Verification of Lactobacillus brevis tolerance to simulated gastric juice and the potential effects of postbiotic gamma-aminobutyric acid in streptozotocin-induced diabetic mice
Amro Abdelazez*, Heba Abdelmotaal, Smith Etareri Evivie,Maha Bikheet, Rokayya Sami, Hassan Mohamed, Xianchen Men*
a Key Laboratory of Dairy Science, Ministry of Education, College of Food Sciences, Northeast Agricultural University, Harbin 150030, China
b College of Agriculture and Forestry, Linyi University, Linyi 276005, China
c Department of Dairy Microbiology, Animal Production Research Institute, Agriculture Research Centre, Dokki, Giza 12618, Egypt
d Department of Microbiology, Soil, Water, Environment, and Microbiology Research Institute, Agriculture Research Centre, Giza 12619, Egypt
e Department of Food Science & Hunan Nutrition, Faculty of Agriculture, University of Benin, Benin City 300001, Nigeria
f Department of Dairy Science, Faculty of Agr iculture, Minia University, Minia 61511, Egypt
g Department of Food Science and Nutrition, College of Sciences, Taif University, P.O.11099, Taif 21944, Saudi Arabia
h Colin Ratledge Center for Microbial Lipids, School of Agriculture Engineering and Food Science, Shandong University of Technology, Zibo 255049, China
i Department of Botany and Microbiology, Faculty of Science, Al-Azhar University, Assiut 71524, Egypt
Keywords:
Diabetes
Gamma-aminobutyric acid
Postbiotics
Lactobacillus brevis
In vitro
C57BL/6J mice
A B S T R A C T
The therapeutic effect of gamma-aminobutyric acid (GABA) on diabetes was spread as one of the alarming epidemics worldwide.The study aims to investigate the function of Lactobacillus brevis KLDS1.0727 and KLDS1.0373 strains as glutamic acid decarboxylase 65 (GAD65) carriers capable of generating GABA by comparing in vitro free and freeze-dried models and GABA intervention in vivo.PCR amplification of gad and in vitro i.e., (growth rate, viability at different pH, bile tolerance, and survivability in simulated gastric juice) were performed. In vivo experiments were conducted in 7 groups of C57BL/6J mice.Each group was injected with streptozotocin (ContSTZ, INSSTZ, LAC1STZ, LAC1MFDSTZ, LAC2STZ, LAC2MFDSTZ) daily except for the control (Cont).One group was injected with insulin (INSSTZ).The body weight and hyperglycemia in the blood were assessed weekly, post-euthanasia blood plasma parameters, insulin, and histological examination were evaluated.Results indicated L.brevis strains demonstrated a great tolerance to bile and simulated gastric juice in vitro (P < 0.05).ContSTZ had the highest average glucose level (6.84 ± 6.46) mmol/L while INSSTZ expressed dramatically decreed in glucose level and displayed a significant decline in the average of weekly blood glucose (–5.74 ± 3.08) mmol/L.The lowest body weight (ContSTZ) was (19.30 ± 0.25) g.Based on the blood plasma analysis, L.brevis strains improved good cholesterol properties, liver and kidney function, where most of these parameters fall within the average the reference range and prevent the development of symptoms of type 1 diabetes in vivo.As recommended, L.brevis should be commonly distributed as a postbiotic GABA in pharmaceutical and nutritional applications.
1.Introduction
The interaction between microbiota and human health disorders is becoming profoundly evident [1].The most basic factors of the intestinal microbiota balance, which dominate the gastrointestinal tract and provides the human body with several types of postbiotic substrates, are dietary habits [2].Several investigations have reported that postbiotics are known as metabiotics [3], pharmacobiotics [4],and immunobiotics [5].Bioactive probiotic compounds produced by heat-killed or live probiotics metabolism released into the environment i.e., enzymes, polysaccharides, organic acids, short-chain fatty acids (SCFA), cell surface proteins, vitamins, and lipids [4-6].
Gamma-aminobutyric acid (GABA) is an amino acid derived mainly from plants, animals, and microorganisms with a non-protein structure [7]and has a wide physiological contribution to prevent epilepsy [8], diabetes [9], asthmatic disorders [10], and cancer [11].GABA permitted as a nutritional ingredient has demonstrated antihypertensive and antidepressant activities [12,13], also has a crucial intervention role in neurological disorders [14-17].Also, as known as “psychobiotics” [18]been investigated its effects on brain function, alleviating anxiety, sleeplessness, and controlling energy metabolism.On the other hand, it is synthesized as an anti-stress protective mechanism in plants and microorganisms [19].
A wide spectrum of microorganisms can synthesize GABA using the GAD pathway, includingEscherichia coli[20],Listeria monocytogenes[21]Aspergillus oryzae[22].Different LAB is capable of produce abundant GABA levels, which greatly increase under certain conditions, where the food industry has leveraged this capacity to develop functional foods enriched with GABA [23].
Diabetes mellitus is associated with hyperglycemia and insulin deficiency.Probiotics and prebiotics can improve diabetic symptoms cure through various modes of action, such as the reduction of oxidative stress or inhibition of pro-inflammatory indicators, among others.Manyin vitroandin vivostudies have demonstrated a reduction in hyperglycemia in diabetic patients due to the use of dairy products such as yogurt, fermented milk, and yogurt enriched with potential synbiotic and postbiotics [24].
Lactobacillus brevisis a heterofermentative Gram-positive organism that can be found in human milk, cheese, sauerkraut,sourdough, silage, and intestinal tract.Additionally, the Food and Drug Administration (FDA) recognized it was generally recognized as safe (GRAS) [25].Furthermore, it one of the strains was often used in potential probiotic products [26].
Abdelazez et al.[9]suggested a further extension ofL.brevisstrains for commercial purposes in pharmaceutical and nutritional applications based on high GABA production, which has therapeutic characteristics of glucose decreasing levels in the diabetic mice model.This hypothesis explores microencapsulated strains ofL.brevisthrough a freeze-dried technique that carries agadenzyme capable of effectively expressing biologically active GABA that has the potential to regulate the adverse symptoms of type 1 diabetesin vivo.Besides,the viability of the simulated gastrointestinal environment has been investigated mimicking the harsh conditions of gastrointestinal juicein vitro.
2.Materials and methods
2.1 Bacterial strains, media, and growth conditions
Key Laboratory of Dairy Science (KLDS, Heilongjiang Province,China) has provided twoL.brevisstrains KLDS1.0727and KLDS1.0373(LAC1and LAC2), inoculated (1%,V/V) and grown in De Man Rogosa Sharpe medium (MRS Difco Laboratories, Sparks, MD,USA).Colonies were streaked on MRS agar plate and incubated at 37 °C for 24 h (Sheldon Manufacturing, Inc., Shel LAB, and Cornelius,OR, USA).The obtained colonies were randomly selected and inoculated on fresh MRS agar plates three times.A single colony of KLDS1.0727and KLDS1.0373was transferred into MRS broth and incubated at 37 °C for 18 h, then preserved in MRS broth supplemented with 30% (V/V) glycerol and frozen at −80 °C (MDF4V; Panasonic,Tokyo, Japan) as concentrated cellular biomass until further analysis.All chemicals and reagents used in this study were obtained from reliable and analytical grade suppliers in Harbin, China.
2.2 Screening of glutamic acid decarboxylase (GAD65) gene library of LAC1 and LAC2 strains
TIANamp Bacterial DNA Kit (Tiangen Biotech Co., Ltd, Beijing,China) was used to produce genomic DNA, with minor modifications to the manufacturer's extraction procedures.LAC1and LAC2strains were lysed and DNA extracted using lysozyme.
2.3 PCR amplification of the 16S rRNA gad sequence
PCR amplification of the 16S rRNA gene fragments was performed using specific primers.gadF: 5’-CCTCGAGAAGCCGA TCGCTTAGTTCG-3’;gadR: 5’-TCATATTGACCGGTATAAGT GATGCCC-3’.It was designed using oligo 6 software (Molecular Biology Insights, Inc.DBA Oligo, Inc.).
Genomic 16S rRNA was used as a template for PCR amplification as described by Abdelazez et al.[9].50 mL of each PCR mixture contained DNA template 1.0 μL, two primer pairs (ComateBio Custom Primers, Jilin, Changchun, China),gadF/gadR (10 μmol/L)2.0 μL, DNA Polymerase (2.5 U/μL) 0.5 μL, 10 ×TaqBuffer DNA polymerase (Sigma, USA) 5.0 μL, 2’-deoxynucleoside-5’-triphosphate(dNTPs) (2.5 mmol/L) 4.0 μL, ddH2O 35.5 μL.
The RT-PCR (GeneAmp PCR System 9700 Thermal Cycling Applied Biosystem, USA) consisting of denaturation stage (95 °C for 5 min), annealing stage (95 °C for 30 s, 55 °C for 1.30 min) and elongation step (1.30 min at 72 °C) was used for DNA amplification.A final extension time (10 min at 72 °C) was inserted after 30 cycles.The amplification products were electrophoresed to 1.5 % agarose gel in the TAE buffer (0.04 mol/L Tris-acetate, 1 mmol/L EDTA, pH 8).The gel was run at a stable voltage of 100 V for 1 h.Gels have been stained with 0.2 μg/mL of ethidium bromide for 15 min.The products of the PCR were visualized under a UV light transilluminator.The 100-base pair of DNA ladders (Gibco-BRL,Grand Island, NY, USA) was loaded into the first lane of each gel to determine the band size.A gel documentation system was used to photograph the gels using UV light (Bio-Rad, Hercules,CA, USA).The BLAST software (http://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to analyze sequence similarity in the GenBank database.
2.4 Cold adaption and microencapsulation of LAC1 and LAC2 using freeze-dried technique with cryoprotective agents
LAC1, LAC2have anaerobically cultivated in the MRS broth at 37 °C for 24 h and collected via centrifugation at 8 000 r/min at room temperature for 15 min, (GL-21 M High-Speed Refrigerated Centrifuge, China) then rinsed three times using 0.9% (m/V) sterile NaCl saline.LAC1, LAC2were formulated at a concentration of 2 × 1011CFU/mL in a sterile phosphate buffer solution (pH 7.2)(Autoclave HVE-50, Japan).Afterward, 1 mL of cell suspension was combined with an equivalent volume of sterile 20% solution of cryoprotective agents, i.e., (20% skim milk, 0.5% yeast extract, 1% peptone, and 4% trehalose.Sigma, USA), equal distilled water was used as a control.
The suspensions were kept at 4 °C for 2 h then transferred at−20 °C for 2 h before freeze-drying using a 24 h program (freeze temperature −40 °C; condenser set point at −60 °C overnight, the freeze dryer ALPHA 1-2 LD plus) [27].Microbiota with no cold adaptation was used as a control.For further experiments, the freezedried cells were transferred to a polyethylene tube that was sealed and stored at −20 °C.Freeze-dried samples were randomly selected periodically and rehydrated in 2 mL NaCl solution for 30 min to evaluate the cell concentration and bacterial viability.For determination, LAC1, LAC2viability was appropriately diluted and then poured onto MRS plates.The plates were incubated for 48 h at 37 °C.The colonies on the plates were counted and converted into bacterial cell numbers per mL.
2.5 In vitro assessment of LAC1, LAC2, and microencapsulated freeze-dried KLDS1.0727 (LAC1MFD), and KLDS1.0373 (LAC2MFD)
In vitroassessment of LAC1, LAC2, LAC1MFD, and LAC2MFDwere conducted for an assessment of growth rate, bile tolerance, the viability in simulated gastric juice (SGJ), and at different pH (2 and 3).Using MRS broth as the blank, the experiments were expressed in triplicate.
2.5.1 Growth rate and acid accumulation of L.brevis strains.
LAC1, LAC2, LAC1MFD, and LAC2MFDwere inoculated (1%,V/V)and grown in MRS broth supplemented with 1%L-monosodium glutamate (MSG) (Sigma, USA) at 37 °C for 36 h anaerobically in glove chambers (Sheldon Manufacturing, Inc., Shel LAB, and Cornelius, OR, USA) enables the ration of gasses (5% carbon dioxide,90% nitrogen, and 5% hydrogen).The data were expressed as optical density (OD620nm) using a UV-visible spectrophotometer (PerkinElmer,Waltham, MA, USA) after dilution using 3 folds of serial dilutions prepared in 0.1% peptone water.Moreover, pH was determined using pH meter (MP 220, Metler Toledo, Greifensee, Switzerland).
2.5.2 Survival of L.brevis strains under different pH conditions
The vitality assessment of LAC1, LAC2, LAC1MFD, and LAC2MFDunder acidic conditions performed as reported by Pieniz et al.[28]with some modifications.Free or freeze-dried strains were grown in MRS broth for 16 h at 37 °C then centrifuged at 4 °C,8 000 r/min for 5 min, and washed twice in PBS.The harvested cell pellets were adjusted to pH 2.0 and 3.0 with 4 mol/L HCl and incubated at aerobically 37 °C for 0, 30, 60 min, 1 mL of cell suspension was diluted sequentially using 0.9% saline solution,counted on MRS agar plates using various folded serial dilutions prepared in 0.1% peptone water and incubated at 37 °C for 48 h.
2.5.3 Tolerance of L.brevis strains to various bile concentrations
The bile solution was prepared separately by dissolving Ox-gale extract powder (Sigma, USA) and filter sterilized by 0.22 μm filter (Critical Syringe Filters; Critical Process Filtration Inc.).LAC1,LAC2, LAC1MFD, and LAC2MFDhave grown in MRS broth for 24 h at 37 °C aerobically, then centrifuged at 4 °C, 8 000 r/min for 5 min, and washed twice in PBS [29].Harvesting strains were exposed to 0.5%,1.0%, and 2.0% of bile.Viable bacterial cells were determined by pour plate counts of all samples using 9-fold serial dilutions prepared in 0.1% peptone water after 0 and 3 h.
2.5.4 Survival of L.brevis strains in SGJ
Determination of survivability of LAC1, LAC2, LAC1MFD, and LAC2MFDin SGJ conditions have been carried out as, the artificial saliva juice (ASJ) was obtained by dissolving 6.2 g NaCl, 2.2 g KCl,0.22 g CaCl2, and 1.2 g NaHCO3in 1 L of distilled water [30].The solution was sterilized and then cooled to 20 °C, and added 3.0 g/L ofα-amylase (Sigma, USA), then adjusted to pH 6.9.ASJ filteredviaa 0.22 μm filter.SGJ has been prepared as described by Khalf et al.[31].1 L of the solution has 3.0 g NaCl, 1.1 g KCl, 0.15 g CaCl2, and 0.60 g NaHCO3.The solution was autoclaving and cooled to 20 °C,followed by adding 3.0 g/L porcine stomach mucosa pepsin (Sigma,USA).pH was set to 3 with 4 mol/L HCl, then the simulated gastric juice was filtered through a 0.22 μm filter.Furthermore, simulated intestinal juice (SIJ) was prepared by dissolving 5.0 g NaCl, 0.60 g KCl, 0.30 g CaCl2, and 0.60 g NaHCO3in 1 L of distilled water.The solution was sterilized then cooled to 20 °C.Afterward, 3.0 g/L Oxgall, 1.0 g/L precreatin, and 3.0 g/L pepsin (Sigma, USA) were added to simulate intestinal juice filtered through a 0.22 μm filter [31].
Abdelazez et al.[9]briefly described three compartments, a simulated mouth, stomach, and small intestine model.After washing 5 mL of bacterial cells, 109CFU was suspended in 5 mL of ASJ (pH 6.9) for 5 min at 37 °C, then suspended in 10 mL of SGJ (pH 3.0) at 37 °C and incubated in 10 mL of SIJ with 1 mol/L NaHCO3(pH 7.6)added and incubated at 37 °C for 2 h, then stired at 50 r/min (HJ-3 Thermostatic Magnetic Stirrer, Jiangsu, China).The final suspended strains were diluted 10-fold and aerobically incubated in MRS agar plates for 36 h at 37 °C.Sustainability was determined as Equ (1).

whereN1is treated strains suspended in ASJ,N2is SGJ,N3is SIJ,andN0is controlled without suspended in SGJ.
2.6 In vivo experiments
2.6.1 The experiment protocol
In vitroexperimental protocol was set out in strict accordance with the recommendations in the institutional animal care and use committee of the Northeast Agricultural University under the approved protocol number specific pathogen-free rodent management(SRM)-06.All experiments were enforced with the China Ministry of Science and Technology Guide for the Care and Use of Laboratory Animals.
2.6.2 The experiment layout
LAC1, LAC2, LAC1MFD, and LAC2MFDinoculum (1%,V/V) were prepared by inoculating at 37 °C in MRS broth, then subcultured every 24 h and aged 18 h in the third generation.Bacterial cells were centrifuged at 2 500 r/min, 4 °C for 10 min to collect cell pellets and washed with PBS three times.During a 4-week trial, washed LAC1and LAC2strains were resuspended separately with PBS to obtain 5 × 108CFU/mL of freshly prepared strains.
The experiment was administered over 4 consecutive weeks in 7 groups (1 control group + 6 diabetic mice group), with 4 pathogenfree male mice in one group (n= 4).Each C57BL/6J mouse was weighted (18–24) g (Vital River Lab.Animal Technology Co., Ltd.Beijing, China, Approvement No.SCXK, JING, 2012-0001), and kept in a conventional animal room for one week under ambient conditions at (23 ± 2) °C and relative humidity at (50 ± 20)% with an artificial light cycle at 12 h light/dark.Mice independently cared for under strict pathogen-free conditions in a ventilation cage and water was givenad libitumand free access to standard pathogen-free food was provided.Afterward, except for the control group (Cont)which received 250 μL of sterile PBS daily, while the remainder of the treated groups injected a high dose of streptozotocin (STZ,180 mg/kg) (Sigma, USA) were freshly prepared in a 50 mmol/L sodium citrate buffer (pH 4.5) and subcutaneously administered under the mentioned regimen by Wu et al.[32]within 10–15 min of one-day dissolution.
Mice with glucose levels below 7 mmol/dL were excluded from the study, while those with the highest were diagnosed as diabetic and were enrolled after three days of STZ injection.The diabetic mice model was divided into 6 groups.STZ control (ContSTZ) was injected with 180 μL of STZ once time as mentioned above.For 4 weeks, the insulin group (INSSTZ) was given daily injections of insulin (Sigma,USA) in standard rodent chow at a dosage of 0.5 unit/kg.Insulin powder was dissolved in acetic acid at a final injection dose of 100 μL.The 4 groups of LAC1STZ, LAC1MFDSTZ, LAC2STZ, and LAC2MFDSTZwere oral gavage using a stainless needle with separate LAC1, LAC2,LAC1MFD, and LAC2MFDdissolved in physiological saline solution with a volume dose inoculum of 250 μL, 105CFU/mL/day.All mice had euthanasia after 4 weeks, blood serum was collected, and the organs viz., liver, pancreas, kidney, and spleen had been fixed in 10% of formaldehyde for histological examination.
2.6.3 Weekly determination of body weight and hyperglycemia of streptozotocin-induced diabetic mice
Body weight was assessed weekly (Precision Electronic Balance(0.0001 g), Hogentogler & CO.INC.USA).Also, overnight fasting 12 h and postprandial 2 h blood glucose levels were measured using a glucometer (Contour H Meter, Bayer HealthCare LLC, USA) in blood samples collected from an ocular vein.The data were expressed as the difference between 2 h postprandial and 12 h overnight fasting as Equ (2).

where NP refers blood glucose of 2 h postprandial and NF means blood glucose of 12 h overnight fasting.
2.6.4 Blood plasma biochemical analyses
The blood samples of 12 h overnight fasting were allowed to clot at 4 °C, then centrifuged at 12 000 r/min for 10 min.Plasma was stored at –80 °C for biochemical testing.Serum biochemical lipids were determined as triglycerides (TG); total cholesterol (CHOL);high-density lipoprotein cholesterol (HDL); low-density lipoprotein cholesterol (LDL).Also, glucose (GLU), and magnesium (Mg2+) were measured.Additionally, liver functions were assessed by measuring serum alanine aminotransferase (ALT), aspartate transaminase(AST), total bile acid (TBA), albumin (ALB), globulin (GLUB), and total protein (TP).The kidney functions were determined based on uric nitrogen (BUN); creatinine (CREA) and uric acid (URIC).All parameters of the test blood serum variables were determined using Beckman Coulter UniCel DxC 800 analyzer (Beckman Coulter,Miami, FL, USA).
2.6.5 Blood serum insulin determination
Purohit [33]described the serum insulin determination using ELISA kits according to the manufacturer’s instructions for insulin kits (Meimian BiotechCo., Ltd., Yancheng, China).
2.6.6 Histological evaluation
Histological assessment was performed as described by Chen et al.[34].Briefly, microscopic inspection of the treated control group and diabetic streptozotocin-induced mice was performed by euthanasia mice and the indicated organs (liver, pancreas, kidney, and spleen) were aseptically removed from all test groups.The organs were rinsed in PBS and placed in 10% buffered formalin, then rinse with graded alcohol concentrations of 75%, 85%, 95%, and 100% and xylene (100%).Subsequently, embedded in paraffin, and sectioned at 5 mm thickness then hematoxylin and eosin staining.The sections were assessed by light microscopy (Olympus, Japan) under 100 ×magnifications.Three images of different sections of the mice organs were used to obtain a mean area/mouse injury tissue.
2.7 Statistical analysis
The data were expressed as the mean ± standard deviation (SD)and analyzed by one-way ANOVA.Graphs were plotted and statistics were computed using GraphPad Prism 5 (GraphPad Software Inc.,San Diego, Ca, USA).The different letters represent significant differences between different groupandP< 0.05 is considered to be statistically significant.
3.Results
3.1 PCR amplification of the 16S rRNA sequence
The results obtained displayed the PCR amplification ofgadband length was 1 407 bp as suggested by Hiraga et al.[35], who investigatedgadofL.brevisIFO 12005 was 1 440 bp.Wu et al.[36]indicated that biochemical analysis and sequencing could confirm the co-existence ofgadmechanism inL.brevis, reflecting thegadability in GABA produced.Based on our preliminary knowledge, we compared LAC1, LAC2, LAC1MFD, and LAC2MFDstrainsin vitroand diabetic mice models.
3.2 Assessment of L.brevis strains in vitro
The various areas of the digestive system vary in different concentrations of acid.The stomach and upper intestines with the highest acidity can be dropped to less than pH 1.5, which affects the formation of microbial communities in the digestive system [37].
3.2.1 Growth rate and pH of L.brevis strains
The growth rate was exhibited as OD620nmand pH of LAC1, LAC2,LAC1MFD, and LAC2MFDstrains at 0–36 h.The results were highly significant atP< 0.05.Data are presented at 0, 18, and 36 h of LAC1((0.105 ± 0.01)%, (1.946 ± 0.03)%, and (0.981 ± 0.05)%) and pH((5.34 ± 0.23)%, (3.83 ± 0.02)%, and (3.22 ± 0.03)%) respectively,LAC2((0.108 ± 0.03)%, (1.850 ± 0.01)%, and (0.911 ± 0.01)%)and pH ((5.35 ± 0.23)%, (3.84 ± 0.43)%, and (3.30 ± 0.25)%)respectively, LAC1MFD((0.095 ± 0.20)%, (1.836 ± 0.23)%, and(1.189 ± 0.03)%) respectively, and pH ((5.26 ± 0.25)%, (3.71 ±0.44)%, and (3.28 ± 0.24)%) respectively, LAC2MFD((0.097 ± 0.05)%,(1.801 ± 0.01)%, and (1.011 ± 0.03)%) and pH ((5.29 ± 0.23)%,(3.81 ± 0.44)%, and (3.36 ± 0.26)%) respectively.
3.2.2 The vitality of L.brevis strains in different pH conditions
Probiotics have a pivotal effect on microbial balance and protection of the digestive system.It should be capable of withstanding extreme stomach conditions and adhere to epithelial cells of GIT [38].Viable probiotic bacterial counts of each LAC1,LAC2, LAC1MFD, and LAC2MFDstrains were determined for 0, 30, and 60 min after incubation under pH 2 and pH 3.The results were highly significant atP< 0.05.Generally, the recoverable CFU of each strain decreased with lower pH and running time.Table 1 showed survival of strains under different pH.After 60 min, the LAC1remained(5.13 ± 3.97) × 105CFU under pH 2 and (1.41 ± 0.07) × 109CFU under pH 3, respectively.Although LAC2remained (2.25 ± 0.35) × 105CFU and (2.14 ± 0.04) × 109CFU, respectively.Conversely, LAC1MFDremained (3.40 ± 0.31) × 105CFU and (1.76 ± 0.08) × 109CFU,respectively.Whereas, LAC2MFDremained (5.75 ± 0.15) × 105CFU and (2.47 ± 0.12) × 109CFU, respectively.

Table 1Survival of LAC1, LAC1MFD, LAC2, and LAC2MFD strains under different pH levels.
3.2.3 Tolerance of L.brevis strains to various bile concentrations
Davoren et al.[39]reported that the concentration of bile in the intestine ranged 0.5%-2.0% in the first hour of digestion.Fig.1 showed high significance ofP< 0.05 at bile concentrations (0.5%,1%, and 2%) at 0 and 3 h.LAC1growth showed a high tolerance of 2% bile at 3 h ((8.50 ± 0.16) × 109CFU) more than that displayed by LAC2((1.06 ± 0.04) × 109CFU) in the same conditions.As well,LAC1MFDindicates (8.00 ± 0.32) × 109CFU, and LAC2MFDindicates(1.15 ± 0.06) × 109CFU.

Fig.1 Bile tolerance of LAC1, LAC1MFD, LAC2, and LAC2MFD strains at (a) 0 h,and (b) 3 h.
3.2.4 Resistance of L.brevis strains in simulated gastrointestinal juice conditions
The effect of simulated gastrointestinal juice was shown in Fig.2.It demonstrated the tolerance of LAC1, LAC2, LAC1MFD, and LAC2MFDin simulated gastrointestinal juice as shown in the mentioned equation above.The findings displayed a high significance atP< 0.05.After exposure to ASJ for 5 min, LAC1and LAC1MFDremained (0.96 ± 1.01) ×109CFU and (0.97 ± 0.00) × 109CFU, respectively, while exposure to SGJ for 120 min were (0.95 ± 0.14) × 109CFU and (2.79 ± 0.21) ×109CFU, respectively, although exposure to SIJ for 120 min were(4.03 ± 0.06) × 109CFU and (0.62 ± 0.10) × 109CFU, respectively.Conversely, after exposure to ASJ, LAC2and LAC2MFDremained (1.00 ±0.06) × 109CFU and (0.98 ± 0.04) × 109CFU.However, exposure to SGJ were (2.16 ± 0.18) × 109CFU and (2.27 ± 0.16) × 109CFU respectively, exposure to SIJ were (4.15 ± 0.44) × 109CFU and(0.62 ± 0.06) × 109CFU, respectively.
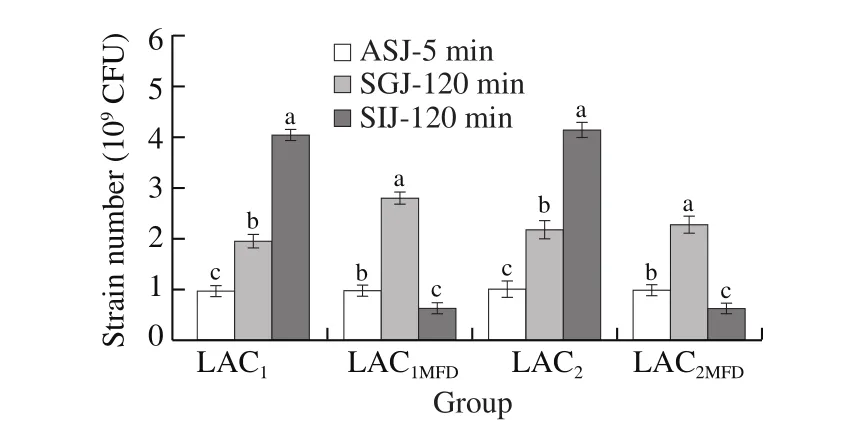
Fig.2 Resistance of LAC1, LAC1MFD, LAC2, and LAC2MFD to simulated gastrointestinal juice.
3.3 Intervention assessment of L.brevis strains on type I diabetes in vivo
3.3.1 Evaluation of body weight and hyperglycemia of the C57BL/6J diabetic mice for 4 consecutive weeks.
Blood glucose and body weight are essential indicators for type 1 diabetes.It was described as a serious metabolic disorder characterized by chronic hyperglycemia due to inadequate insulin secretion.In diabetic patients, glycemic regulation is evaluated by determining glucose levels [40].Fig.3a indicated the highest body weight of Cont and LAC1STZ((21.1 ± 0.50) g and (21.12 ± 0.63) g) and the lowest body weight of ContSTZand LAC1MFDSTZ((19.30 ± 0.25) g and (20.78 ±0.50) g), respectively.Additionally, based on Equ (2), Fig.3b showed the highest hyperglycemic was ContSTZ((6.84 ± 6.46) mmol/L),while INSSTZexpressed a dramatic drop in glucose ((–5.74 ±3.08) mmol/L).However, LAC1MFDSTZ, LAC2STZ,and LAC2MFDSTZwere slightly similar, which were (4.03 ± 1.20), (3.97 ± 2.86) and (3.64 ±1.70) mmol/L, respectively.Conversely, glucose in LAC1STZwas(3.05 ± 1.24) mmol/L.Briefly, the effect of STZ on diabetic C57BL/6J mice had a serious effect on body weight and hyperglycemia due to its deleterious effect on pancreatic cells and the liver.
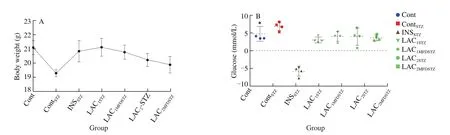
Fig.3 Determination of (a) body weight and difference of (b) glucose level during 4 consecutive weeks.
3.3.2 Biochemistry assessment of blood serum parameters
Blood serum biochemistry such as TG, CHOL, HDL, LDL,GLU, INS, and Mg2+were shown in Fig.4, the high significances were expressed in all treated groups.Group Cont showed the highest levels of INS, TG, CHOL, HDL and LDL as (13.52 ± 0.18) IU/L,(2.54 ± 0.90), (7.25 ± 0.93), (4.2 ± 0.89), and (1.14 ± 0.46) mmol/L,respectively.INSSTZshowed the lowest levels of Mg2+((1.48 ± 0.60) mmol/L)and INS ((10.60 ± 0.17) IU/L) and the highest level of blood plasma glucose of (9.4 ± 2.83) mmol/L.ContSTZshowed the lowest HDL ((1.55 ± 0.63) mmol/L) and the highest level of Mg2+((1.75 ± 0.71) mmol/L).LAC1STZexpressed the lowest levels of TG and LDL ((0.96 ± 0.39) and (0.38 ± 0.16) mmol/L) respectively.LAC2MFDSTZdisplayed the lowest levels of GLU and CHOL ((2.6 ±1.06) and (3.27 ± 0.33) mmol/L) respectively.
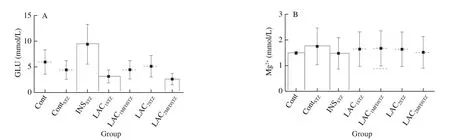
Fig.4 Biochemical blood plasma assessment.
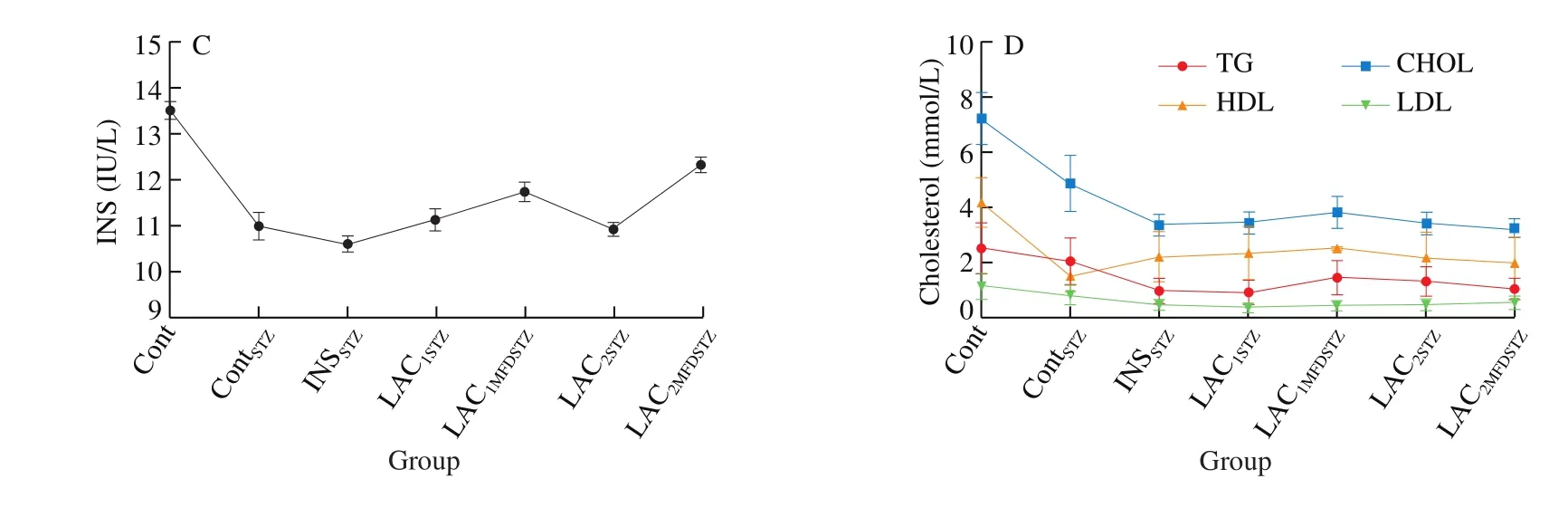
Fig.4(Continued)
3.3.3 Assessment of liver functions

Fig.5 Liver function parameters evaluation.
3.3.4 Evaluation of kidney functions
The kidney functions were showed in Fig.6.the highest BUN level was in LAC1STZ((11.8 ± 0.81) mmol/L) and the lowest was in INSSTZ((8 ± 0.26) mmol/L).Meanwhile, the highest level of CREA was in Cont ((44.3 ± 0.05) mmol/L) and the lowest level in LAC1STZ((37.4 ± 0.26) mmol/L).The highest level of URIC level was in ContSTZ((187.7 ± 1.48) ) mmol/L and the lowest in Cont((76.5 ± 1.23) mmol/L).

Fig.6 Kidney function parameters evaluation.
3.3.5 Histological evaluation
Histological evaluation was showed in Fig.7 as liver, pancreas,kidney, and spleen.Cont and ContSTZshowed that the liver has a normal morphology, cytoplasmic nuclei have clear boundaries,with no congestion, fat denatured, or inflammatory cell infiltration.Although INSSTZshowed slightly denatured and there was mild fatty degeneration.LAC1STZand LAC1MFDSTZshowed a hepatic nucleus and shrunk.Whereas LAC2STZand LAC2MFDSTZhad hepatic mild steatosis,and inflammatory cells gathered piles scattered inflammatory cells.Histological examination of Cont pancreatic cells didn’t display any abnormal morphology while ContSTZshowed significant atrophy in pancreatic islet cells with massive vacuolar degeneration although,LAC1MFDSTZand LAC2STZhad no abnormal manifestations.The spleen tissue revealed that Cont had no unusual appearance.Meantime,the number of lymphocytes displayed by ContSTZand LAC1STZwere decreased and their structure has been lost.INSSTZshowed increased trabecular and lymphatic declines.Not surprisingly, the number of lymphocytes was reduced in LAC1STZalthough LAC2STZshowed spleen trabecular and phagocyte were increased.The red pulp in LAC2STZsignificantly increased, and leukomonocyte was decreased.The kidney’s histological anatomy showed Cont didn’t have any abnormal morphology.Conversely, ContSTZshowed pronounced glomerular atrophy and shrinkage of the visually fused renal tubules.Moreover, the glomerulus was normal, and the renal tubules slightly fused into INSSTZand LAC1STZ.Furthermore, LAC2MFDSTZwas divided into tubular epithelial cells while LAC2STZand LAC1MFDSTZhad no abnormalities.
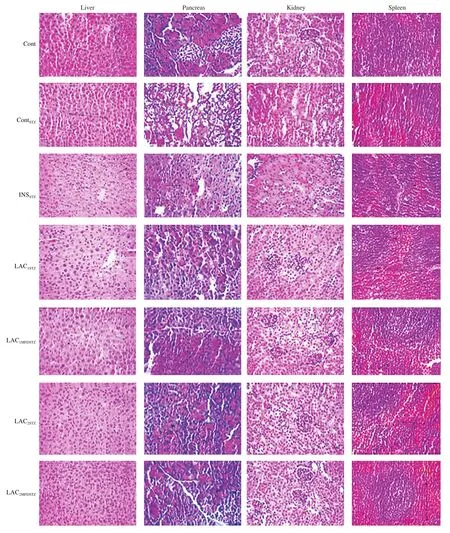
Fig.7 Histological assay of mice organs.
4.Discussion
The use of probiotics is preferable by many different modes of action to preserve a balanced intestinal microbiota equilibrium,even preventing pathogen adhesion or colonization in intestinal epithelial cells [41], also during antibiotic therapy [42].The potency of probiotics ranges from person to person based on the intestinal microbiota [43].These beneficial effects include antagonistic activity against pathogens [44,45], immune enhancement [46], modulation of stress [47], protection of the urogenital system [46], bacterial overgrowth, intestinal motility disorders, neutralization of toxic products, and intestinal microbiota [44].The pH variance promotes GAD pathway activation because it is a special mechanism that maintains cell balance [48].Despite the effectiveness of the direct addition of MSG, other alternatives have to be sought to reduce economic costs [19].
Lim et al.[7]indicated that a higher quantity of GABA could be produced in 35 h using different strains ofL.plantarumwhile the production byL.brevisreached 30 h later.Hasegawa et al.[49]indicated that the production of an adequate amount of GABA on an industrial scale was achieved by microencapsulation ofL.breviscells with higher GAD activity usingL-MSG.Numerous studies indicated thatgadnucleotide size 1 407 bp and encode a 468 amino acid [7].These results were consistent with our previous HPLC chromatogram analysis indicated the LAC1and LAC2were (1.98 ± 0.07) and (0.05 ± 0.05) g/L,respectively, which is considered the main difference between these strains.Also, PCR amplification ofgadgene band showed the same size 1 407 bp of both teste strains [9].
The critical point of the freeze-drying technique is pre-freezing temperature.However, the optimal pre-freezing temperature that varies among strains and protectants still unclear [50,51].Drying methods such as spray drying, vacuum drying, and fluidized bed drying are suitable methods based on the reduction of the water activity of microorganisms, particularly over a long period of storage [52]as well as, the freeze-drying technique consists of removing water from previously frozen cells by sublimation.Ice crystals form during this process, causing bacterial cell damage and thereby reducing the cell’s ability to survive [53].
Osmotic stress occurs during the freezing process [54].This could clarify the decline of some viable freeze-driedL.brevisstrains during some experiments in this study.The obtained results were in line with Liao et al.[55]who investigated that the pH values decreased swiftly in the first 9 h then slowly declined, attributed to the strains that reached the stationary phase.Immediately after ingestion, bacteria face various challenges such as low pH and stomach enzyme activity.On a fasting stomach, pH can drop to 1.2–1.5 [56], although pH 2 is the most common pH level [57], and viability of probiotics at pH 3 is the lowest absolute standardin vivo.Generally, LAB growth reaches the stationary phase when the pH is less than 4.5.Also, before reaching the intestines, probiotic bacteria should survive in transit through the stomach where pH 1.5–2 [58].The obtained results were in close agreement with Gautam and Sharma [59], who reported the viability ofL.brevisUN after incubation for 0, 30, and 60 min at different pH (pH 1–3) that showed a survival rate of about 91.87% at pH 1.0 after 3 h, while it reached 100% in control after 3 h.
陈山利有点慌,想躲避,然而,他却动弹不得,只得紧紧地闭住眼睛,动弹不得地感受着,从她唇边荡开的凉意,如同雪莲,携带着丝丝柔情,向下覆盖他火烧般灼疼的伤口。
Di Ciaula et al.[60]indicated that bile tolerance is one of the critical factors that can limit the number of viable microbiota in GIT.The findings in agreement with Hyacinta et al.[61], who studied the tolerance and longevity of theL.brevisstrains in the acidic environment and at varying concentrations of 0.5%, 1%, and 2% of bile as a comparable concentration in the small intestine.It is also on the same side as previously obtained by Abdelazez et al.[9],who investigated the viability ofL.brevisstrains under the harsh conditions of simulated gastrointestinal juice.
Bagheri et al.[8]reported that supplementation withL.rhamnosus,L.reuteriandBifidobacterium infantisenhanced GABA function and improved oxidative balance in animal models.Moreover, Tian et al.[62]stated that the inflammatory response and pre-diabetes development can be inhibited by administering the GABA molecule as a therapeutic agent.
Mbiti et al.[63]indicated the blood test reference ranges are a set of values that a health professional uses to interpret a set of medical test results from blood samples.Also, the reference ranges may change due to varying by age, sex, health, and ethnicity,improvements of techniques, reagents, equipment, and measurement units.Individual results should always be interpreted using the reference range provided by the test laboratory.
Wasserman [64]stated that a blood glucose test can measure the liver’s ability to produce glucose, and it is usually the last function lost if the liver fails.The first morning hours are usually the lowest in glucose levels and then increase within 2 h of eating the first meal,although higher levels than standard glucose concentrations may indicate the presence of some medical disturbances.The findings are consistent with those of Sacks et al.[65,66], who proposed that diabetes was traditionally defined as fasting blood glucose levels of greater than 7 mmol/dL to 11.1 mmol/dL or higher with hyperglycemia symptoms.
The obtained results were in close agreement with Members et al.[67], who suggested that the HDL and LDL reference range were 2.07–3.63 mmol/Lb since all treated groups were lower than the reference ranges except for Cont that had the highest HDL level.Sacks et al.[65]reported the blood plasma glucose reference range between 3.57–6.12 mmol/Lb, resulting in all test groups falling within the reference range, except for INSSTZ.
GABA mode of action in the pancreas, which is produced by β-cells and activates ionotropic GABAAand metabotropic GABABreceptors in both α- and β-cells once released.GABA released from β-cells prevents glucagon release from α-cellsviaa paracrine signal, while an autocrine signal, based on extracellular glucose levels, enhances or decreases insulin secretion from β-cells [68].Thus, GABA not only increases the proliferation of β-cells but also suppresses immune responses, reversing the disease in the severely diabetic mice model [69,70].
The results obtained are closely similar to Graham et al.[71], who suggested a reference range of insulin of approximately 3–19 IU/mL,although all the results obtained fall within the reference range of insulin.In contrast, all test groups had the highest level more than Mg2+reference range 0.70–1.10 mmol/L.
McGill [72]mentioned the increase or decrease in AST and ALT are the most important symptoms of liver health and this may happen due to liver damage, which causes changes in the permeability of the cell membrane.The obtained results were in agreement with Renuka et al.[73], who reported that using STZ increases the level of AST, ALT, and ALP in blood plasma.Additionally, it was in close consistency with Newsome et al.[74]who investigate the reference range of TP is 60.0–80.0 g/dL, and ALB is 35.0–55.0 g/Lb, and GLUB is 25.0–40.0 g/ Lb.Except for LAC2STZ, all test groups fall within TP reference range 60.0–80.0 g/Lb as investigated by Bernardi et al.[75].While all groups fall within the ALB reference range 32.0–50.0 g/L.Newsome et al.[74]indicated that the GLUB reference range is 25.0–40.0 g/Lb and ALT and AST were 1.0–40.0 IU/L.All results obtained fall within the GLUB reference range, except for LAC2STZ, which is slightly higher than the reference range.Also, all test groups had a sharp increase in ALT, AST more than the reference range.Vice versa, all test groups were within the reference range of ALB (1.0–10.0 μmol/L).
The kidney function is an indicator of the activity, vitality,and overall health of the kidney [76].Besides, serum creatinine measurement is a simple test and is the most common indicator of kidney function that is used to define the cause of acute kidney injury or dehydration.All treated groups didn’t fall within the reference range BUN which ranged 1.5–5.9 mmol/Lb,while all test groups fall within the CREA reference range of 53.0-132.0 mmol/Lb [77].Stevens and Levin [78]indicated the URIC reference range was 142.0–401.0 mmol/L.Therefore, the results didn’t display a worrying increase in uric acid.
Briefly, the discrepancy between free or freeze-dried treatments ofL.brevisstrains do not mean that they are not tolerant of unfavorablein vitroconditions, but rather that they can withstand, adapt and expand based on the freezing temperature until exposed at the beginning and during freeze-dried treatments, but not as the activity of free strain that has not been exposed to it.
5.Conclusion
Probiotic health effects were investigated for a long time and several studies have demonstrated their efficacy byin vitroandin vivoresearch.Postbiotics, such as GABA, enhance the potential of probiotic functions which play a pivotal role in human health, leading to increased demand for GABA-enhanced functional foods.Our hypothesis explores the survivability of twoL.brevisstrains using a microencapsulated freeze-dried technique in simulated gastrointestinal juicein vitroand GABA intervention in the diabetic mice model.The results demonstrated the ability ofL.brevisstrains in reducing the hyperglycemia in the blood plasma of C57BL/6J mice and improve the overall components of the blood plasma due to the presence of thegadantiporter.Herein, we appeal for more expanding usage of postbiotics in functional foods and nutraceuticals enriched with GABA, as well as in novel pharma-biotics applications that would prevent symptoms of some chronic diseases in the future.
Acknowledgments
The authors gratefully thanks to Taif University Researchers Supporting Project Number (TURSP-2020/140), Taif University, Taif,Saudi Arabia.As well as Dr.Mahmoud Helal, Faculty of Engineering,Taif University for his assistance in the statistical analysis.
Conflict of Interest
The authors declare no conflict of interest.
Funding
This work was supported by grant from the National Key Research and Development Program of China (2018YFE0120500).
- 食品科学与人类健康(英文)的其它文章
- Effects of dietary fiber on human health
- Tea polyphenol - gut microbiota interactions: hints on improving the metabolic syndrome in a multi-element and multi-target manner
- Resveratrol and its derivates improve inflammatory bowel disease by targeting gut microbiota and inflammatory signaling pathways
- Milled flaxseed-added diets ameliorated hepatic inflammation by reducing gene expression of TLR4/NF-κB pathway and altered gut microbiota in STZ-induced type 1 diabetic mice
- Fermented soy whey induced changes on intestinal microbiota and metabolic influence in mice
- Effects of soy hull polysaccharide on dyslipidemia and pathoglycemia in rats induced by a high-fat-high-sucrose diet

