Understanding the battery safety improvement enabled by a quasi-solid-state battery design
Luyu Gan(甘露雨) Rusong Chen(陈汝颂) Xiqian Yu(禹习谦) and Hong Li(李泓)
1Beijing Advanced Innovation Center for Materials Genome Engineering,Institute of Physics,Chinese Academy of Sciences,Beijing 100190,China
2Center of Materials Science and Optoelectronics Engineering,University of Chinese Academy of Sciences,Beijing 100049,China
3Beijing Frontier Research Center on Clean Energy,Huairou Division,Institute of Physics,Chinese Academy of Sciences,Beijing 101400,China
The rapid development of lithium-ion batteries (LIBs) is faced with challenge of its safety bottleneck, calling for design and chemistry innovations. Among the proposed strategies, the development of solid-state batteries(SSBs)seems the most promising solution,but to date no practical SSB has been in large-scale application. Practical safety performance of SSBs is also challenged. In this article,a brief review on LIB safety issue is made and the safety short boards of LIBs are emphasized. A systematic safety design in quasi-SSB chemistry is proposed to conquer the intrinsic safety weak points of LIBs and the effects are accessed based on existing studies. It is believed that a systematic and targeted solution in SSB chemistry design can effectively improve the battery safety,promoting larger-scale application of LIBs.
Keywords: battery safety,thermodynamics,kinetics,solid-state batteries
1. Introduction
Lithium-ion batteries (LIBs) have been commercialized for over 30 years.[1]Thanks to the considerably improved performance in energy density,power density,cycle life,production consistency, cost,etc., the application of LIBs has expanded from traditional consumer electronics to electric vehicles(EVs),energy storage,and other application scenarios.However,a notable increase in LIB safety accidents is reported in recent years accompanied with the rapid evolution of LIB market, which can seriously depress consumer confidence in the application of LIBs.[2]
Great efforts to explore the cause of safety accidents and improve safety performance of LIBs have been made.[3]Researchers have discovered that some severe exothermic reactions may happen under specific abuse conditions. Multiple strategies on different levels have been proposed to promote the battery safety,[2]but the safety concerns of LIBs are still haunting the whole industry and academics especially when battery chemistry with higher energy density and power density is being introduced into practice, such as layered oxide cathode with high nickel content and anode with high lithium capacity.[4–6]An innovation in battery chemistry is required to balance the safety properties and electrochemical performance of LIBs. Among the new chemistry presented for next generation LIBs, using solid electrolytes (SEs) in place of present liquid organic electrolytes (LEs) to build solid-state batteries(SSBs) seems the most promising solution.[7]In this article the mechanisms of battery safety accidents accompanied with solutions at present stage are briefly reviewed. A further discussion on the effects of solid electrolytes and solidification of LIBs on battery safety is made to show the actual potential of this strategy.
2. Basic understanding of LIB safety issue
Safety has been the most important topic for LIBs since their birth.[8]Safety research of LIBs can date back to 1990s when scientists applied quantitative measurement to study the thermal stability of LIB materials and cells.[9,10]They discovered that the sudden rise of temperature in LIBs and subsequent fire or explosion is resulted by a chain of exothermic side reactions in batteries at elevated temperatures.[11,12]The phenomenon of the uncontrollable rise of battery temperature is named “thermal runaway (TR)” of LIBs.[12]The detailed process is basically made clear in the next decades.[13]
In most battery chemistries nowadays, the first exothermic side reaction to break the stability of the battery is the decomposition of solid electrolyte interface(SEI)on anode surface formed at the initial cycle. Once the SEI decomposes at a certain temperature (commonly 80°C–120°C) the intercalated anode has chance to react with the electrolyte releasing more heat.[14]These two reactions are not fast enough to trigger TR, but are able to raise the battery’s temperature continuously-if the battery temperature management system(BTMS) fails-to trigger a specific reaction which can release heat in an enomous and uncontrollable rate. TR of a battery happens as a result.To our best knowledge,critical exothermic reactions directly resulting in TR include reactions caused by oxygen released from charged cathodes (200°C–400°C, depending on cathode chemistry),[15]reactions of plated lithium metal with electrolyte(60°C,lithium metal is induced by improper charging protocol or battery design),[16]and combustion of organic electrolyte (depending on the fulfillment of combustion triangle),[13]as shown in Fig. 1. Internal short circuit (ISC) can also cause battery thermal runaway by producing joule heat in a high rate and heating a local hot spot to more than 400°C.[17,18]In practical usage there are different causes to trigger different exothermic reactions.[14,18,19]As concluded in Fig. 1, besides an external heat source directly triggering possible reactions,thermal runaway can also be caused by severe mechanical damage or improper charging protocols.
Lots of solutions have been proposed to improve battery safety from the aspects of controlling the basic reactions in one battery or preventing TR’s propagation in a system. However,the unchanged basic chemistry for LIBs have decided its upper limit of safety. That is, as long as reactive LEs exist,their reactions with lithiated anode, leakage, and combustion have great possibilities to happen at elevated temperatures and dominate the entire accidents in terms of the distribution of reaction heat.[20]Another unavoidable risk is the decomposition of charged layered oxide cathode which will release oxygen to ignite organic electrolyte or oxidize lithiated anode inside the battery. In most cases, once inner oxygen release happens the severe exothermic reactions cannot be stopped before all possible reactants are consumed. In general, the inherent properties of present LIB chemistry decide its intrinsic safety problem.

Fig.1. Possible routes of thermal runaway of present lithium-ion batteries.
3. Safety design for solid-state batteries
As concluded above,organic electrolyte and oxygen from cathode have decided the upper safety limit of present LIBs and have become the theoretical bases for the substitution of liquid electrolyte with inflammable solid electrolytes. Great efforts are being made to develop SSBs worldwide currently and different types of SSBs, such as sulfide-, oxide-, and polymer-based, are being developed at the same time.[21–23]The development of all-solid-state batteries (ASSBs), where no liquid substance is applied, is obsessed by their weak interfacial chemical/electrochemical/mechanical contact and the difficulty in mass production.[24]As a result, a strategy to reduce the proportion of LEs in the battery (a hybrid-solid–liquid battery or quasi-solid-state battery, quasi-SSB) is proposed,so that the electrochemical and safety performances can be balanced.[25]Quasi-SSBs and ASSBs can be collectively referred to as SSBs.
Accompanied with the development and research of SSBs,some disputes have gradually emerged that SSBs are not intrinsically safe and may release large amount of heat at certain circumstances.[26,27]The argument is reasonable because it is impossible for an energy storage device to keep thermodynamically stable. However, the improvement of safety can still be confirmed considering that the reduction of LEs can reduce the combustion outside the SSBs. At the present stage,a comprehensive innovative design on battery chemistry can be realized in quasi-SSBs to enhance the safety performance of batteries. Based on the understanding of LIB safety,a systematic safety design on quasi-SSB chemistry is proposed, containing “multi-layer prevention of reactive oxygen”, “multifunctional separator”, and “multi-level protection of lithium anode”, as shown in Fig. 2. The functions of these strategies will be discussed respectively.
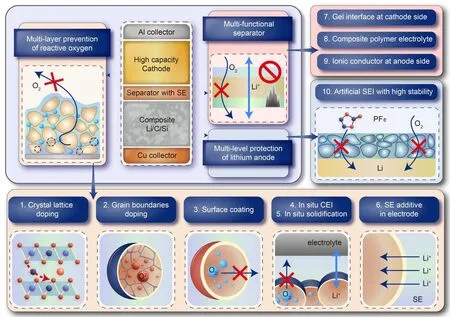
Fig.2. A rational battery safety strategy enabled by hybrid-SSBs or ASSBs.
3.1. Multi-layer prevention of reactive oxygen
Layered oxide, such as LiCoO2, LiNi1-x-yMnxCoyO2,and lithium-rich oxide are one series of the most practical cathodes.[28]However, the charged layered oxides will decompose with oxygen release at high temperatures (200°C–300°C),which can easily cause internal combustion and trigger TR.[9,29]As the release of oxygen is spontaneous,the prevention of oxygen at every step must be adopted,from releasing to transferring to reacting.In a practical quasi-SSB design,a“multi-layer prevention of reactive oxygen”can be made.
3.1.1. Doping in crystal lattice and at grain boundaries
Doping is a conventional strategy in LIBs to improve the stability of layered cathodes and is still an effective and necessary approach in development of quasi-SSBs. Although numerous reports have confirmed the improvement of thermal stability through bulk phase doping,the mechanism is still under debate.[30–32]In terms of the effects,proper doping in cathode can delay the oxygen release of deintercalated cathodes kinetically;[33]the heat release onset of cathode-LE reaction can be raised by 20°C–50°C and the rate by up to 90%.[34]Recent study also demonstrated that in Ti–Mg–Al co-doping process,Mg and Al can be doped into crystal lattice while Ti is segregated at grain boundaries and on the surface,which is able to inhibit the oxygen activity.[35]
3.1.2. Coating with solid electrolytes
Coating is another means widely used to promote cathode performance and thermal stability.[36]Unlike the doping method altering the bulk phase, coating mainly modifies materials’ surface, either by forming a stable surface or by decreasing the reactive surface area, and inhibits the interfacial reactions between charged cathode and electrolyte. Metal oxides and phosphates on cathode surface coating have been successfully realized in early 2000s.[37–39]Li4Ti5O12and LiNbO3have been proved to be effective coatings in sulfide-based ASSBs.[40,41]In recent years SE coating with good lithium-ion conductivity draws more attention because it can effectively maintain the performance of the cathode while inhibiting the interfacial reaction, which can be applied both in LIBs and SSBs.[42–44]LiCoO2coated by Li1.5Al0.5Ti1.5(PO4)3(LATP)exhibits a better discharge performance and longer cycle life as a result of an in situ formed ionic/electron mixed conductor layer at the surface. The inorganic-rich and more stable layer also elevates the cathode-LE reaction temperature by 20°C.[45]
3.1.3. In situ cathode SEI (CEI) and solidification
In-situsolidification is considered as one of the most promising methods for improving interfacial stability of LIBs and SSBs, where LEs arein-situconverted into polymer-like or inorganic SEs within the battery.[46,47]Combustion behavior can be effectively restrained with the conversion of liquid phase to solid or polymer. However,a total conversion of LEs may lead to poor rate performance;a more practical way is to form thin, dense, and stable CEI through addition of proper CEI former.[48]A well-constructed interface can effectively prevent the interfacial reaction and can act as a physical barrier to block the transfer paths of released oxygen.[49]A polymerbased LiCoO2/Li ASSB within situsolidification does not release heat till 280°C with a heating rate of 0.13°C/min. The initial self-heating temperature is much higher than conventional LIBs (80°C–120°C) and the control sample withoutin situsolidification.[50]Both of the two techniques can be applied in manufacture at electrode or cell level,thus very attractive to industry.
3.1.4. Solid electrolytes as additive in electrode
Aside from the basic role of ionic conductor and inflammable substance, SEs may have more complex effects on battery safety performance. Only a simple addition of specific SE into positive electrode can also improve safety performance of a battery as reported.[51]Solid electrolyte Li6.5La3Zr1.5Ta0.5O12(LLZTO) can act as a lithium-ion supplier to re-lithiate the surface of charged LiCoO2at elevated temperatures, which can postpone its structural decomposition and the associated release of oxygen during heating. The 1-wt% addition of LLZTO in LiCoO2/graphite full cell can postpone TR of a conventional LIB from 150°C to 200°C.
3.2. Multi-functional separator
In conventional LIBs separator is porous polyethylene/polypropylene(PE/PP)polymer film which has good capability of carrying LEs and isolating positive and negative electrode. However, conventional separator has a low melting point at~130°C and low mechanical strength. Thermal impact and mechanical damage can easily break the separator, cause ISC and result in TR.[17,52]Protocol ASSBs using pure SEs as separator have been widely reported to pass mechanical short circuit test,e.g.,nail penetrating,bending, and cutting,[53–55]but considering fabrication and performance bottlenecks of ASSBs,[56]a practical separator design for quasi-SSBs is needed at current stage. We propose a “multi-functional separator” modified with SEs to enhance battery safety. The SE reinforced separator will be denser to further block oxygen transfer paths and its enhanced mechanical strength will reduce the risk of ISC.Besides,each part of the separator also has its own function.
3.2.1. Gel interface at cathode side
The gel interface at cathode side should be multifunctional. High lithium-ion conductivity and wide electrochemical window is needed to ensure the stable performance at high voltage.[57]Such interface should also be flexible so that good electrode-separator contact is maintained. Furthermore,it may also act as oxygen absorbent to be another oxygen barrier, considering the reaction between polymer and charged cathode can be milder in some cases than cathode/LE reactions.[58,59]Polymer or organic SEs,e.g., PVDF-HFP,PMMA, and LATP, can all be applied in gel interface of separator.[60]
3.2.2. Ionic conductor coating at anode
An ionic conductor(LATP,LLZTO)pasted layer at anode side is able to form a dense stable SEI,which can better control the lithium-ion deposition behavior. Such improvement makes it possible to apply lithium metal as anode to enhance SSB’s energy density without risky lithium dendrite.[61]Risks of plated lithium in graphite or silicon will also be reduced because such formed SEI has a higher stability than that in LIBs,according to the research that the interface of LATP/LLZTO and lithium metal is stable until~290°C.Furthermore,the reaction only releases less than 10%of the heat by conventional LIBs.[62]
3.2.3. Composite polymer electrolyte
Composite polymer SEs (polymer electrolyte plus oxide or sulfide SE, for example) as the basic structure of multifunctional separator are the best balance of ionic conductivity,processing difficulties, and mechanical strength.[55,63]Their dense property not only blocks oxygen, but also reduces the total content of LEs in batteries,thus greatly reducing the fire hazard of battery TR.Besides,it is also worth mentioning that there are much fewer reports on innovative separator compared to electrode materials and electrolytes;their effects on battery safety still need further study.
3.3. Multi-level protection of lithium anode
As the lithiated anode is the most reactive in charged LIBs and SSBs,all methods mentioned above can be seen as a protection of lithiated or lithium anode. Considering the anode itself, SEI is the last defense of the anode. In conventional LIBs SEI is not stable at 120°C, but in SSBs the stability of intentionally designed SEI may be improved prominently up to 300°C. As long as the anode does not react with oxygen,even the reaction of lithium anode with SEs is acceptable, as demonstrated in Subsection 3.2.2.
3.4. Further discussion
In this part, we mainly propose a rational safety design strategy of quasi-SSBs and explain the effects of SEs in quasi-SSB chemistry on battery safety. The application scenarios,possible advantages, and disadvantages of different methods are concluded in Table 1. An ideal battery thermal performance is illustrated in Fig. S1 (supplementary information).As the temperature rises,it is inevitable for battery to become instable, but practical usage safety can be realized if the operating temperature range can be wider (normal operation at 100°C indicates an effective hydrocooling system), the heat generation rate is controlled(in battery TR the rate can reach 1000°C/min),and the total heat released is reduced. A further question is even though TR of a SSB happens and releases more heat due to its higher energy density,will it be more dangerous than present LIBs if no(or less)fire,explosion,or toxic gas release takes place in SSBs? The thermal safety of SSBs still needs testing in real applications.
Despite the expected functions,new reactions or mechanisms may also exist in SSB chemistry,resulting in new safety problems or unexpected improvement. For example,addition of SEs into positive electrode not only stabilizes the oxygen at cathode surface,but also alters the CEI properties compared to normal cells, thus enhancing its rate performance.[53]Manyto-many structure activity relationship is a charming characteristic in battery research,which needs comprehensive study and deep thinking.

Table 1. Comparisons of different safety designs.
4. Conclusion
In this article the safety issue of LIBs is briefly reviewed and a rational safety design in SSBs is concluded. The effects of the design are reviewed and mechanisms are briefly analyzed. The core solution to battery safety issue is to block hazardous oxygen and reduce the overall flammability. The positive results recently reported may encourage the developers of SSBs. To date, although some prototype hybrid-SSBs or ASSBs are already exhibited, various technical routes of SSBs are still competing. Practical SSBs still need further development and testing of real application as well as in-depth research on related basic science problems. Practical high performance and evaluable high safety may be the key topics at present.
Acknowledgment
Project supported by the National Key Research and Development Program of China(Grant No.2021YFB2500300).
- Chinese Physics B的其它文章
- A design of resonant cavity with an improved coupling-adjusting mechanism for the W-band EPR spectrometer
- Photoreflectance system based on vacuum ultraviolet laser at 177.3 nm
- Topological photonic states in gyromagnetic photonic crystals:Physics,properties,and applications
- Structure of continuous matrix product operator for transverse field Ising model: An analytic and numerical study
- Riemann–Hilbert approach and N double-pole solutions for a nonlinear Schr¨odinger-type equation
- Diffusion dynamics in branched spherical structure

