Assessing radiation dose for postoperative radiotherapy in prostate cancer:Real world data
Asunción Hervás-Morón, Jose Domínguez-Rullán, Victor Duque Santana, Mireia Valero, Carmen Vallejo,Sonsoles Sancho, Juan David García Fuentes, Miguel Cámara Gallego, Fernando López-Campos
Asunción Hervás-Morón, Jose Domínguez-Rullán, Victor Duque Santana, Mireia Valero, Carmen Vallejo, Sonsoles Sancho, Fernando López-Campos, Department of Radiation Oncology, Hospital Universitario Ramón Y Cajal, Madrid 28034, Spain
Juan David García Fuentes, Miguel Cámara Gallego, Department of Medical Physics, Hospital Universitario Ramón Y Cajal, Madrid 28034, Spain
Abstract BACKGROUND Approximately 30% of patients with localized prostate cancer (PCa) who undergo radical prostatectomy will develop biochemical recurrence. In these patients, the only potentially curative treatment is postoperative radiotherapy (PORT) with or without hormone therapy. However, the optimal radiotherapy dose is unknown due to the limited data available.AIM To determine whether the postoperative radiotherapy dose influences biochemical failure-free survival (BFFS) in patients with PCa.METHODS Retrospective analysis of patients who underwent radical prostatectomy for PCa followed by PORT-either adjuvant radiotherapy (ART) or salvage radiotherapy (SRT)-between April 2002 and July 2015. From 2002 to 2010, the prescribed radiation dose to the surgical bed was 66-70 Gy in fractions of 2 Gy; from 2010 until July 2015, the prescribed dose was 70-72 Gy. Patients were grouped into three categories according to the total dose administered: 66-68 Gy, 70 Gy, and 72 Gy. The primary endpoint was BFFS, defined as the post-radiotherapy prostatespecific antigen (PSA) nadir + 0.2 ng/mL. Secondary endpoints were overall survival (OS), cancer-specific survival (CSS), and metastasis-free survival (MFS; based on conventional imaging tests). Treatment-related genitourinary (GU) and gastrointestinal (GI) toxicity was evaluated according to Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer criteria. Finally, we aimed to identify potential prognostic factors. BFFS, OS, CSS, and MFS were calculated with the Kaplan-Meier method and the log-rank test. Univariate and multivariate Cox regression models were performed to explore between-group differences in survival outcome measures.RESULTS A total of 301 consecutive patients were included. Of these, 93 (33.6%) received ART and 186 (66.4%) SRT; 22 patients were excluded due to residual macroscopic disease or local recurrence in the surgical bed. In this subgroup (n = 93), 43 patients (46.2%) were Gleason score (GS) ≤ 6, 44 (47.3%) GS 7, and 6 (6.5%) GS ≥ 8; clinical stage was cT1 in 51 (54.8%), cT2 in 35 (39.3%), and cT3 in one patient (1.1%); PSA was < 10 ng/mL in 58 (63%) patients, 10-20 ng/mL in 28 (30.6%), and ≥ 20 ng/mL in 6 (6.4%) patients. No differences were found in BFFS in this patient subset versus the entire cohort of patients (P = 0.66). At a median follow-up of 113 months (range, 4-233), 5- and 10-year BFFS rates were 78.8% and 73.7%, respectively, with OS rates of 93.3% and 81.4%. The 5-year BFFS rates in three groups were as follows: 69.6% (66-68 Gy), 80.5% (70 Gy) and 82.6% (72 Gy) (P = 0.12):the corresponding 10-year rates were 63.9%, 72.9%, and 82.6% (P = 0.12), respectively. No significant between-group differences were observed in MFS, CSS, or OS. On the univariate analysis, the following variables were significantly associated with BFFS: PSA at diagnosis; clinical stage (cT1 vs cT2); GS at diagnosis; treatment indication (ART vs SRT); pre-RT PSA levels; and RT dose 66 -68 Gy vs. 72 Gy (HR: 2.05; 95%CI: 1.02-4.02, P = 0.04). On the multivariate analysis, the following variables remained significant: biopsy GS (HR: 2.85; 95%CI: 1.83-4.43, P < 0.001); clinical stage (HR: 2.31; 95%CI: 1.47-4.43, P = 0.01); and treatment indication (HR: 4.11; 95%CI: 2.06-8.17, P < 0.001). Acute grade (G) 1 GU toxicity was observed in 11 (20.4%), 17 (19.8%), and 3 (8.3%) patients in each group (66-68 Gy, 70 Gy and 72 Gy), respectively (P = 0.295). Acute G2 toxicity was observed in 2 (3.7%), 4 (4.7%) and 2 (5.6%) patients, respectively (P = 0.949). Acute G1 GI toxicity was observed in 16 (29.6%), 23 (26.7%) and 2 (5.6%) patients in each group, respectively (P = 0.011). Acute G2 GI toxicity was observed in 2 (3.7%), 6 (6.9%) and 1 (2.8%) patients, respectively (P = 0.278). No cases of acute G3 GI toxicity were observed.CONCLUSION The findings of this retrospective study suggest that postoperative radiotherapy dose intensification in PCa is not superior to conventional radiotherapy treatment.
Key Words: Prostate cancer; Postoperative radiotherapy; Dose intensified; Radiation dose; Biochemical relapse free survival
lNTRODUCTlON
In the year 2020, prostate cancer (PCa) was the 4thmost common cancer worldwide, with an annual incidence of 1414259 cases, and the 8thleading cause of cancer mortality, with 375304 deaths[1]. Radical prostatectomy (RP) is one of the primary treatments for localized PCa, with good long-term results[2]. However, up to 30% of surgically-treated patients will develop biochemical recurrence, which is primarily observed in patients who present high-risk factors in the surgical specimen, positive surgical margins, Gleason score (GS) ≥ 8, extracapsular extension, and/or involvement of the seminal vesicles[3].
In this clinical context, the main international clinical guidelines recommend postoperative radiotherapy (PORT)[4]. There are two main treatment modalities for PORT, adjuvant radiotherapy (ART) or salvage radiotherapy (SRT). ART is defined as the prophylactic administration of RT after RP but before recurrence (when prostate-specific antigen [PSA] levels remain undetectable) in patients with a high risk of recurrence due to adverse pathologic features. By contrast, SRT involves the administration of RT to the prostate bed in patients with confirmed biochemically-recurrent PCa (without evidence of distant metastasis) after surgery[5].
Despite the recent publication of several studies[6-9], the optimal timing of PORT (i.e., ARTvsSRT) remains unclear in some patient subgroups. The optimal dose for both ART and SRT has not been established, nor is it clear whether dose escalation is appropriate in these patients. Although several studies suggest that dose intensification may be more effective than conventional doses in terms of biochemical control[10-15], other studies have found that dose intensification does not provide any benefits compared to conventional dosing and is also associated with greater toxicity[16].
In this context, the aim of the present retrospective study was to describe long-term clinical outcomes and treatment-related toxicity (acute and chronic) according to the PORT dose (66-68 Gy, 70 Gy, and 72 Gy) in patients treated at our hospital between 2002 to 2015.
MATERlALS AND METHODS
This was a retrospective analysis of patients with PCa who underwent radical prostatectomy followed by PORT (ART or SRT) at the Ramón y Cajal University Hospital in Spain between April 2002 and July 2015. From 2002 to 2010, the dose to the surgical bed was 66-70 Gy; in 2011, the dose was increased to 70-72 Gy. In all cases, the doses were delivered in fractions of 2 Gy, 5 d a week according to the protocol established in that centre at that time and the clinical criteria of the radiation oncology specialist.
Treatment planning was performed with the patient in the supine position, with a full bladder and empty rectum. Contouring of the surgical bed was performed in accordance with Radiation Therapy Oncology Group guidelines[17,18]. Until April 2006, three-dimensional (3D) conformal radiotherapy was used. Thereafter, patients were treated with intensity-modulated radiotherapy (IMRT).
Follow-up was performed by specialists from the Radiotherapy Oncology or Urology Departments at our hospital. The first follow-up visit, consisting of a clinical evaluation and PSA determination, was conducted three months after treatment completion. Subsequent visits were performed every 3-6 mo during the first five years and annually thereafter.
Acute toxicity was defined as any toxicity from the start of radiotherapy until six months after treatment finalisation. Treatment-related toxicity observed > six months after treatment completion was defined as chronic toxicity.
The primary aim of this study was to evaluate BFFS, defined as the PSA nadir + 0.2 ng/mL after completion of RT. Patients were classified into three groups according to the total radiotherapy dose administered to the surgical bed (66-68 Gy, 70 Gy, and 72 Gy). Secondary objectives were as follows: overall survival (OS), cancer-specific survival (CSS), and metastasis-free survival (MFS)-assessed by conventional imaging tests (computed tomography [CT] and bone scan); and genitourinary (GU) and gastrointestinal (GI) toxicity according to Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer criteria[19]. Finally, we evaluated the following variables as potential prognostic factors: PSA level prior to the start of RT; clinical and pathological stage; GS; margin status; radiotherapy dose; hormonal therapy; perineural invasion and treatment indication (ARTvsSRT).
Statistical analysis was performed with the SPSS statistical software, v.20 (IBM-SPSS Corp). BFFS, OS, CSS and MFS were calculated from the start of RT, using the Kaplan-Meier method and the log-rank test with a significance level ofP <0.05. Univariate and multivariate Cox regression models were performed to explore between-group differences in survival measures.
RESULTS
We evaluated 301 consecutively-treated patients. Of these, 93 (33.6%) received ART (≤ six months after surgery) due to unfavourable histological factors (involved or close margins or stage pT3b-T4). A total of 186 patients (66.4%) were treated with SRT after biochemical recurrence. Twenty-two patients were excluded due to residual macroscopic disease or local recurrence in the surgical bed. Lymph node dissection was performed simultaneously with radical prostatectomy in 135 patients (48.6%). The clinicopathologic characteristics of the patients by radiotherapy dose to the surgical bed are shown in Table 1.
At a median follow-up of 113 mo (range, 4-233), 5- and 10-year survival rates, respectively, were as follows: BFFS: 78.8% and 73.7%; OS: 93.3% and 81.4%; CSS: 95.9% and 88.4%; and MFS: 96.8% and 91.8%. Local recurrence in the surgical bed was observed in four cases (1.5%), lymph node recurrence in 22 patients (8.3%), and distant metastases in 27 patients (10.1%).
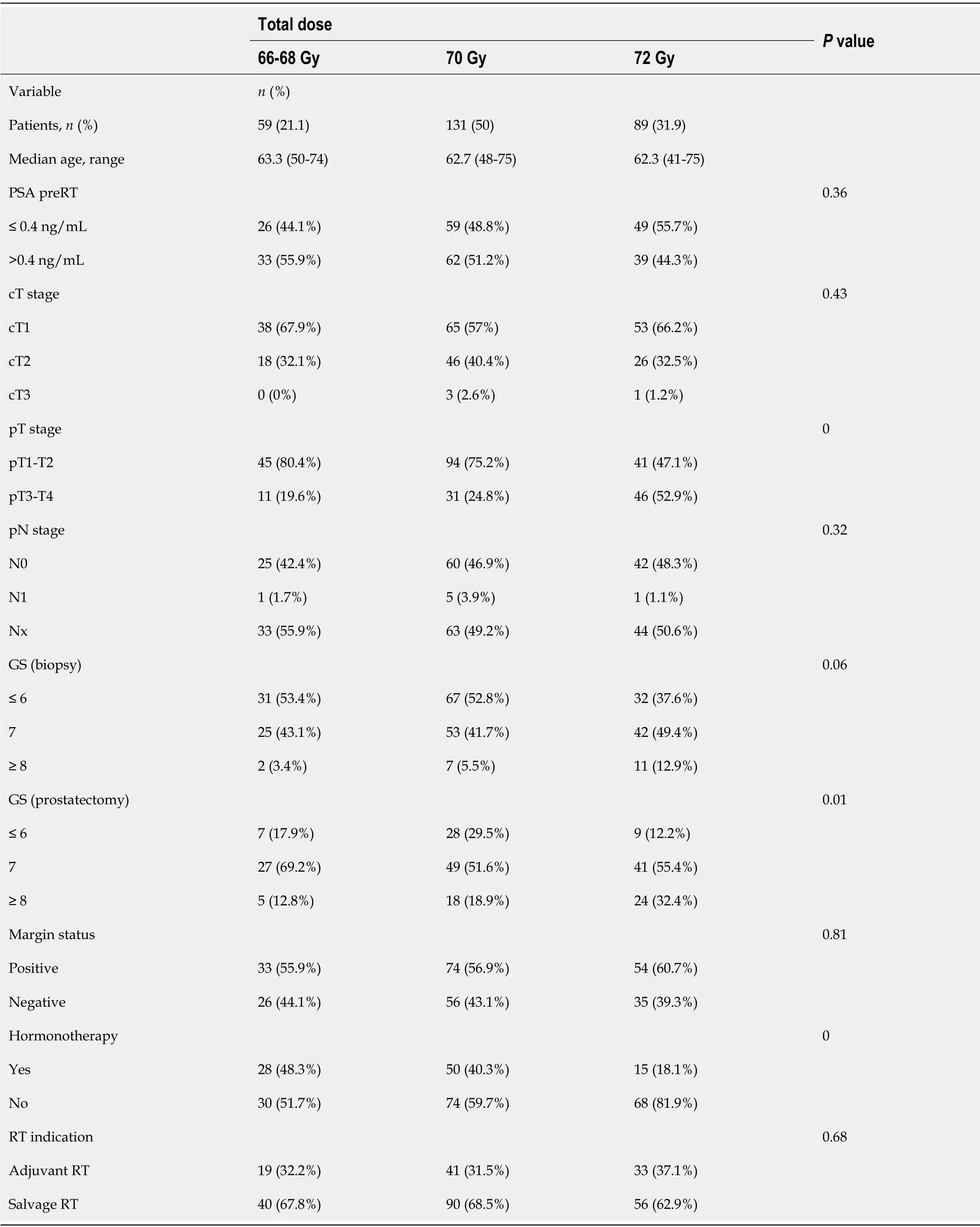
Table 1 Clinicopathologic characteristics of the patients according to the radiotherapy dose to the surgical bed
At 5 and 10 years, BFFS was 89.1% and 89.1% in the ART groupvs73.3% and 65.5%, respectively, in the SRT group (Figure 1). By total dose, the median BFFS (Figure 2) was not reached in any of the subgroups; the 5- and 10-year BFFS rates in these three groups were 69.6%, 80.5% and 82.6% (P= 0.12) and 63.9%, 72.9% and 82.6% (P= 0.12), respectively; the 5- and 10-year CSS rates in these three groups were 100%, 98.4% and 98.8% and 89.3%, 96.4% and 97.3% (P= 0.067), respectively; the 5- and 10-year OS rates in these three groups were 93.1%, 94.5% and 91.5% and 76.6%, 81.3% and 88.9% (P= 0.519), respectively, Figures 3 and 4.
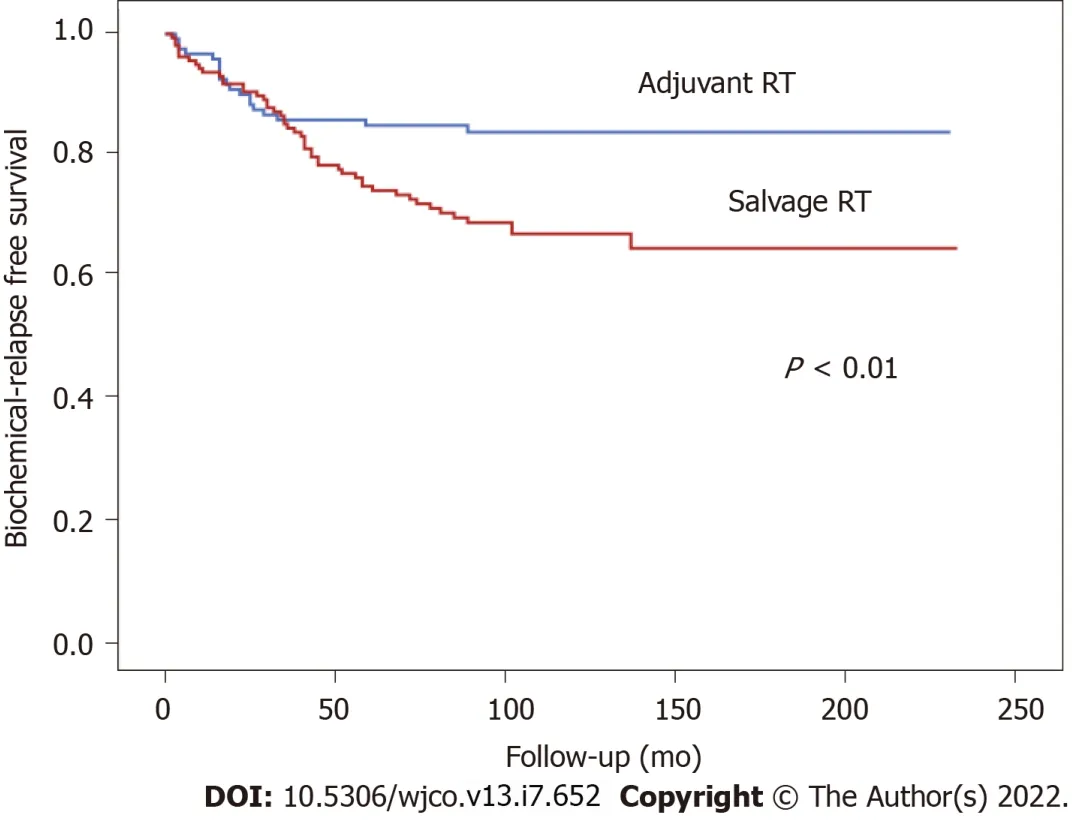
Figure 1 Biochemical relapse free survival according to radiotherapy indication (adjuvant versus salvage radiotherapy).

Figure 2 Biochemical failure-free survival according to radiotherapy dose to the surgical bed.
On the univariate analysis, the following variables were significantly associated with BFFS: PSA at diagnosis (hazard ratio [HR]: 1.05; 95% confidence interval [CI]: 1.77-5.11,P= 0.00); clinical stage cT1vscT2 (HR: 3.01; 95%CI: 1.67-4.75,P <0.001); GS at diagnosis 6vs7 (HR: 2.31; 95%CI: 1.31-4.08,P= 0.004) and 6vs8-9 (HR: 7.88; 95%CI: 3.76-16.52,P <0.001); (ARTvsSRT; HR: 3.40; 95%CI: 1.74-6.66,P= 0.00); PSA level prior to RT (HR: 1.25; 95%CI: 1.14-1.38,P <0.001); and RT dose 66-68 Gyvs72 Gy (HR: 2.05; 95%CI: 1.02-4.02,P= 0.04). None of the following variables were associated with BFFS: preoperative androgen blockade (P= 0.66), perineural invasion (P= 0.15), or involved margins (P= 0.36).
On the multivariate Cox regression analysis, the following variables remained significantly associated with BFFS: GS in the biopsy (HR: 2.85; 95%CI: 1.83-4.43,P <0.001); clinical stage (HR: 2.31; 95%CI: 1.47-3.43,P= 0.01); and the indication for external beam radiation therapy (ARTvsSRT), (HR: 4.11; 95%CI: 2.06-8.17,P <0.001).
On the univariate analysis, the following variables were significantly associated with OS: Age (HR: 1.07; 95%CI: 1.02-1.12,P= 0.003); GS in the surgical specimen: GS 6vs8-9 (HR: 2.36; 95%CI: 1.01-5.52,P= 0.048); PSA prior to RT: ≤ 4vs> 4 ng/mL (HR: 1.81; 95%CI: 1.07-3.06,P= 0.027); and distant metastases (HR: 2.49; 95%CI: 1.37-4.53,P= 0.003). On the multivariate analysis, only age (HR: 1.09; 95%CI: 1.03-1.13,P= 0.002) and distant metastases (HR: 2.82; 95%CI: 1.54-5.16,P= 0.001) remained significant.

Figure 3 Overall survival according to total radiotherapy dose to the surgical bed.

Figure 4 Metastasis-free survival according to the total radiotherapy dose to surgical bed.
Acute grade (G)1 GU toxicity was observed in 11 (20.4%), 17 (19.8%), and 3 (8.3%) patients in each group (66-68 Gy, 70 Gy, and 72 Gy), respectively, (P= 0.295). Acute G2 GU toxicity was observed in 2 (3.7%), 4 (4.7%) and 2 (5.6%) patients, respectively, (P= 0.949). Only one patient (in the 72 Gy group) developed G3 toxicity (Table 2). Acute G1 GI toxicity was observed in 16 (29.6%), 23 (26.7%) and 2 (5.6%) patients, respectively, (P= 0.011). Acute G2 toxicity was observed in 2 (3.7%), 6 (6.9%) and 1 (2.8%) patient, (P= 0.278). No cases of acute G3 GI toxicity were observed in any of the groups (Table 2). Chronic GU toxicity was as follows: G1-2 in 3 patients (11.5%) and G3 in one (3.8%) patient in the 70 Gy group, and in 11 (13.4%) and 1 (1.2%) of those who received 72 Gy, respectively (P= 0.338). Chronic G1-G2 GI toxicity was observed in 2 (7.7%) of the patients who received 70 Gy and in one (2%) who received 72 Gy (P= 0.262), with no G3 chronic GI toxicity in any of the groups (Table 3).
DlSCUSSlON
In this retrospective study, higher total postoperative radiation doses to the surgical bed were not associated with better BFFS or OS outcomes, a finding that is consistent with data from randomised clinical trials[16].
In this series, the patients’ clinical characteristics were indicative of an aggressive disease profile: 139 patients (48%) were stage pT3-T4, 47 (16.8%) had a GS ≥ 8, 161 (58.9%) had positive margins in the surgical specimen, and 124 (49%) had a pre-RT PSA > 0.4ng/mL. In addition, 93 patients (34.8%) received short-term androgen blockade prior to surgery, as this was standard clinical practice at some centres based on the available evidence at that time, even though preoperative androgen deprivation therapy is no longer prescribed in these cases[20]. Given the time period (2002-2015) of this study, none of the patients were prescribed concurrent hormonal therapy with postoperative radiotherapy, even though this approach is now common clinical practice-due to the proven clinical benefits-in wellselected patients who meet the clinical criteria[21,22].

Table 2 Acute gastrointestinal and genitourinary toxicity (Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer criteria) according to total radiotherapy dose to the surgical bed

Table 3 Chronic gastrointestinal and genitourinary toxicity (Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer criteria) according to total radiotherapy dose to the surgical bed
The patients in this series did not undergo prophylactic nodal radiation due to the conflicting and controversial evidence in the literature[23-25]. Nevertheless, only 22 patients (8.3%) developed nodal recurrence; of these, 70% received SRT, 64% presented perineural invasion, and 45% were stage pT3-4. Given the low rate of nodal recurrence in this series, we were unable to identify any significant predictors.
A recent randomised trial compared ART to SRT (64 Gy) without hormonal therapy or prophylactic nodal irradiation[8].The 5-year BFFS was 87%, which was higher than the 78.8% observed in our series, probably due to the less aggressive disease profile and the application of treatment volumes that differed from those recommended in the Radiation Therapy Oncology Group contouring guidelines[17,18].
In our series, 5- and 10-year BFFS was significantly better in the patients who received ARTvsSRT, a finding that is consistent with previous reports[3,26-30]. Although numerous studies have sought to determine the optimal timing of EBRT after radical prostatectomy, this remains uncertain[3,27,31,32] Several recent phase III trials-RADICALS, Groupe d' Etude des Tumeurs Uro-Genitales (GETUG)-AFU 17 and RAVES, and the ARTISTIC meta-analysis-have compared ART to SRT, demonstrating that early SRT is superior to ART[6-9]. However, because those trials included a limited number of patients with highly unfavourable clinicopathologic characteristics (involved margins, pathologic lymph nodes, stage pT3b and/or GS ≥8) more studies are needed to determine whether early SRT is indicated in all surgically-treated patients, or whether some patient subgroups might benefit from ART.
In the comparison of treatment outcomes according to the radiotherapy dose to the surgical bed, dose intensification did not improve the results, a finding that contrasts with several retrospective reports that reported better clinical outcomes in patients who received higher doses[10-15]. However, it is important to emphasize that we did not randomise patients and, moreover, there were important differences among the subgroups in terms of the clinicopathologic characteristics. In fact, this is a study limitation given that patients who received 72 Gy had more severe disease (stage pT3-T4, higher GS) and were less likely to receive hormonal therapy than patients included in the other two subgroups (70 Gy and 66-68 Gy). Both the European Association of Urology (EAU) and the GETUG have developed criteria to identify patients with a high risk of developing metastatic disease in this clinical scenario[21,33]. Nonetheless, in our multivariate analysis, most of these criteria failed to predict the effectiveness of PORT. Consequently, a more comprehensive analysis in a larger sample that stratifies patients according to their baseline clinical characteristics could help to better elucidate the true potential of dose intensification, thus allowing for more individualized treatment.
In terms of toxicity, previous studies have found that dose-escalated PORT is associated with a significant increase in both GU and GI toxicity[16]. However, various factors could influence this association, including the technique (i.e., 3D-CRTvsIMRT), irradiation or not of the uninvolved nodal areas, the contouring criteria for the treatment volumes, pretreatment urinary function, as well as several other factors described elsewhere[34]. We found no significant between-group differences in acute or chronic GI or GU toxicity, regardless of the radiotherapy dose, a finding that is consistent with the randomised trial conducted by Qiet al[35]. In that trial, the authors compared outcomes in patients (n= 144) randomised to receive either 66 or 72 Gy to the surgical bed. They found no significant between-group differences in acute and/or chronic GI or GU toxicity. In our study, the use of more advanced radiotherapy techniques (IMRT/rotational techniques) in approximately 50% of the patients may have contributed to the good treatment outcomes. However, these findings should be interpreted cautiously given the retrospective study design and small sample size. Given these limitations, we cannot draw any definitive conclusions. Consequently, larger, more comprehensive studies are needed.
CONCLUSlON
The findings of this study suggest that dose-intensified postoperative radiotherapy in patients with PCa is not superior to conventional dosing. Consequently, there is a clear need for randomised clinical trials with well-selected patients to determine the optimal individualized radiotherapy dose scheme in patient subgroups with highly aggressive disease.
ARTlCLE HlGHLlGHTS
Research background
Approximately 30% of patients with localized prostate cancer (PCa) who undergo radical prostatectomy will develop biochemical recurrence. In these patients, the only potentially curative treatment is postoperative radiotherapy (PORT) with or without hormone therapy. However, the optimal radiotherapy dose is unknown due to the limited data available.
Research motivation
Our article analyses the changing landscape of the management of prostate cancer patients who receive postoperative radiotherapy, shedding light on an area, optimal radiation dose, applicable to clinical practice, for which the current evidence base is constantly fluctuating with a growing need to optimize the treatment of these patients.
Research objectives
To determine whether the postoperative radiotherapy dose influences biochemical failure-free survival(BFFS) in patients with prostate cancer.
Research methods
Retrospective analysis of patients who underwent radical prostatectomy for PCa followed by PORTeither adjuvant radiotherapy or salvage radiotherapy-between April 2002 and July 2015. From 2002 to 2010, the prescribed radiation dose to the surgical bed was 66-70 Gy in fractions of 2 Gy; from 2010 until the present, the prescribed dose was 70-72 Gy. Patients were grouped into three categories according to the total dose administered: 66-68 Gy, 70 Gy, and 72 Gy. The primary endpoint was BFFS, defined as the post-radiotherapy prostate-specific antigen (PSA) nadir + 0.2 ng/mL. Secondary endpoints were overall survival (OS), cancer-specific survival (CSS), and metastasis-free survival (MFS; based on conventional imaging tests). Treatment-related genitourinary (GU) and gastrointestinal (GI) toxicity was evaluated according to Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer criteria. Finally, we aimed to identify potential prognostic factors. BFFS, OS, CSS, and MFS were calculated with the Kaplan-Meier method and the log-rank test. Univariate and multivariate Cox regression models were performed to explore between-group differences in survival outcome measures.
Research results
301 consecutive patients were included. At a median follow-up of 113 mo (range, 4-233), 5-and 10-year BFFS rates were 78.8% and 73.7%, respectively, with OS rates of 93.3% and 81.4%. The 5-year BFFS rates in the three groups were as follows: 69.6% (66-68Gy), 80.5% (70Gy) and 82.6% (72Gy) (P = 0.12): at 10 years, the corresponding rates were 63.9%, 72.9% and 82.6% (P = 0.12), respectively. No significant between-group differences were observed in MFS, CSS, or OS. No significant differences were found in GU or GI toxicity between the 3 radiation-dose groups except acute grade 1 GI toxicity that was observed in 16 (29.6%), 23 (26.7%) and 2 (5.6%) patients in each group (66-68Gy, 70Gy and 72Gy),respectively (P = 0.011).
Research conclusions
Postoperative radiotherapy dose intensification in PCa is not superior to conventional radiotherapy treatment.
Research perspectives
A more comprehensive analysis of the radiation dose in prostate cancer patients who receive postoperative radiotherapy could help to better elucidate the true potential of dose intensification, thus allowing for more individualized treatment.
FOOTNOTES
Author contributions:Hervás A, Domínguez-Rullán JA, Duque Santana V, Valero M, Vallejo C, Sancho S, García Fuentes JD, Cámara Gallego M and López-Campos F contributed to writing, review, editing and supervision; all authors have read and approve the final manuscript.
lnstitutional review board statement:The study was reviewed and approved by the Ethics Research Committee of Hospital Universitario Ramón y Cajal.
lnformed consent statement:Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement:The manuscript has been read and approved for submission by all the named authors. All authors declare no conflict of interests for this article.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Spain
ORClD number:Asunción Hervás-Morón 0000-0002-7439-1820; Jose Domínguez-Rullán 0000-0003-4887-5749; Victor Duque Santana 0000-0001-7505-8176; Mireia Valero 0000-0002-5271-0990; Carmen Vallejo 0000-0001-8437-1930; Sonsoles Sancho 0000-0002-3141-2011; Juan David García Fuentes 0000-0001-9395-2155; Miguel Camara Gallego 0000-0002-1511-1518; Fernando López-Campos 0000-0002-4077-0507.
S-Editor:Wang LL
L-Editor:A
P-Editor:Wang LL
 World Journal of Clinical Oncology2022年7期
World Journal of Clinical Oncology2022年7期
- World Journal of Clinical Oncology的其它文章
- Nanomedicine approaches for treatment of hematologic and oncologic malignancies
- Simple approach for the histomolecular diagnosis of central nervous system gliomas based on 2021 World Health Organization Classification
- Edmonton Symptom Assessment Scale may reduce medical visits in patients undergoing chemotherapy for breast cancer
- Awareness, knowledge, and attitudes towards sun protection among patients with melanoma and atypical mole syndrome
- Short term safety of coronavirus disease 2019 vaccines in patients with solid tumors receiving systemic therapy
- Decreased incidence of febrile neutropenia in Michigan following masking and social distancing orders for the COVlD-19 pandemic:A population based cohort study
