Tix-Ni1-x-MOFs的制备及其CO选择性催化还原NOx研究
石 勇,李 橙,黄 磊,熊 巍,肇启东,孙健恒,丁 越
Ti-Ni1-x-MOFs的制备及其CO选择性催化还原NO研究
石 勇1*,李 橙1,黄 磊1,熊 巍1,肇启东2,孙健恒1,丁 越1
(1.大连理工大学环境学院,工业生态与环境工程教育部重点实验室和精细化工国家重点实验室,辽宁 大连 116024;2.大连理工大学盘锦校区,化工学院盘锦分院,辽宁 盘锦 124221)
采用溶剂热法和微波法合成了不同比例的Ti-Ni1-x-MOFs材料,并用于以CO为还原剂的选择性催化脱硝反应.结果表明,双金属Ti-Ni1--MOFs的NO还原率显著高于单金属Ni-MOF,且反应温度窗口更宽,其中,Ti0.2-Ni0.8-MOF表现出最佳的脱硝效率,在200~400oC温度范围达到100%的转化率.通过XRD,FT-IR,SEM,TGA,XPS,N2吸脱附等表征手段发现,Ti掺杂Ni-MOF后有利于改善原子分散性,Ti、Ni间金属的相互作用有利于产生丰富的高效Ni-O-Ti位点,加强Ni2++Ti4+↔ Ni3++Ti3+氧化还原循环,从而明显提高了NO+CO催化反应性能.与溶剂热法相比,微波法制备Ti0.2-Ni0.8-MOF具有合成效率高、结晶度好、晶粒细小均匀的优势,并进一步提高了其低温脱硝效果.
CO-SCR;金属有机骨架;Ti-Ni1-x-MOFs;微波法;溶剂热法
化石燃料的大量燃烧导致氮氧化物(NO)的过度排放,从而引起了如温室效应、光化学烟雾、酸雨和臭氧损耗等环境及健康问题.工业中广泛采用的选择性催化还原技术(SCR)是通过使用还原剂与NO发生化学反应,生成氮气与水,具有二次污染小、净化效率高等优点.其中CO-SCR是以烟气中与NO同时存在的CO作为SCR反应的还原剂,不仅可以同时去除这2种有害气体达到“以废治污”的目的,还能有效解决还原气体的成本和运输储存等问题,所以被认为是目前最具吸引力和应用前景的脱硝技术.为了避免烟尘中重金属和SO2引起的磨损或中毒效应,SCR脱硝设备通常置于除尘器和脱硫装置下游,这使得脱硝反应温度低于300°C,所以研发高效稳定的低温CO-SCR催化剂成为当今脱硝领域内的重点课题[1-4].
金属-有机骨架(MOFs)是金属离子连接有机配体形成的新型多孔功能材料.由于MOFs材料的高表面积、有序的多孔结构以及可灵活修饰功能化等优异性能在低温催化脱硝领域引起广泛关注.MOFs材料不仅有利于底物反应分子的吸附和富集,而且杂化结构可形成多催化中心.由于Ni-MOF成本低、比表面积大、热稳定性强、电子传输效率高等特点[5-7],可在宽温度窗口下NO转化率超过90%,优于同构型的Co-MOF,Cu-MOF,Fe-MOF等材料[8-13].但单金属Ni-MOF也存在Ni分散性差且还原温度高的问题,可通过引入另一种过渡金属到骨架中来改善单金属催化剂缺陷,使两种金属原子在同一晶胞中共存产生更规则的结构、强的相互作用以及提高原子分散度.Ti金属能提供丰富的酸性位点,TiNi金属间的协同作用不仅可以产生新的活性位点增强活性,还使材料具有良好的活性物质均匀分散性[14-17],有利于催化反应的进行.已有研究通过控制Ni2+/Ti4+的物质的量之比进而调节金属团簇组分,使得Ni-Ti双金属MOFs不仅具有大量反应位点,而且高电子亲和度的Ni可加快电荷转移速率进而增强CO2还原催化活性[18].然而,Ni-Ti双金属MOFs催化剂在CO-SCR中的应用研究较少,利用Ni-Ti双金属掺杂改善Ni-MOF金属催化剂缺陷,优化其性能结构并提高材料的低温催化性能仍是很大的挑战.
Ni-Ti-MOFs的性能通常取决于材料粒径、结晶度、分散性和均匀性,这些性质受制备工艺的影响,因此通过优化合成方法调控MOFs材料的微观形貌、晶体尺寸具有一定的可行性.传统溶剂热法具有操作简单步骤少的特点,但反应时间较长[19].通过微波法制备的双金属M/Fe-MOFs(M=Ni,Mg,Sn),不仅反应速率快,可控制反应参数,而且有效减少副反应,提高选择性[20-21].然而,微波合成法和溶剂热法对Ni-Ti-MOFs的晶粒尺寸、形貌及热稳定性能的影响还未有过系统研究.因此,通过研究对比溶剂热法和微波合成法Ni-Ti双金属MOFs的结构性能,有利于优化合成工艺来进一步提高该材料的热稳定性及低温催化性能.
本文首先通过溶剂热法制备出系列Ti改性Ni基MOFs材料Ti-Ni1-x-MOFs(x代表Ti:(Ti+Ni)的物质的量之比),对其进行NO+CO催化性能研究.然后根据活性结果筛选出最优比例的Ti0.2-Ni0.8-MOF进行微波法制备.利用XRD、BET、TGA、SEM、FT-IR、XPS技术针对材料物理化学性质进行了表征,比较研究了溶剂热法和微波法这两种材料合成方法对双金属Ti0.2-Ni0.8-MOF性能的影响规律和反应机理.
1 材料与方法
1.1 溶剂热法制备Tix-Ni1-x-MOFs催化剂
按照不同的Ti物质的量占比将总物质的量为5mmol的Ti(SO4)2·9H2O(AR,国药集团),NiCl2·6H2O (AR,国药集团)与1,3,5-苯三甲酸(5mmol,1.051g)溶于50mLN,N-二甲基甲酰胺和10mL无水乙醇的混合溶液中,Ti:(Ti+Ni)的物质的量比值为0, 0.14, 0.2, 0.33, 0.5, 1.0,超声搅拌直至混合均匀.然后将溶液转移到100mL的特氟隆内衬的高压反应釜中,放置在150°C烘箱内反应24h.降至室温后,取出淡绿色粉末状样品,采用N,N-二甲基甲酰胺与乙醇重复洗涤3次去除样品表面的溶剂分子,再将样品放在乙腈溶液中浸泡2d,最后放置在真空烘箱中100°C干燥24h,得到淡绿色样品并依次标记为Ti-Ni1-x-MOFs催化剂.
1.2 微波法制备Ti0.2-Ni0.8-MOF-M催化剂
采用微波辐射法制备Ti0.2-Ni0.8-MOF催化剂进行实验对比.将Ti(SO4)2·9H2O(1mmol,0.240g),NiCl26H2O(4mmol,0.951g)与1,3,5-苯三甲酸(5mmol, 1.051g)依次加入到30mL N,N-二甲基甲酰胺溶剂中,充分混合均匀并超声分散30min.然后将该混合溶液密封在100mL的反应釜中,放置在100W功率下的微波反应器加热2h,待乳白色悬浮液自然降至室温后分别用乙醇和N,N-二甲基甲酰胺溶剂洗涤3次进行分离纯化,放入乙腈溶液浸泡48h,最后放置在真空烘箱中100℃干燥24h,得到淡绿色样品,标记为Ti0.2-Ni0.8-MOF-M催化剂.
1.3 CO-SCR反应活性测试
称量0.2g催化剂样品,用压片机将其粒径压至20~40目,然后将制备好的催化剂放置在内径为8mm的U型石英管中,在Ar气氛下预处理2h用以去除表面杂质,同时降低烟气分析仪的本底浓度以提高监测的灵敏性.通过气体配比模拟实际情况下的烟气,总气体流量为100mL/min,气体空速约为30,000h-1.反应初始入口气体浓度为:[NO]=500× 10-6, [CO]=1000×10-6,氩气作为反应平衡气.采用温控加热仪进行温度调节,反应在25~400℃温度范围内进行.使用烟气分析仪(Testo 350)记录进气口与出口气体中的NO和CO浓度.NO转化率依据以下公式计算:
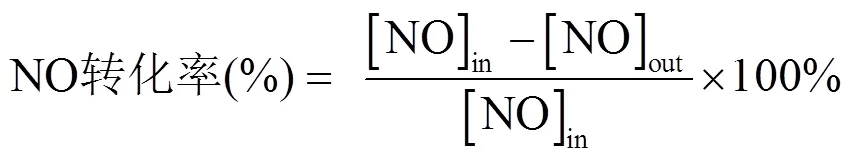
1.4 催化剂性能表征
X射线衍射(XRD)表征使用Rigaku D/MAX 2500v/PC仪器进行,以分析催化剂结晶性和结构.场发射扫描电子显微镜(SEM)用于表征材料的微观形貌和表面结构,使用日本日立公司生产的S-4200场发射扫描电子显微镜.N2吸附脱附的表征使用NOVA 1200(Quanta Chrome)吸附仪在液氮温度77K下进行得出催化剂的孔径分布.热重分析(TGA)表征使用TGA/SDTA851型热重分析仪.使用Thermo 139ESCALAB 250XI型电子能谱仪对催化剂进行X-射线光电子能谱(XPS)表征.使用布鲁克VERTEX-70型傅立叶变换红外光谱仪对催化剂进行原位红外测试(FT-IR),分析样品的分子结构和官能团.
2 结果与讨论
2.1 催化剂活性测试
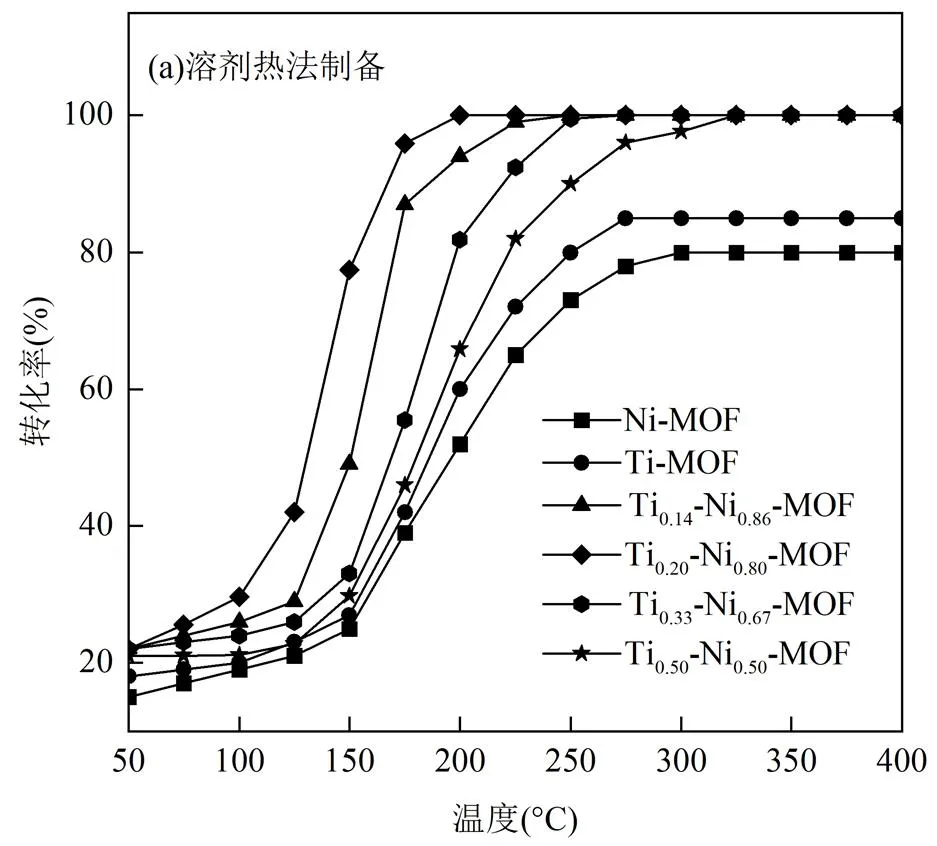
如图1(a)所示,双金属Ti-Ni1-x-MOFs的NO还原率都显著高于单金属Ni-MOF,且反应温度窗口更宽.随着Ti占比增加,双金属Ti-Ni1-x-MOFs的催化活性先升高后下降.其中,Ti0.2-Ni0.8-MOF在200~400℃的宽温度窗口内达到100%的最高NO还原率,200℃时的催化效率较Ni-MOF提高36%,说明适量的Ti在Ni基MOFs材料中的掺杂起到了促进剂的作用.这说明Ni和Ti两种物质之间具有良好的分散性和协同效应[15],不仅Ni-O-Ti位点高度分散,而且有利于提高晶格氧活性,促进氧化还原循环,从而降低反应能垒,改善NO和CO的吸附和活化.随着Ti含量进一步增加,Ti-Ni1-x-MOFs的脱硝性能未呈正相关上升趋势,可能是由于团聚造成的底物与材料接触面积下降.
活性测试表明,Ti0.2-Ni0.8-MOF催化剂在整个温度范围内具有最优NO还原效率,所以用微波法合成Ti:(Ti+Ni)=0.2的双金属材料,记为Ti0.2- Ni0.8-MOF-M.如图1(b)所示,在100~150℃温度范围内,微波法制备的MOF材料低温效果更佳,125℃时的NO还原率提高16%,除此之外,微波法还使反应热时间由24h缩减至2h,加快了Ti0.2-Ni0.8- MOF的结晶速率,这是由于微波通过分子与电磁场的相互作用将能量直接传递给反应溶液,并在开始成核时局部超热,从而快速加热结晶,缩短了合成时间.
2.2 催化剂表征
如图2所示,观察到5个主要衍射峰分别为2=7.3°,11.0°,14.8°,18.4°和22.5°,衍射峰位置与文献所报道的Ni-MOF模拟峰位一致[22],分别对应于Ni-MOF的(001)、(110)、(213)、(150)和(143)晶面,表明两种制备方法使得Ti均匀掺杂到Ni基MOFs中,晶体结构仍保持稳定.双金属MOFs的衍射峰强度比单金属更高,证明双金属材料结晶度较强.与溶剂热法相比,微波法制备的Ti0.2-Ni0.8-MOF的峰更窄,更尖锐,在14.8°和22.5°处峰强较弱可能是由于MOFs中TiNi金属组分及有机配体对微波功率的吸收程度不同产生选择性生长导致的[23-24].
如图3所示,在25~275℃低温阶段内,Ti0.2-Ni0.8- MOF-M的质量损失最小,表明微波制备使得低温下MOFs材料的热稳定性更强.失重曲线可分为P1、P2、P3共3个阶段.50~170℃温度范围下,催化剂的5%~10%质量损失是MOFs材料表面以及孔道内部吸附的溶剂分子挥发所致.在170~320℃的质量损失是由于金属活性位点结合的水分子脱除,促使金属活性位点暴露,催化剂的反应活性随之增强,其中, Ti0.2-Ni0.8-MOF-M的质量损失(35%)略大于Ti0.2- Ni0.8-MOF(32%),表明微波法制备的双金属材料暴露出更多的Ni-O-Ti位点,形成丰富的催化活性中心,从而明显促进NO+CO反应的吸附与还原.320~ 400℃的P3阶段产生的质量损失是由于高温阶段有机配体的受热分解坍塌[25-27].
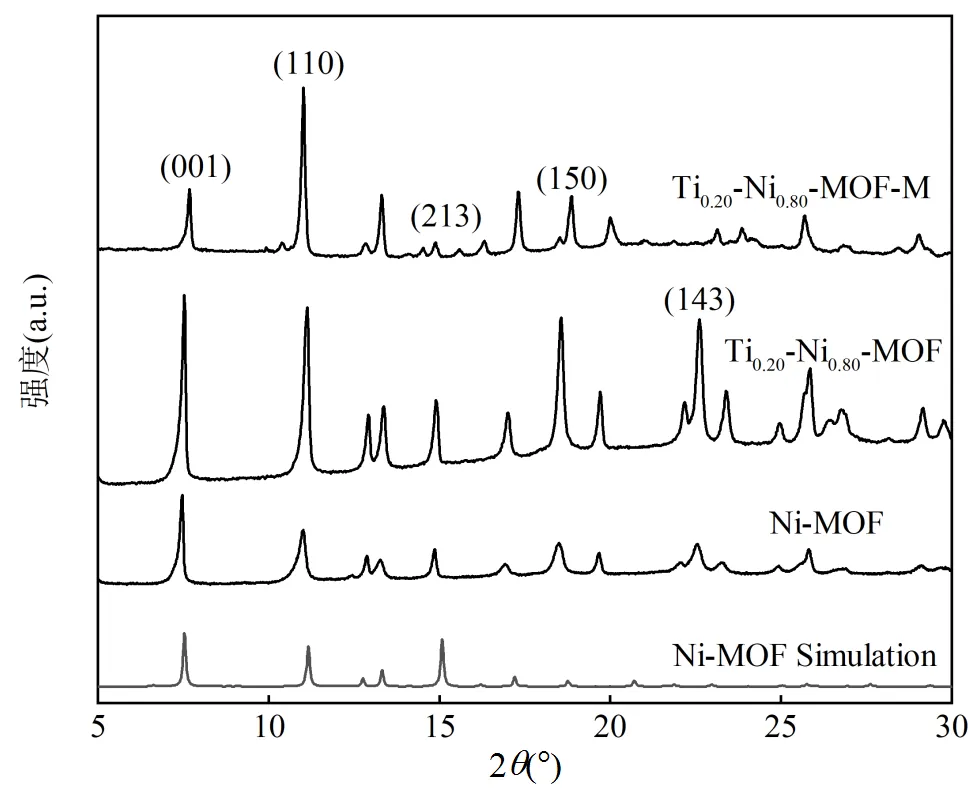
图2 催化剂XRD谱图
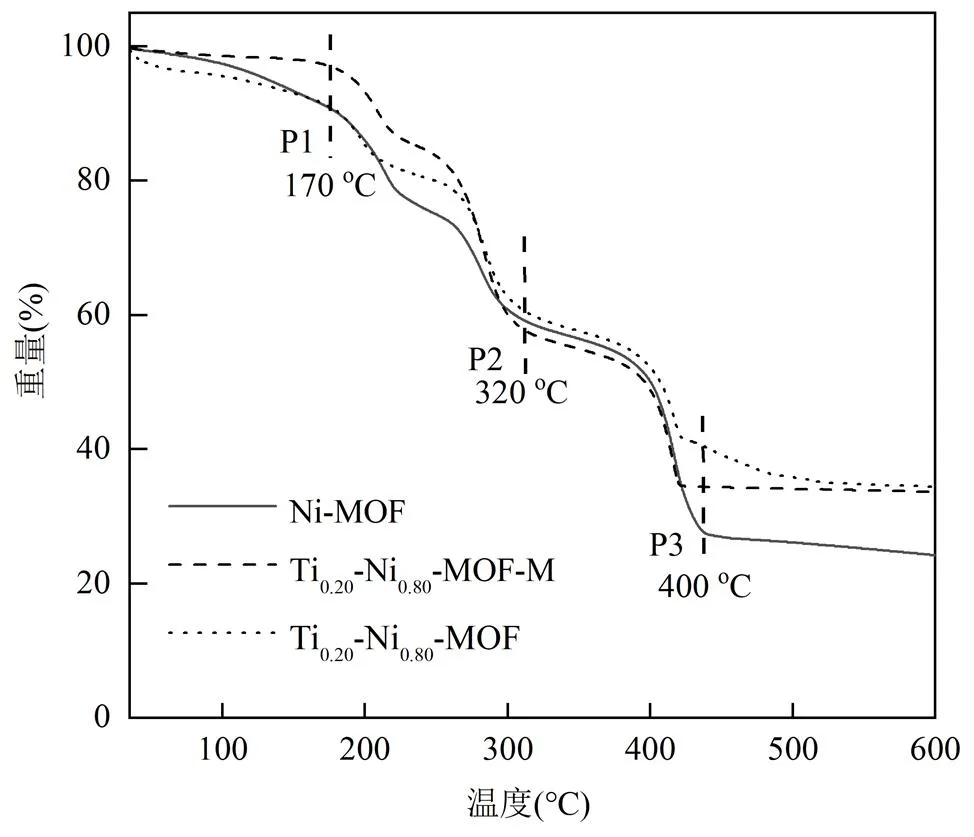
图3 催化剂TGA谱图
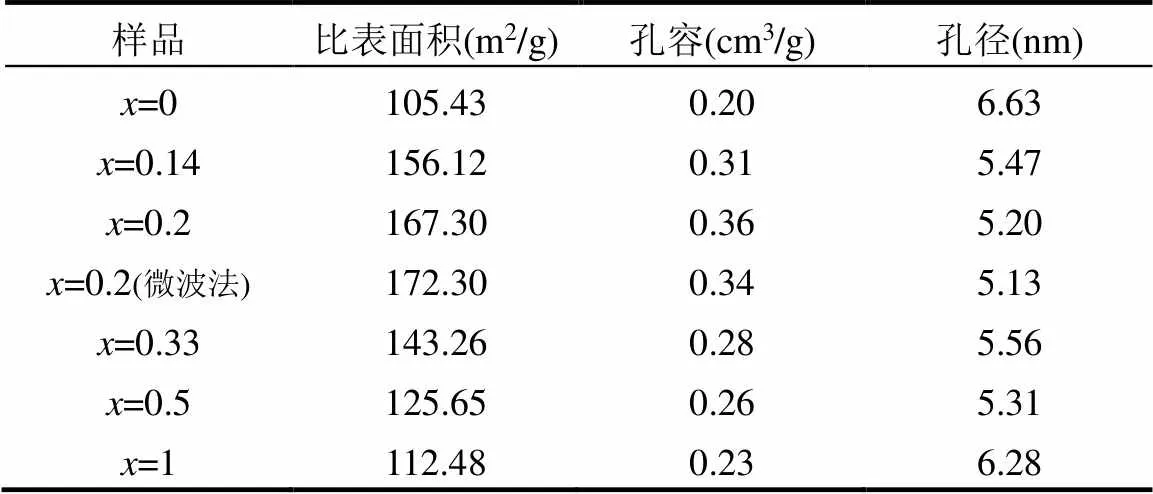
表1 Tix-Ni1-x-MOFs催化剂的孔隙结构参数
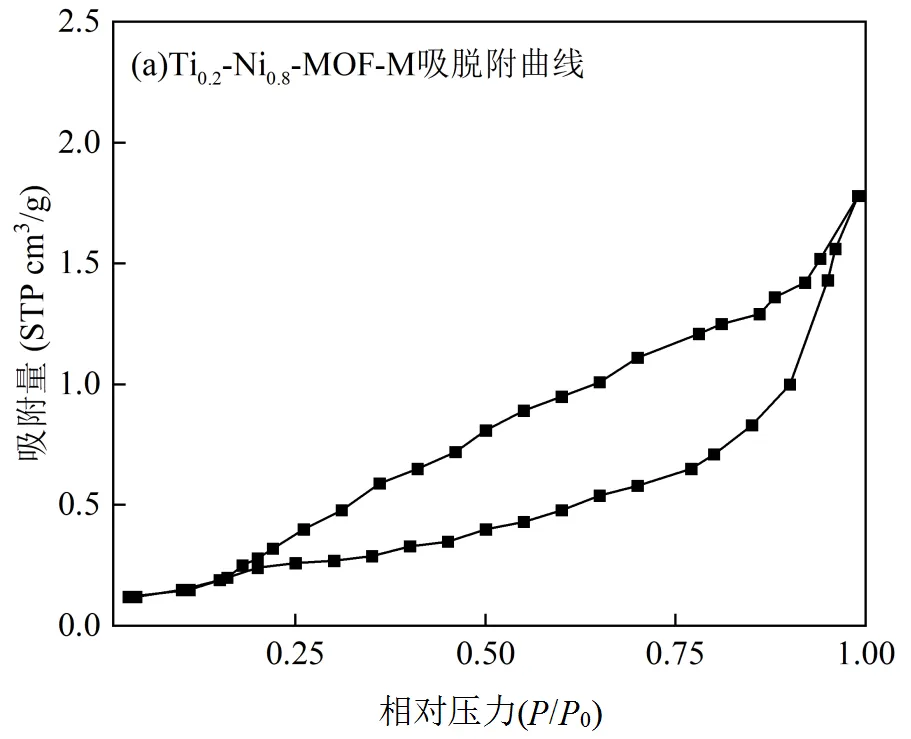
由表1可知,Ti掺杂Ti-Ni1-x-MOFs材料的比表面积均高于单金属材料,当Ti占比x = 0~0.2时,比表面积和孔容与Ti含量成正比,Ti0.2-Ni0.8-MOF的比表面积(167.30m2/g)和孔容(0.36cm3/g)达到最大.催化剂的高比表面积不仅产生对NO与CO的高效吸附,还可有效提供NO+CO与Ni-O-Ti位点相互作用的场所[28-29],从而提高催化效率.如图4,Ti0.2- Ni0.8-MOF-M催化剂吸脱附等温线与III型等温线相似,在高相对压力段(P/P0 = 0.7~1.0)吸附量迅速上升,达到最高值,反映出多分子层吸附现象.Ti0.2- Ni0.8- MOF-M催化剂孔径分布主要集中在2~5nm范围,说明其内表面活性位点主要分布在晶粒堆积的空隙及晶内孔道,这种空隙结构更有利于反应过程中的扩散传质[30].
如图5所示,2种方法制备的双金属MOFs材料均呈现类似的层状长方体结构,其中,溶剂热法制备的晶粒长度分布在0.5~3um,而优化制备后的晶粒尺寸分布在1~1.5um.这是由于溶剂热法的反应时间长,所以晶粒生长尺寸较大.微波制备反应时间短,可较好克服高温条件下团聚引起的粒径增大却不阻碍大量成核位点的产生[24,31],故产物平均粒径减小且更加均匀.

图5 催化剂SEM图
(a)Ti0.2-Ni0.8-MOF, (b)Ti0.2-Ni0.8-MOF-M
如图6所示,3条MOFs材料的红外谱图中均观察到(-C=O)伸缩振动特征峰从1712cm-1蓝移至1610cm−1,表明配体与金属(Ni)络合,制备所得金属有机骨架成键增强并且结构趋于稳定.在3496cm-1处的伸缩振动表明制备的材料中存在吸附与配位结合的(H2O)分子,而在1338,1386和1445cm−1处3个较强的特征峰是由金属离子与水分子中的羟基结合形成的(M-OH)键的弯曲振动.此外,对比双金属MOFs和Ni-MOF红外谱图,在722cm−1处的特征峰归因于(Ni-O)键的振动[32-33],双金属的峰强度增加,研究表明,Ti、Ni相互作用使得Ni原子电子云密度增加导致瞬间偶极矩变化增大,振动加强,活性升高.在双金属Ti0.2-Ni0.8-MOF-M和Ti0.2-Ni0.8-MOF红外谱图中的500~700cm−1的特征峰归因于(Ti-O)键的伸缩振动带,Ti掺杂使材料产生了新络合峰,更有利于NO+CO的吸附.
由图7、表2可知,其中双金属MOFs中均存在Ti、Ni、O等元素且Ti:Ni原子比接近1:4.
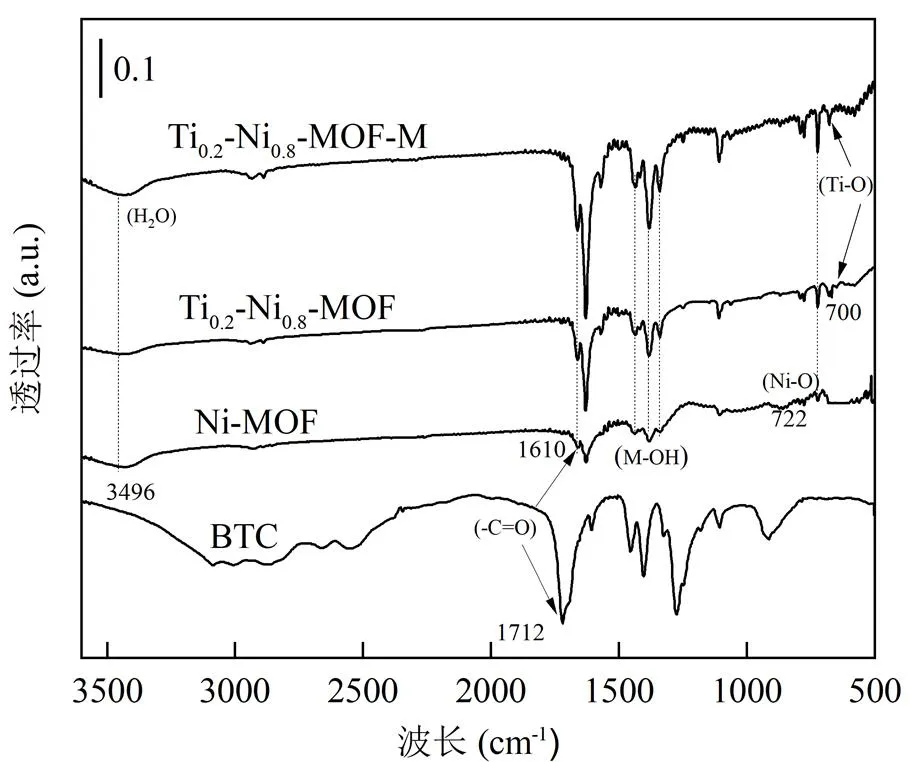
图6 代表性的FT-IR谱图
图7(b)中结合能在458.0~458.6eV和463.7~ 464.4eV分别对应于Ti2p3/2和Ti2p1/2峰.其中,将Ti 2p3/2分为458.97和459.33eV两处特征峰,分别属于Ti3+和Ti4+物种.与Ti0.2-Ni0.8-MOF相比, Ti0.2-Ni0.8- MOF-M的Ti3+峰的结合能增加0.2eV,这是由于微波法制备的双金属材料具有电子亲和性更强的Ti-O-Ti键,有利于产生良好的Ti、Ni间诱导和协同效应[34-35].微波法通过快速均匀地加热材料促进晶粒生长,优化Ni-Ti-MOFs的粒径、结晶度、分散性和均匀性.
图7(c)中结合能在856.4和873.8eV的特征峰分别归属于Ni的2p3/2和2p1/2轨道.通过高斯方法拟合分峰,得到Ni2p3/2光谱在856.0和856.8eV处的两个特征峰,分别为Ni2+和Ni3+物种.此外,在861.2~ 861.8eV处还出现了震激峰,这是由于镍内层电子光电离发射后的弛豫效应.研究表明Ni3+是促进氧化还原性质增强的有利物种[36-37],Ni电子云密度的增加可以促进催化剂表面CO的吸附和氧化,从而获得高催化性能.通过表2可知,Ni3+/(Ni3++Ni2+)的比例按以下顺序递减:Ti0.2-Ni0.8-MOF-M(42.5%)>Ti0.2- Ni0.8-MOF(41.7%)>Ni-MOF(29.1%),与CO-SCR活性测试结果相吻合.此外,相较于Ni-MOF,Ti0.2- Ni0.8-MOF和Ti0.2-Ni0.8-MOF-M的Ni3+特征峰向着更低的结合能分别偏移了0.5和0.2eV,研究表明, Ti4+/Ti3+氧化还原电子转移使Ni原子电子云密度增加[38],推断在催化反应中发生了Ni2++Ti4+↔Ni3++ Ti3+氧化还原循环,引发并促进了Ti、Ni之间的协同效应,增强MOFs材料的低温脱硝活性.

图7 催化剂XPS谱图
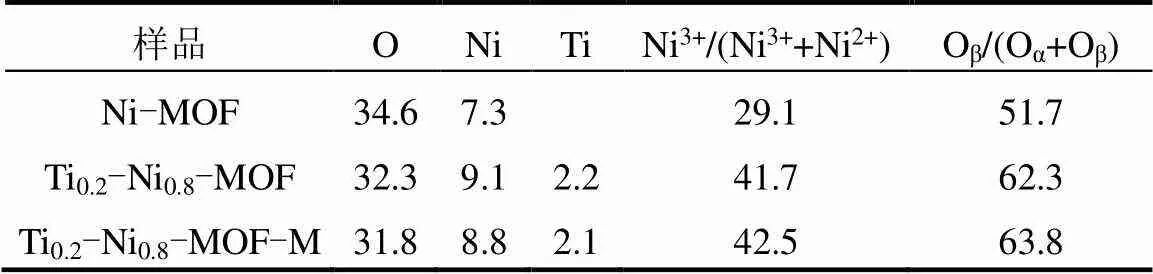
表2 基于XPS表征的Tix-Ni1-x-MOF的化学成分(%)
O1s的XPS能谱图被拟合成如图7(d)所示的两个峰.高结合能(532.4~532.8eV)处的峰属于表面化学吸附氧(Oα,如OH-、CO32-),低结合能(531.3~ 531.7eV)处的峰属于表面晶格氧(Oβ).如表2所示,双金属Ti0.2-Ni0.8-MOF中的Oβ/(Oα+Oβ)的比值(微波法63.8%、溶剂热法62.3%)大于单金属Ni-MOF(51.7%).晶格氧Oβ比Oα具有更好的反应活性[39-41],较高的Oβ/(Oα+Oβ)比值促进Ni-Ov-Ti中氧空位(Ov)的产生[42-43],有效提高NO+CO催化循环效率.
由Ni2++Ti4+↔ Ni3++Ti3+氧化还原循环引发的双金属协同效应,使Ni-O-Ti活性位点提供晶格氧来吸附CO和NO物种.在此过程中产生的晶格氧空位Ov可以与N-O键断裂产生游离的O结合,并再次生成,从而重新启动催化循环.
2.3 催化剂循环性测试
对Ti0.2-Ni0.8-MOF-M,Ti0.2-Ni0.8-MOF和Ni- MOF重复进行5次CO-SCR测试,如图8所示,经过循环测试后,Ni-MOF的NO还原率保持在80%以上,说明催化剂结构发生了局部的破坏和失稳.而2种方法制备的Ti0.2-Ni0.8-MOF均保持在初次测量的90%以上,这可能是由于Ti的适量掺杂加强了原子分散度导致金属骨架结构更加稳定.
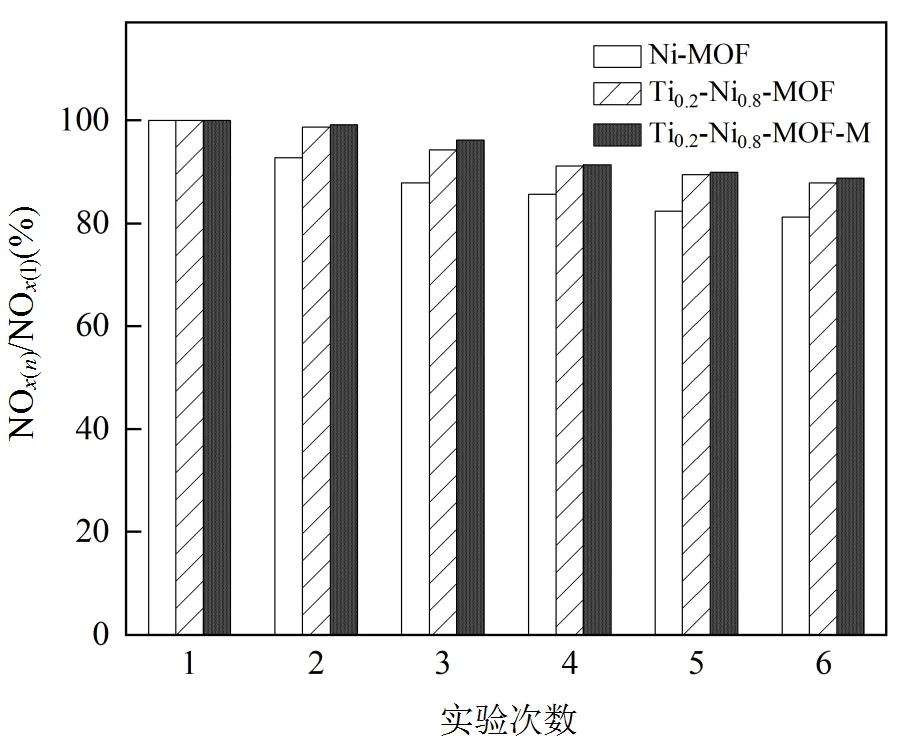
图8 催化剂循环测试
3 结论
3.1 Ti在Ni基MOF材料中的适量掺杂不仅增大双金属Ti0.2-Ni0.8-MOF的比表面积和孔容,有利于Ni-O-Ti活性位点高度分散,并且Ti4+/Ti3+间的氧化还原电子转移促进了Ni3++Ti3+↔Ni2++Ti4+循环反应,降低了反应能垒.其中Ti0.2-Ni0.8-MOF在200~400oC温度范围可达到100%的转化率.
3.2 采用微波法替代溶剂热法,不仅提高了合成效率,而且晶粒更加小且均匀,使Ti0.2-Ni0.8-MOF-M材料的比表面积增大,暴露出更加丰富高效的Ni-O- Ti活性位点,催化效率、热稳定性及循环性能均明显提高.
[1] 蒋春来,宋晓晖,钟悦之,等.2010~2015年中国燃煤电厂NO排放特征[J]. 中国环境科学, 2018,38(8):2903-2910.
Jiang C L, Song X H, Zhong Y Z, et al. Characteristics of NOemissions from coal-fired power plants in China from 2010 to 2015 [J]. China Environmental Science, 2018,38(8):2903-2910.
[2] 李 阳,陈敏鹏.长江经济带农业源非二氧化碳温室气体排放的时空特征[J]. 中国环境科学, 2020,40(5):2030-2039.
Li Y, Chen M P. Spatial and temporal characteristics of non-carbon dioxide greenhouse gas emissions from agricultural sources in the Yangtze River Economic Belt [J]. China Environmental Science, 2020, 40(5):2030-2039.
[3] 陈小根,张茹杰,沈伯雄,等.以CO为还原剂的选择性催化还原NO催化剂研究进展 [J]. 现代化工, 2020,40(5):5.
Chen X G, Zhang R J, Shen B X, et al. Research progress in catalysts for selective catalytic reduction of NO with CO as reductant [J]. Modern Chemical Industry, 2020,40(5):5.
[4] 付玉秀,仲雪梅,常化振,等.铈钴复合氧化物催化剂催化CO-SCR反应机理研究[J]. 中国环境科学, 2018,38(8):2934-2940.
Fu Y X, Zhong X M, Chang H Z, et al. Mechanism study on CO-SCR over Ce-Co-Omixed oxides catalysts [J]. China Environmental Science, 2018,38(8):2934-2940.
[5] 黄锦玉,孙 波,孙义高,等.镍系低温SCR脱硝催化剂载体与助剂的研究进展 [J]. 现代化工, 2021,41(1):34-37.
Huang J Y, Sun B, Sun Y G, et al. Research progress in supports and additives of Ni-based catalysts for low temperature SCR De-NO[J]. Modern Chemical Industry, 2021,41(1):34-37.
[6] Sun X, Yong S, Wang Z, et al. A new type Ni-MOF catalyst with high stability for selective catalytic reduction of NOwith NH3[J]. Catalysis Communications, 2018,114:104-108.
[7] 戴 波,张 松,顾明言,等.Ce改性钒磷氧催化剂的制备及其脱硝性能研究[J]. 中国环境科学, 2019,39(1):126-133.
Dai B, Zhang S, Gu M Y, et al. Preparation of supported VPO catalyst modified by Ce and the investigation of its denitration performance [J]. China Environmental Science, 2019,39(1):126-133.
[8] Jiang H, Wang S, Wang C, et al. Selective catalytic reduction of NOwith NH3on Cu-BTC-derived catalysts: influence of modulation and thermal treatment [J]. Catalysis Surveys from Asia, 2018,22(2):1-10.
[9] Shi Y , Li C , Liu X , et al. MIL-100(Fe) as a new catalyst for selective catalysis reduction of NOwith ammonia [J]. Integrated Ferroelectrics, 2017,181(1):14-25.
[10] 石 勇,牛丹阳,武卓敏,等.Ag/Cu3(BTC)2复合催化剂的制备及其NH3-SCR催化性能[J]. 中国环境科学, 2018,38(7):2445-2450.
Shi Y, Niu D Y, Wu Z M, et al. Synthesis of Ag/Cu3(BTC)2composite catalysts and their catalytic performance for NH3-SCR [J]. China Environmental Science, 2018,38(7):2445-2450.
[11] 刘 晶,熊志波,周 飞,等.新型铈钨钛复合氧化物催化还原脱硝机理[J]. 中国环境科学, 2018,38(5):1670-1676.
Liu J, Xiong Z B, Zhou F, et al. The NH3-SCR mechanism of a novel cerium-tungsten-titanium mixed oxide catalyst prepared through the hydrothermal co-precipitation method modified by H2O2complex [J]. China Environmental Science, 2018,38(5):1670-1676.
[12] Li C, Shi Y, Yu F, et al. Preparation of metal-organic framework Cu+/Ni-MOF catalyst with enhanced catalytic activity for selective catalytic reduction of NOx[J]. Ferroelectrics, 2020,565(1):26-34.
[13] Song K, Liang S, Zhong X, et al. Tailoring the crystal forms of the Ni- MOF catalysts for enhanced photocatalytic CO2-to-CO performance [J]. Applied Catalysis B: Environmental, 2022,309:121232.
[14] Dey K, Sauerland S, Ouladdiaf B, et al. Magnetostructural coupling in ilmenite-type NiTiO3[J]. Physical Review. B, 2021,103(13):134438.
[15] Wu X, Wang R, Du Y, et al. Performance enhancement of NH3-SCR via employing hydrotalcite-like precursor to induce the decoration of NiO by TiO2phase [J]. Molecular Catalysis, 2019,467:150-160.
[16] Liu B, J Liu, Xin L, et al. Unraveling reactivity descriptors and structure sensitivity in low-temperature NH3-SCR reaction over CeTiOxcatalysts: A Combined Computational and Experimental Study [J]. ACS Catalysis, 2021,11(13):7613-7636.
[17] Deng K. Optimized microwave-based synthesis of thermally stable inverse catalytic core–shell motifs for CO2hydrogenation [J]. ACS Applied Materials & Interfaces, 2020,12(29):32591–32603.
[18] Chen S, Xu X, Gao H, et al. Fine-tuning the metal oxo cluster composition and phase structure of Ni/Ti bimetallic MOFs for efficient CO2reduction [J]. The Journal of Physical Chemistry C, 2021, 125(17):9200-9209.
[19] Zhai M, Cheng Y , Jin Y , et al. Solvothermal synthesis of flower-like structure Cu-Mn bimetallic sulfide on Ni-foam for high-performance symmetric supercapacitors [J]. International Journal of Hydrogen Energy, 2019,44(26):13456-13465.
[20] Nguyen H T, Tran K T, Van Tan L, et al. Microwave-assisted solvothermal synthesis of bimetallic metal-organic framework for efficient photodegradation of organic dyes [J]. Materials Chemistry and Physics, 2021,272:125040.
[21] Chen C, Feng X, Zhu Q, et al. Microwave-assisted rapid synthesis of well-shaped MOF-74 (Ni) for CO2efficient capture [J]. Inorganic Chemistry, 2019,58(4):2717-2728.
[22] Israr F, Kim D K, Kim Y, et al. Scope of various solvents and their effects on solvothermal synthesis of Ni-BTC [J]. Química Nova, 2016,39(6):669-675.
[23] Ipadeola A K, Ozoemena K I. Alkaline water-splitting reactions over Pd/Co-MOF-derived carbon obtained via microwave-assisted synthesis [J]. RSC Adv., 2020,10(29):17359-17368.
[24] Devarayapalli K C, Vattikuti S V P, Tvm S, et al. Facile synthesis of Ni-MOF using microwave irradiation method and application in the photocatalytic degradation [J]. Materials Research Express, 2019, 6(11):1150.
[25] Xie S, Qin Q, Liu H, et al. MOF-74-M (M = Mn, Co, Ni, Zn, MnCo, MnNi, and MnZn) for low-temperature NH3-SCR and in situ DRIFTS study reaction mechanism [J]. ACS Applied Materials & Interfaces, 2020,12(43):48476-48485.
[26] Wang D, Huang B, Shi Z, et al. Influence of cerium doping on Cu-Ni/activated carbon low-temperature CO-SCR denitration catalysts [J]. RSC Adv., 2021,11(30):18458-18467.
[27] Xie S, Li L, Jin L, et al. Low temperature high activity of M (M = Ce, Fe, Co, Ni) doped M-Mn/TiO2catalysts for NH3-SCR and in situ DRIFTS for investigating the reaction mechanism [J]. Applied Surface Science, 2020,515:146014.
[28] Gao S, Sui Y, Wei F, et al. Facile synthesis of cuboid Ni-MOF for high-performance supercapacitors [J]. Journal of Materials Science, 2018,53(3):6807–6818.
[29] Liu Z, Sun G, Chen C, et al. Fe-doped Mn3O4spinel nanoparticles with highly exposed Feoct-O-Mntetsites for efficient selective catalytic reduction (SCR) of NO with ammonia at low temperatures [J]. ACS Catalysis, 2020,10(12):6803-6809.
[30] Shu S A, Guo J, Li J A, et al. The enhanced performance of Ti doped MnOfor the removal of NO with NH3[J]. Journal of the Taiwan Institute of Chemical Engineers, 2019,100:168-177.
[31] He H, Zhang C, Wang Y, et al. Low-temperature selective catalytic reduction of NO with CO over Nix-MOF-5 [J]. Journal of Materials Science, 2022,57(4):2502-2513.
[32] Ramasubbu V, Kumar P R, Mothi E M, et al. Highly interconnected porous TiO2-Ni-MOF composite aerogel photoanodes for high power conversion efficiency in quasi-solid dye-sensitized solar cells [J]. Applied Surface Science, 2019,496(Dec.1):143646.1-143646.11.
[33] Cao J, Rohani S, Liu W, et al. Influence of phosphorus on the NH3-SCR performance of CeO2-TiO2catalyst for NO removal from co-incineration flue gas of domestic waste and municipal sludge [J]. Journal of Colloid and Interface Science, 2022,610:463-473.
[34] Leukkunen P M, Rani E, Sasikala D A, et al. Synergistic effect of Ni-Ag-rutile TiO2ternary nanocomposite for efficient visible- light- driven photocatalytic activity [J]. RSC Adv., 2020,10(60):36930- 36940.
[35] Chen L, Rui L, Li Z, et al. Effect of Ni doping in NiMn1-xTi10(x = 0.1-0.5) on activity and SO2resistance for NH3-SCR of NO studied with in situ DRIFTS [J]. Catalysis Science & Technology, 2017,7(15): 3243-3257.
[36] Liu Z, Liu H, Feng X, et al. Ni-Ce-Ti as a superior catalyst for the selective catalytic reduction of NOxwith NH3[J]. Molecular Catalysis, 2018,445:179-186.
[37] Hu Z, Yong X, Li D, et al. Synergism between palladium and nickel on Pd-Ni/TiO2for H2-SCR: A transient DRIFTS study [J]. Journal of Catalysis, 2020,381:204-214.
[38] Liu Q, Tian Y, Ai H. Methanation of carbon monoxide on ordered mesoporous NiO-TiO2-Al2O3composite oxides [J]. RSC Advances, 2016,6(25):2971-2978.
[39] Y Zhang, Zhao L, Duan J, et al. Insights into deNOprocessing over Ce-modified Cu-BTC catalysts for the CO-SCR reaction at low temperature by in situ DRIFTS [J]. Separation and Purification Technology, 2019,234:116081.
[40] Wang Q, Xu H, Huang W, et al. Metal organic frameworks-assisted fabrication of CuO/Cu2O for enhanced selective catalytic reduction of NOby NH3at low temperatures [J]. Journal of Hazardous Materials, 2019,364:499-508.
[41] Du Y, Liu J, Li X, et al. SCR performance enhancement of NiMnTi mixed oxides catalysts by regulating assembling methods of LDHs- Based precursor [J]. Applied Organometallic Chemistry, 2020,34(4): e5510.
[42] Huang L, Shi Y, Xiong W, et al. Facile design of highly effective Fe-modified bimetallic Fex–Ni1−x-MOFs catalysts with rodlike structures for low-temperature NO reduction by CO [J]. Journal of Materials Science, 2021,56(16):9914-9928.
[43] Ding Y, Shi Y, Xiong W, et al. Insights into N-coordinated bimetallic site synergy during NO selective catalytic reduction by CO [J]. ACS Applied Materials & Interfaces, 2021,13(48):57182-57192.
Preparation of Ti-Ni1-x-MOFs and their selective catalytic reduction of NOby CO.
SHI Yong1*, LI Cheng1, HUANG Lei1, XIONG Wei1, ZHAO Qi-dong2, SUN Jiang-heng1, DING Yue1
(1.Key Laboratory of Industrial Ecology and Environmental Engineering and State Key Laboratory of Fine Chemicals, School of Environmental Science and Technology, Dalian University of Technology, Dalian 116024, China;2.Panjin Branch of School of Chemical Engineering, Dalian University of Technology Panjin Campus, Panjin 124221, China)., 2022,42(11):5080~5087
Ti-Ni1-x-MOFs catalysts with different proportions were successfully synthesized by solvothermal method and microwave method, applied for selective catalytic reduction reaction of NOwith CO. The doping of Ti significantly improved NOreduction performance of Ni-MOF catalysts and widened reaction temperature window. Ti0.2-Ni0.8-MOF showed the best denitration efficiency and reached 100% conversion in the temperature range of 200~400℃. Multiple characterizations were conducted to ascertain the properties of bimetallic Ti-Ni1-x-MOFs materials (e.g., TGA, XRD, SEM, FT-IR, XPS and BET). Ti-doping in Ni-MOF can improve the atomic dispersion, and indicate a strong metal-metal interaction between Ti and Ni which was conducive to produce more efficient Ni-O-Ti sites and oxygen vacancies, strengthen the Ni2++Ti4+↔ Ni3++Ti3+redox cycle, and thus improve the catalytic performance of NO reduction reaction by CO. Compared with solvothermal method, the preparation of Ti0.2-Ni0.8-MOF by microwave method exhibits the advantages of high synthesis efficiency, good crystallinity, fine and uniform grains, which further enhancing its low-temperature denitration effect.
CO-SCR;metal-organic frameworks;Ti-Ni1-x-MOFs;microwave method;solvothermal method
X511
A
1000-6923(2022)11-5080-08
石 勇(1975-),男,河南南阳人,副教授,博士,在大连理工大学从事环境催化材料合成、污染物监测及大气污染控制等方面的研究.发表论文70余篇.
2022-04-25
国家自然科学基金资助项目(21677022,22006007);工业生态与环境工程教育部重点实验室开放基金资助项目(KLIEEE-21-03)
* 责任作者, 副教授, yongshi@dlut.edu.cn

