Spinal motor networks.
Role of V2a Interneurons in Locomotor Pattern Generation
As previously mentioned, V2a interneurons play a role in rhythm generation and pattern formation for mouse locomotion.Using transgenic Chx10 mice, Crone et al.(2008) indicated the role of V2a interneurons in leftright alternation during fictive locomotion (Crone et al., 2008).Further work by the same group using this transgenic line has demonstrated that loss of left-right limb coordination occurs only at high-speeds.Alternating hindlimbs (trotting) and synchronous movement (galloping) is achieved by the functional inhibition or excitation of the commissural interneuron (CIN)pathway, respectively.Thus, the occurrence of synchronicity in Chx10-null mice denotes an intact excitatory CIN system and suggests that loss of V2a excitatory inputs may impact the V0 class of CINs (necessary for maintaining left-right alternation) and subsequent functional inhibition.This V2a-V0 inhibitory network is likely predominant only at higher speeds in mouse (Crone et al., 2009).A follow-up study by Zhong et al.(2010) confirmed that Chx10-deletion related abnormalities in left-right limb coordination are only present during high-frequency locomotion.The same group demonstrated with electrophysiology that V2a interneurons fire rhythmically with ventral-root recorded motor activity (approximately 50%) and increase their rhythmicity during higher frequency fictive locomotion to maintain left-right alternation at high speeds in mouse (Zhong et al., 2010).
In a subsequent report, Zhong et al.(2010) identified three electrophysiological subclasses of V2a interneurons, namely, tonic, phasic,and delayed onset, all of which are rhythmically activated during fictive locomotion.These subclasses form synapses with other V2a interneurons of the same subclass and demonstrate electrical coupling, supporting a physiologically-relevant neuronal circuit.Although a consecutive study by Doughtery and Kiehn failed to confirm electrical coupling of V2a interneurons during fictive locomotion, they similarly reported 40–65% rhythmic firing of V2a interneurons in mice (Dougherty and Kiehn, 2010a, b) with the majority in phase with either flexor (L2/L3) or extensor (L4/L5)-related ipsilateral ventral root, partially corroborating Zhong’s observations.Further, Doughtery and Kiehn confirmed the earlier findings of Crone et al.that V2a interneurons mainly fire in response to excitatory synaptic inputs with little to no inhibitory inputs, suggesting that the role of V2a interneurons in rhythmic patterning is predominantly one of maintenance rather than initiation.
The functional confirmation of V2a-related rhythmic patterning has been described in forelimb/hindlimb alternation in rodents by Hayashi et al.(2018).Dougherty et al.(2013) demonstrated flexor- or extensor-biased V2a subtype firing.Hayashi et al.(2018) described a subpopulation of postmitotic V2a interneurons that downregulate Chx10 expression in mice; they also reported that Chx10+V2a interneurons are primarily located in the lumbar spinal cord but that Chx10-deficient V2a interneurons are relegated to more cervical regions and possess supraspinal projection capacity.This rostrocaudal Chx10 gradient likely confers the forelimb ability in the more rostral regions while maintaining the autonomous, central pattern generating activity of the hindlimbs.The implication of two distinct V2a subpopulations in the regulation of left-right alternation at high locomotor frequencies has been further demonstrated by genetic ablation (Rybak et al., 2015).Doughtery et al.(2013) elaborated on the 25% of V2a interneurons that express Shox2 but lack Chx10.Unlike the inhibition of Chx10+V2a interneurons,which demonstrate deficits in left-right alternation, inhibition of Shox2-expressing interneuron outputs produces deficits in rhythmogenic activity while maintaining intact left-right alternation (Dougherty et al., 2013).These findings may shed light on a unique subpopulation of rhythm-generating excitatory interneurons in the spinal cord.
To characterize the integration of sensory modulation of rhythm generating circuits, Li et al.(2019) focused on Shox2-expressing interneurons (a subset of which express Chx10), and they found that low-threshold stimulation of either flexor or extensor ankle afferents produces mostly inhibitory postsynaptic currents.This input does not differ for flexor or extensor afferent stimulation nor does the response differ by interneuron type (rhythm-generating or pattern-forming premotor neurons).Moreover, afferent stimulation is sufficient to reset fictive locomotion in mouse (Li et al., 2019) in a flexorbiased manner, which is contrary to the opposing effects generally observed in locomotion in response to either flexor/extensor afferent stimulation(Rossignol et al., 2006).The phenomenon reported by Li et al.(2019) may be a transient developmental effect as their rodent population was less than four days old.
Insight into asymmetric generating locomotor commands comes from a rodent study describing imbalances in descending excitation from reticulospinal neurons on either the right or left side.This unilateral Chx10+V2a interneuron excitation causes contraction of ipsilateral muscles and simultaneous suppression of ipsilateral force generation, thereby reducing ipsilateral axial turning in mouse (Cregg et al., 2020).This mechanism is in contrast to the inhibitory networks governing limb alternation.Interestingly,just as Chx10 is expressed in diverse subsets of cells in the spinal cord,brainstem expression occurs in reticulospinal neurons as well as in local,excitatory interneurons in mice (Chopek et al., 2021).Similar to the spinal cord, Chx10interneurons in the brainstem form synapses with one another and express rhythm-generating electrophysiology in mouse embryonic hindbrain (Bubnys et al., 2019).In addition, Chx10interneurons in the brainstem synapse and enhance the output from reticulospinal neurons that drives locomotor commands.In an anterograde labeling study, researchers have confirmed the direct synapse of corticospinal fibers from the motor cortex onto Chx10-expressing spinal interneurons and noted their importance in skilled reaching behaviors through corticospinal tract (CST) transection and chemogenetic ablation of Chx10in mice (Ueno et al., 2018).Excitatory interneurons establish an internal feedback loop in the mammalian skilled reaching circuit.Specifically, skilled reaching deficits resulting from chemical ablation of V2a interneurons elicit the recruitment of a fast cerebellar-motor feedback loop (Azim et al., 2014).This compensatory loop is based on the existence of “internal copy” circuits in cervical propriospinal neurons but may be present in other spinal cord regions to compensate other motor dysfunctions.
Role of V2a Interneurons in Motor Disease/Injury
Studies using amyotrophic lateral sclerosis (ALS) mouse models have shown a susceptibility of V2a interneuron degeneration in more advanced stages of the neurodegenerative disease (Salamatina et al., 2020).Both spinal and brainstem V2a interneurons degenerate in ALS model mice, and previous preclinical animal studies have indicated that V2a interneuron degeneration may contribute to accessory respiratory muscle (ARM) failure during the late stages of ALS (Romer et al., 2017).Another important study by Butts et al.(2017) showed that transplantation of human iPSC-derived V2a interneurons into the spinal cord of healthy mice are viable after transplantation (Butts et al., 2017).Follow-up experiments by Zholudeva et al.(2018b) have subsequently demonstrated that transplanted iPSC-derived V2a interneurons and neural progenitor cells (NPCs) are viable in rat cervical spinal cord of injured rats.Preclinical fetal graft experiments have also demonstrated that transplanted cells retain their phenotype within the injured spinal cord,allowing the possibility of replacing interneuron populations that may be diminished after disease or injury.The loss of V2a interneurons mirrors the pattern of MN loss, likely due to the direct connectivity of the two, but the V1 inhibitory interneurons that also directly synapse on MNs demonstrate only a 50% loss comparatively at the same disease stage.While the precise reasons for this selective loss of spinal motoneurons in ALS are unknown, the direct connectivity of V2a interneurons to MNs versus the multisynaptic connections of V1 interneurons supports a “trans-synaptic” disease etiology (Salamatina et al., 2020).In another ALS mouse model, investigators have found that loss of V2a interneurons during the later stages of the disease contribute to failure of recruitment of accessory respiratory muscles (ARMs) used for ventilation during increased respiratory demand (Romer et al., 2017).This loss occurs after an initial compensatory period when V2a interneuron drive increases ARM activity and increases ventilation.
Spontaneous synaptic loss after TBI alters spinal interneuron networks,thereby playing a large role in coordinating postinjury neuroplasticity and synaptic transmission in mice (Zavvarian et al., 2020).This has been hypothesized to occur due to the neurotransmitter imbalance that results from spontaneous postinjury sprouting that inactivates preserved synapses(Chen et al., 2018).During high cervical SCI, there is a high degree of spinal Chx10-expressing V2 interneurons involved in injured phrenic pathways,which is detectable by retrograde tracing as early as two weeks postinjury in mice (Zholudeva et al., 2017).The multiple microcircuit organization reveals that the excitatory drive from the locomotor network is selectively conveyed from a specific V2a interneuron class to a defined pool of MNs.The modular microcircuit organization endows the spinal cord with an intrinsic gearshift that allows an increase in the locomotor speed by incrementally recruiting slow, intermediate, and fast V2a interneuron-MN modules.Each module includes a distinct subclass of excitatory V2a interneurons that make selective monosynaptic connections with slow, intermediate, or fast MNs (Ampatzis et al., 2014).This study was the first to use a Chx10-eGFP mouse line in an SCI model.Husch et al.(2012) demonstrated that after SCI, V2a interneurons are hypersensitized to 5-HT by 100–1000X due to overexpression of 5-HT2C receptors but that their excitability is not affected, which may serve as a compensatory mechanism in response to lesion-dependent downregulation of serotonin transporters in mice.Restoring serotonergic activity to motor circuits after SCI is important for recovery of locomotor function (Slawinska et al., 2014).More recently, a Sem7A knockout mouse line subjected to lumbar SCI has demonstrated that the axon guidance molecule, semaphorin7A, plays an important role in the proper targeting of serotonergic fibers postinjury.Semaphorin7A-deficient mice undergoing spinal thoracic lesion have irregular serotonergic projections and increased MN contact, resulting in impaired recovery of locomotor function (Fourneau and Bareyre, 2022).
Role of Chx10 V2a Interneurons in Functional Recovery after Spinal Cord Injury
Chx10 V2a interneurons with dense local connections within the spinal cord efficiently drive motor neuron firing (Hayashi et al., 2018).Thus, Chx10 V2a interneuron therapy may be beneficial for the recovery/repair of neural networks and functional ability.However, as the functions of Chx10-lacking V2a interneurons are mainly exhibited within motor command circuits projecting from Chx10-expressing V2a interneurons to supraspinal structures,it is unlikely that singular V2a interneuron supplementation/transplantation will sufficiently correct host circuitry and connectivity.In contrast, other interneuron subtypes, such as V1 interneurons, normally exhibit ipsilaterally projecting axons and may therefore be better monotherapeutic candidates forrepair of spinal circuitry caudal to the injury (Hayashi et al., 2018; Zholudeva et al., 2018a).
Advancement of the understanding of the role of Chx10-expressing V2a interneurons in SCI recovery has been facilitated byin vitro
derivation and culture.The generation of V2a interneurons from mouse embryonic stem cells has been published by a few groups (Brown et al., 2014; Iyer et al., 2016;Thompson et al., 2017).Iyer et al.(2016) specifically demonstrated the ability of mouse embryonic stem cell (mESC)-derived V2a interneurons to form functional synapses with MNs in coculture.Another group has generated functional V2a interneurons and MNs from conditionally immortalized human fetal spinal cord cells, which retain their molecular identity (Cocks et al., 2013).Importantly, one group has generated V2a interneurons from human pluripotent stem cells that survive and grow synapses afterin vivo
host transplantation, demonstrating a viable, transplantable V2a interneuron derivation protocol (Butts et al., 2017).Epidural stimulation, physical rehabilitation, pharmacological therapy, and gene therapy have all been successfully applied to modulate postinjury neuroplasticity of spinal interneurons (Zavvarian et al., 2020).In particular,transplantation of V2a interneurons after SCI is believed to improve functional recovery.To date,in vivo
transplantation of mouse ESC-derived V2a interneurons using hyaluronic acid (HA) hydrogels has produced viable V2a interneurons in the host that grow local and distal processes around the lesion site (Thompson et al., 2018).One step further, Thornton et al.(2018)demonstrated in a rat model of spinal cord transection that a combination of epidural stimulation, motor training, and olfactory ensheathing cell transplantation increases the number of postinjury Chx10+V2a interneurons,suggesting a protective postinjury protocol in rats.A more nonspecific approach has been utilized with a one-time docosahexaenoic acid (DHA)bolus to mice prior to cervical hemisection, which is sufficient to promote robust sprouting of corticospinal and serotonergic fibers.Corticospinal fibers sprouting to the denervated side contact V2a interneurons, while increased serotonergic fibers and synaptophysin directly contact MNs.DHA has been proposed to promote sprouting postinjury by upregulating miR-21, which targets PTEN, a well-known repressor of anatomical plasticity in mice (Liu et al., 2015).Other nontargeted strategies, which have (incidentally) improved the proportion of postinjury V2a interneurons and MNs, is transplantation of human-induced pluripotent stem cell (hiPSC)-derived preoligodendrocyte progenitor cells.Interestingly, transplantation in combination with glial scar ablation results in a lower yield of both V2a interneurons and MNs, suggesting that the local environment at transplantation can modulate the fate of the transplanted cells in rats (Patil et al., 2021).This is in line with emerging evidence that glial scar ablation may contribute to increased neuronal cell death and demyelination due to the increased presence of macrophages and other proinflammatory factors (Adams and Gallo, 2018).Finally, Zholudeva et al.(2018b) reported that transplantation of NPCs enriched with mouse ESC-derived V2a interneurons into injured C3–C4 injury sites migrate and project neurites one month after cervical injury;in particular, the V2a/NPC transplant recipients had greater recovery from respiratory challenge even though only 12% of their transplanted V2a interneurons expressed mature neuronal markers (Zholudeva et al., 2018b).A summary of therapeutic stimulation/transplantation of Chx10+V2a interneurons is shown in Figure 4.Evidence of noncervical transplantation of Chx10+V2a interneurons and associated functional outcomes also remains to be observed.In humans, the targeting of damaged spinal circuits by less invasive therapies, such as epidural stimulation or physical rehabilitation, also remain to be better understood and optimized.The V2a spinal interneurons have become a therapeutic target for ongoing work, and stem cell-derived V2a interneurons are now even being engineered and transplanted for repair of injured phrenic pathways (Fortino et al., 2022).

Figure 4 | Role of Chx10+V2a interneurons in functional recovery after SCI.
Discussion
Summary of the results
Though the role of Chx10-expressing V2a spinal interneurons has been widely described, this review is the first research summary of the interneuron subtype in relation to its regulatory role in spinal locomotion and spinal cord disease.We surmise that V2a interneurons originate from the p2 progenitor domain and are induced toward their fate by early Notch and TGF-β signals,which trigger the endogenous inhibition of MN fates.Subsequently, a spatiotemporally precise developmental program is facilitated by a series of transcription factors, endowing the appropriate balance of V2a/V2b commitment.
Anatomical studies have placed Chx10+V2a interneurons in spinal cord lamina VII, providing ipsilateral glutamatergic input to MNs, V0 interneurons, and inhibitory interneurons.V2a interneurons receive input from reticulospinal neurons, serotonergic inputs, and mechanosensory feedback from specialized neurons to refine their output.This robust integration positions Chx10+V2a interneurons for functional roles in the initiation of movement and regulation of speed, frequency, amplitude, and motor timing.Moreover,these interneurons hold a key position within the CPG, contributing to the regulation of forelimb/hindlimb coordination.
Numerous studies have suggested that Chx10+V2a interneurons play a crucial role in the pathological changes occurring during spinal injury/degeneration as investigated using animal models of amyotrophic lateral sclerosis, TBI, and SCI.Despite the high degree of understanding of the roles of Chx10+V2a,its role in the etiology of motor disease is less understood.The study of Chx10+V2a interneurons in therapeutic contexts may help clarify this role.Much of this work has been accomplished in SCI models.Currently, V2a interneurons derived from hESCs, cihFSCCs, hiPSCs, and mESCs have been used in transplantation techniques, yielding favorable outcomes.Future goals include an emphasis on combinatorial strategies that amplify the survival, migration, and functional integration of transplanted cells through epidural stimulation, hydrogels, and cotransplantation with support cells(Table 1).Refinement of these strategies is expected to optimize spinal circuit remodeling and functional recovery.

Table 1 | Therapeutic advances in Chx10+ transplantation strategies for SCI
Although the application of stimulation/transplantation of Chx10+V2a interneurons in SCI treatment has shown encouraging results through aforementioned experiments, the effectiveness of these approaches in patients cannot be fully verified owing to the differences between humans and other animals used in preclinical studies.To date, there is no clinical report suggesting that treatment with Chx10+V2a interneurons is associated with clinical improvements in patients with neurological disorders.Further, the current methods for primary culture of Chx10+V2a interneurons are inadequate to produce a sufficient number of cells to be utilized in a clinical study.
Conclusions and Perspectives
We conclude through our review of the literature that V2a interneurons may be a potential key therapeutic target for neurodegenerative or spinal injury disease, but their mechanism remains to be comprehensively defined.Emerging technologies, including optogenetics, chemogenetic ablation, and single-cell sequencing, hold promising possibilities.Some persisting challenges for the clinical application of V2a interneuron therapies include the lack of a comprehensive framework to address the diverse action of V2a interneurons across spinal segments.Additionally, the precise action of V2a interneurons across the different MN activation patterns required for specific behaviors remain incompletely defined.
Finally, the production of sufficient Chx10+V2a interneurons for clinical application is limited under current primary culture methods.Ultimately,although promising preclinical research has been published, there is a current lack of clinical evidence to support the therapeutic efficacy of V2a interneuron transplantation in patients with spinal or neurological disease.
Acknowledgments:
The authors thank the colleagues from the Department of Institute of Neural Tissue Engineering, Mudanjiang College of Medicine for invaluable discussions and suggestions.
Author contributions:
WYL, LXD, ZGL and YW designed and wrote the manuscript.FGZ and XYW helped to search the literature.All authors have read and approved the manuscript.
Conflicts of interest:
The authors declare that there are no conflicts of interest.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:
Henrik Boije, Uppsala University, Sweden; Shane Gao,Shanghai East Hospital, China.
Additional file:
Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- Vimentin as a potential target for diverse nervous system diseases
- Blocking postsynaptic density-93 binding to C-X3-C motif chemokine ligand 1 promotes microglial phenotypic transformation during acute ischemic stroke
- The critical role of the endolysosomal system in cerebral ischemia
- The effects and potential of microglial polarization and crosstalk with other cells of the central nervous system in the treatment of Alzheimer’s disease
- Artificial nerve graft constructed by coculture of activated Schwann cells and human hair keratin for repair of peripheral nerve defects
- Novel therapeutic strategies targeting mitochondria as a gateway in neurodegeneration

