外源硫诱导下的Desulfovibrio desulfuricans sub sp. EPS特性及对Zn(Ⅱ)的吸附
甘 雨,宋卫锋,杨佐毅,连泽阳,马双念,黄祥武,羊仁高,温炎标
外源硫诱导下的sp. EPS特性及对Zn(Ⅱ)的吸附
甘 雨,宋卫锋*,杨佐毅,连泽阳,马双念,黄祥武,羊仁高,温炎标
(广东工业大学环境科学与工程学院,广东 广州 510006)
研究了3种外源硫(Na2SO4、Na2SO3和Na2S2O3·5H2O)对sp.(.sp.)的胞外聚合物(EPS)的胁迫/诱导作用.结果表明,在还原性硫源0.50g/L Na2SO3的条件下,EPS产量最高,为2104.39mg/g VSS,蛋白质含量为1888.52mg/g VSS,较胁迫/诱导前均提高了300%以上;其对Zn(Ⅱ)的吸附性能最好,为954.4mg/g EPS,提高了98.17%.三维荧光(3D-EEM)结果表明,胁迫/诱导后EPS中类酪氨酸均大量增加;傅里叶红外光谱(FTIR)结果表明,胁迫后-OH、C=O、C-O-C等官能团均大量增加,在Zn(Ⅱ)的吸附中发挥了重要作用;X光电子能谱(XPS)结果表明,在还原性硫源(Na2SO3和Na2S2O3·5H2O)胁迫/诱导后,EPS中C-O/C-N、C=N和某种含氧基团(X)大量增加,可能是吸附Zn(Ⅱ)的主要基团.
外源硫;胁迫/诱导;EPS;Zn(Ⅱ)
胞外聚合物(EPS)存在于细胞外和微生物聚集体内部,为细胞聚集以至形成颗粒污泥的关键,主要由蛋白质、多糖、腐殖质、核酸、脂质和磷脂组成,其中蛋白质和多糖是主要成分[1-2].产生EPS是细菌的一种自我保护机制,有利于细菌在不良条件中生存[3],其中含有丰富的官能团,如羧基(-COOH)、氨基(-NH2)、羟基(-OH)和羰基(C=O)等,可以和重金属离子结合,防止重金属离子进入细胞内[4].EPS的产生受多种因素影响,如营养元素碳、氮、磷,以及生长环境如pH值、温度等[5].近年来,关于EPS胁迫/诱导研究主要集中在重金属方面[6-8],胁迫后EPS中官能团的浓度与重金属的浓度具有相关性,如Zn(Ⅱ)与-OH、C-O-C等官能团[9],还发现类色氨酸参与了Cr(Ⅵ)的还原[10].重金属胁迫/诱导不仅能提高EPS产量还可以降低其他重金属的毒性[11],SRB在Zn(Ⅱ)胁迫/诱导后,EPS对Zn(Ⅱ)、Cu(Ⅱ)和Cd(Ⅱ)的吸附量都增加了[12].但由于重金属的毒性,难以得到大量的EPS.研究者逐渐将视野转向营养物质,如碳源[13]和氮源[14].一些特殊的物质还可以定向增加官能团的含量,在外源硫Na2S的胁迫/诱导下EPS中蛋白质增加了将近一倍,尤其是蛋白质中的巯基(-SH) 增加了约48.2%,EPS重金属的吸附能力也变得更加突出[15].不仅如此,氧化/还原性物质也可以起到一定的胁迫/诱导作用[16].因此,EPS的胁迫/诱导因子多种多样,不应局限于重金属.
目前,国内外对EPS的研究主要集中在好氧菌EPS的胁迫/诱导效应上[17-18],对厌氧菌EPS的胁迫/诱导效应研究较少.硫酸盐还原菌(SRB)属于革兰氏阴性厌氧菌,以乳酸或丙酮酸等碳源为电子供体将硫酸盐、亚硫酸盐、硫代硫酸盐等物质还原为硫化氢[19],释放出的硫离子与吸附在EPS上的重金属离子形成金属硫化物沉淀.含硫无机盐作为SRB的营养物质,为SRB的生命活动提供能量,如亚硫酸盐、硫代硫酸盐可发生歧化反应生成硫酸盐并释放能量[20],也必将促进EPS的合成.硫源对于SRB产生EPS有重要意义,但相应的的研究报道几乎没有.
研究EPS与重金属作用的意义不仅体现在重金属的去除,也体现在生物合成金属硫化物中[21],其中Zn(Ⅱ)具有重要价值,如SRB胞外聚合物中的多糖和蛋白质均可以提供Zn(Ⅱ)的结合点位,可以更高效的进行吸附,而Cu(Ⅱ)只能与多糖结合[22].不仅如此,EPS与Zn(Ⅱ)的作用是生物合成具有特殊性能的ZnS量子点的重要一环[21].在以往研究中,使用EDTA-4Na+降低Zn(Ⅱ)的毒性[23],但是EDTA也会与EPS发生螯合,一定程度上阻碍了生物合成.胁迫/诱导可能会提高SRB对Zn(Ⅱ)的吸附能力,也就可能通过胁迫/诱导促进生物合成ZnS.
本研究所使用菌种为脱硫弧菌脱硫亚种(sp.),以3种外源硫(Na2SO4、Na2SO3、Na2S2O3·5H2O)为胁迫/诱导因子,在同一浓度梯度下对.sp.进行培养,探究其生长情况、EPS产量及组分和吸附性能的变化,并通过多种测试分析方法揭示胁迫/诱导作用规律.研究发现EPS的产量大量增加,且作为主要成分的蛋白质产量极高,对高效处理重金属废水有启发作用.
1 材料与方法
1.1 材料
菌种为.sp.,由北京百欧博伟生物科技有限公司提供,经平板划线法厌氧培养3~4代后接种至液体培养基厌氧活化培养,然后用甘油于-80.00℃中保存待用.
厌氧血琼脂培养基:从广州翔博生物科技有效公司购得,由一次性无菌塑料平皿和琼脂培养基组成.其中,每1.00L琼脂含10.00g酪蛋白胰酶消化物、400.00mL半胱氨酸、1.00g玉米淀粉、14.00g琼脂、0.01g维生素K1、5.00g酵母浸出粉,5.00g氯化钠、70.00mL羊血或马血,加蒸馏水至1.00L配制而成.
Starkey培养基[24]:称取0.50g K2HPO4、1.00g NH4Cl、1.00g Na2SO4、0.10g CaCl2·2H2O、2.00g MgSO4·7H2O 、2.00g DL-乳酸钠、1.00g酵母粉,用超纯水定容至1.00L,调节pH值为(7.00±0.20),于锥形瓶中121℃高压灭菌20min,冷却至常温.使用前加入用无菌水配置的抗坏血酸,使其在培养基中的浓度为0.10g/L.初始Starkey培养基中不含SO32-和S2O32-,初始SO42-浓度为1.46g/L.
外源硫溶液:诱导/胁迫所使用的外源硫为SO42-、SO32-和S2O32-,以Na2SO4、Na2SO3和Na2S2O3·5H2O溶液的形式加入到培养基中.
1.2 活化与培养
将.sp.冻干粉用0.20mL无菌水溶解,用平板划线法接种至厌氧血琼脂培养基中,装入厌氧袋并持续吹入氮气5min,于30℃恒温培养箱中厌氧活化培养48h,再挑取单个菌落重复上述厌氧活化培养步骤3~4次,确保菌种复苏,恢复活性.
然后挑取单个菌落接种至Starkey液体培养基中,持续吹入氮气1min后密封,于35℃,150r/min的条件下恒温震荡培养48h后,用甘油于-80℃(Haier 医用低温保存箱DW-86L338J)中保存待用.
将菌种解冻至常温后以5.00%()接种至Starkey液体培养基中,持续吹入氮气1min后密封,于35℃,150r/min的条件下恒温震荡培养24h后,再以10.00%()分别接种到浓度为0,0.10,0.20,0.30, 0.40,0.50,0.60,0.70,0.80,0.90g/L不同外源硫的Starkey液体培养基中,持续吹入氮气1min后密封,于35℃,150r/min的条件下恒温震荡厌氧培养72h,达到稳定期[24].上述接种过程均在无菌条件下进行.
1.3 EPS提取与主要成分测定
采用NaOH法提取EPS[24].取30.00mL经外源硫胁迫/诱导培养后的菌液,在8000r/min,4℃的条件下离心10min;弃掉上清液,加入30.00mL的0.90%NaCl,震荡洗涤菌体,再于上述相同条件下离心10min;弃掉上清液,加入20.00mL的0.90%NaCl,震荡洗涤菌体,再以5.00%()加入1.00ml的1.00mol/L NaOH,摇匀,于4℃条件下静止3h;于4℃下,分别在16000r/min和8000r/min的条件下,各离心20min和15min;取上清液于0.45μm过滤器中过滤,再置于4000Da透析袋中透析24h,提纯EPS后于-20℃下保存备用.
EPS产量用蛋白质、多糖、核酸三者之和表示,3种成分分别用考马斯亮蓝法、硫酸蒽酮法、二苯胺法进行测定.EPS产量的实验结果取3次平行实验的平均值.
1.4 EPS表征分析
1.4.1 三维荧光光谱(3D-EEM)分析 用Edinburgh FLS1000型荧光分光光度计测定,对EPS主要成分进行比较分析,激发光(x)和发射光(m)扫描范围分别为200~450nm、200~550nm.
1.4.2 X射线光电子能谱(XPS)分析 采用K- Alpha型X射线光电子能谱仪(赛默飞,英国)对EPS进行测试分析.能谱扫描范围为0.00~1200.00eV,能谱采用C 1s(284.80eV校正),分辨率:pass energy 100.00eV,C 1s和O 1s等分辨率为40.00eV.所有的峰都用C 1s峰的结合能在284.80eV校准.
1.4.3 傅里叶红外光谱(FTIR)分析 采用Thermo Scientific Nicolet iS20型傅里叶变换红外光谱仪对EPS进行测试分析.扫描范围为400~4000cm-1,分辨率为4cm-1,样品扫描次数为32.
1.5 Zn(Ⅱ)吸附实验
配制浓度为20.00mg/L的Zn(Ⅱ)溶液,调节pH值为5.00,取不同胁迫/诱导条件得到的等质量(0.10mg)的EPS于锥形瓶中,并加入上述Zn(Ⅱ)溶液15.00mL.于35℃、150r/min条件下震荡吸附2h,然后将混合物装入分子量为4000Da的透析袋中,于200.00mL的超纯水中透析12h.用火焰原子吸收分光光度计测定透析后样品中Zn(Ⅱ)的浓度.吸附实验的结果均取3次实验结果的平均值.EPS吸附量的公式如下:
= (00-CV)/(1)
式中:为重金属吸附量,mg/g EPS;0为金属离子初始浓度,mg/L;0为初始溶液体积,L;C为吸附后金属离子浓度,mg/L;V为透析液体积,L;为EPS质量,g.
2 结果与讨论
采用3种不同的外源硫作为胁迫/诱导因子,在0~0.90g/L的浓度梯度下对.sp.进行胁迫/诱导,相应的EPS分别记为Na2SO4-EPS,Na2SO3-EPS,Na2S2O3-EPS,空白样记为Control-EPS.
2.1 胁迫/诱导下D. desulfuricans sp.的生长情况
为了方便对比,本文中以外源硫的浓度来表示胁迫/诱导强度,由图1可看出,.sp在3种不同外源硫的胁迫/诱导下,总体趋势是细胞干重随着胁迫/诱导浓度的增加先减少后增加.其中,Na2SO3对于细菌的生长影响最大,在Na2SO3胁迫/诱导下细胞干重基本上低于相同浓度的Na2SO4和Na2S2O3·5H2O,当Na2SO3浓度达到0.50g/L时,细胞干重为0.40g/L,相较于胁迫/诱导前,降低了44.51%.当胁迫/诱导浓度达到1.20g/L时,在Na2SO4和Na2S2O3·5H2O条件下,细菌大量繁殖,细胞干重大幅增加.Na2SO4胁迫/诱导下细胞干重最高,达到1.42g/L,增幅为106.37%.Na2SO3的条件下,细胞干重增长趋势十分缓慢.
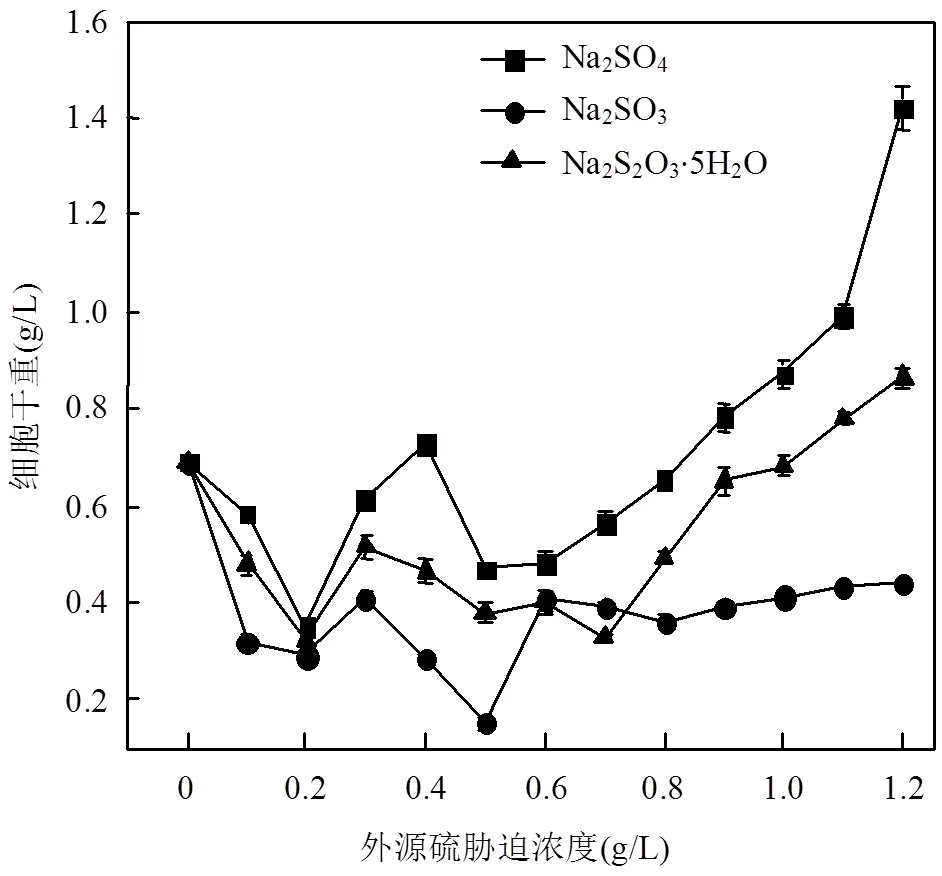
图1 不同浓度外源硫胁迫/诱导下D. desulfuricans sp.单位体积培养基的细胞干重
2.2 胁迫/诱导下D. desulfuricans sp.EPS组分变化
由图2可知,虽然在Na2SO3胁迫/诱导下.sp.细胞干重减少了,但在0.50g/L时,其单位质量微生物的EPS产量却是最高的,EPS的产量从胁迫/诱导前的494.38mg/g VSS提高到2104.39mg/g VSS,提高了325.66%,其中蛋白质的产量从451.44mg/g VSS提高到了1888.52mg/g VSS,提高了318.33%,整体上单位体积培养基的EPS产量相差并不明显.
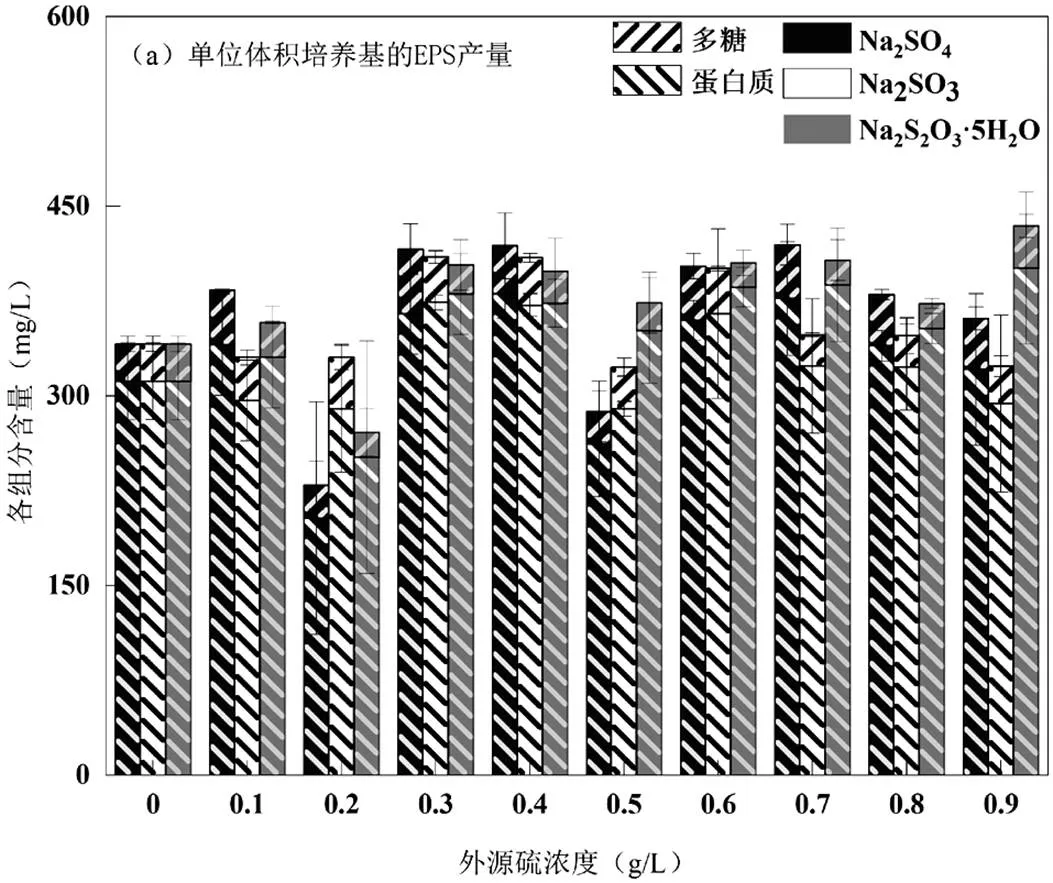
Na2SO4为0.60g/L时,EPS产量从494.38mg/g VSS提高到833.13mg/g,提高了68.52%,其中蛋白质从451.44mg/g VSS提高到744.28mg/g VSS,提高了74.71%;Na2S2O3·5H2O为0.70g/L时,EPS的产量从胁迫/诱导前的494.38mg/g VSS提高到1247.39mg/g VSS,提高了152.31%,其中蛋白质产量从451.44mg/g VSS提高到了1186.94mg/g VSS,提高了162.92%.另外,3种条件下EPS中的多糖变化不明显,但其产量同样在相应的最佳胁迫/诱导浓度下达到最大(Na2SO4:88.86mg/g VSS、Na2SO3:215.87mg/g VSS、Na2S2O3·5H2O:60.46mg/g VSS),而DNA未检测出.
由上述可知,在3种外源硫中,还原性硫源Na2SO3对.sp.的胁迫/诱导最为突出, Na2S2O3·5H2O次之,Na2SO4较差.Na2SO3超过最佳胁迫/诱导浓度,EPS和蛋白质的产量会迅速降低至最高值的46.81%、47.51%,随着浓度增加会变得更低.由此可见Na2SO3既能显著提高.sp.EPS产量,超过一定范围也能显著抑制EPS的产 生.
在SRB还原硫酸盐途径中,SRB需要消耗能量还原硫酸盐,SRB可以将亚硫酸盐或者硫代硫酸盐歧化,生成硫酸盐,同时释放能量供其生命活动所需.在本研究中,Na2SO3胁迫效果最好极有可能是其能够提供更多的能量以合成氨基酸,而硫代硫酸盐歧化释放的能量较少,所以胁迫效果次之.已有研究表明,亚硫酸盐歧化释放的能量远大于硫代硫酸盐[20].
2.3 胁迫/诱导下D. desulfuricans sp. EPS的吸附性能
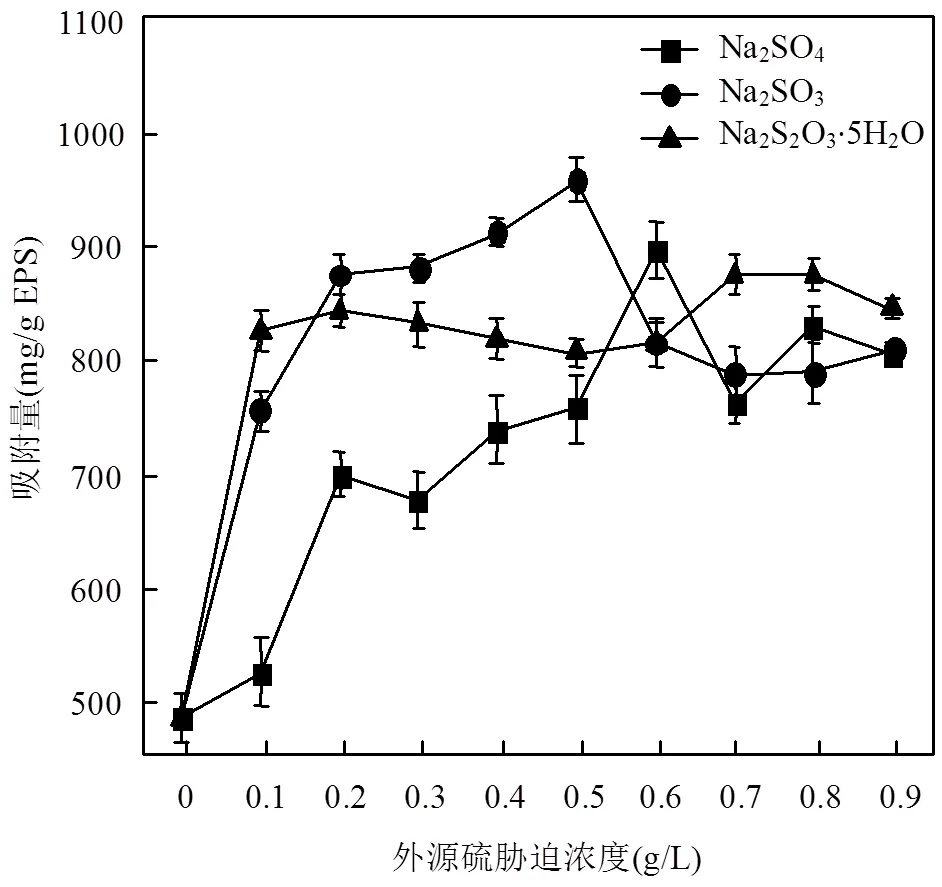
图3 不同浓度外源硫胁迫下EPS对Zn(Ⅱ)的吸附量
由图3可知,胁迫/诱导下的EPS对Zn(Ⅱ)的吸附量在整体上均体现出先增加后减少的趋势. Na2SO4最佳胁迫/诱导浓度为0.60g/L时,吸附量从胁迫/诱导前的481.6mg/g EPS增加到了893.6mg/g EPS,提高了85.54%;Na2SO3为0.50g/L时,吸附量增加到954.4mg/g EPS,提高了98.17%;Na2S2O3·5H2O为0.70g/L时,吸附量增加到871.8mg/g EPS,提高了81.02%.
Na2SO3-EPS对Zn(Ⅱ)的吸附能力较强, Na2SO4-EPS的吸附能力次之,Na2S2O3-EPS的吸附能力较差.结合EPS产量和组分变化规律,Na2SO3对.sp.的胁迫/诱导效果最为明显,不仅大幅提高了EPS产量(尤其是蛋白质),且对Zn(Ⅱ)的吸附能力也成倍增加.当胁迫/诱导浓度超过一定范围,不仅EPS产量减少,且吸附Zn(Ⅱ)能力也减弱.
总体上看,Na2SO3、Na2SO4和Na2S2O3·5H2O胁迫/诱导可以提高EPS的重金属吸附性能,且吸附性能变化趋势与组分变化规律基本一致.
2.4 胁迫/诱导前后EPS三维荧光分析
用三维荧光光谱研究3种外源硫的最佳胁迫/诱导前后的EPS.由于EPS样品中含水,测试结果均减去空白水样的背景值.
由图4和表1可知,Peak A强度较高而Peak B强度较低,它们分别为可溶性微生物产物(SMP)和酪氨酸,均含有C=O、-NH2/NH、-COOH、-OH等官能团,可以与重金属离子进行螯合和吸附.
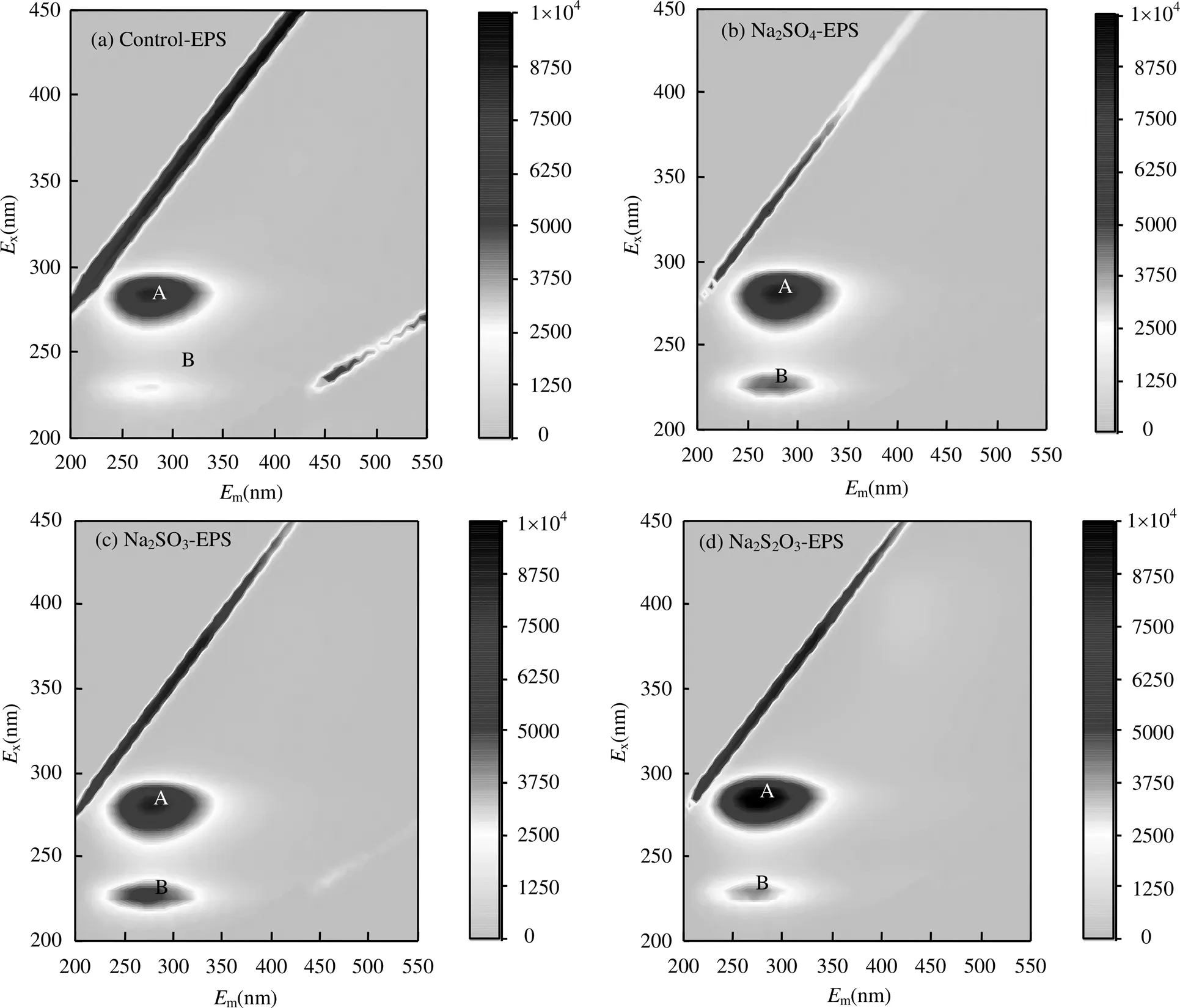
图4 外源硫胁迫/诱导前后EPS三维荧光谱图

表1 荧光光谱中的谱峰信息及其对应成分
Na2SO4的胁迫/诱导下,反映蛋白类物质的Peak B强度与Control-EPS相比,提高65.51%; Na2SO3的胁迫/诱导下,Peak B强度提高103.45%; Na2S2O3·5H2O胁迫/诱导下,Peak B强度提高44.83%,而Peak A强度增长缓慢.由此推断,类酪氨酸为胁迫/诱导后主要增加的蛋白质类产物,在提高EPS重金属吸附性能中发挥关键作用,SMP虽荧光强度高,含量多[27],但是从Na2SO4-EPS和Na2S2O3-EPS的重金属吸附能力变化上分析,SMP并不是发挥主要作用组分.
2.5 胁迫/诱导前后EPS傅里叶红外光谱分析
由图5可知,EPS在外源硫胁迫/诱导前后的关键官能团变化明显,胁迫后原有特征峰峰值变大,且增加了新特征峰.数学模型证明,谱峰强度与样品中官能团的浓度或数量具有正相关性[28],胁迫/诱导后EPS中官能团浓度均大量增加.
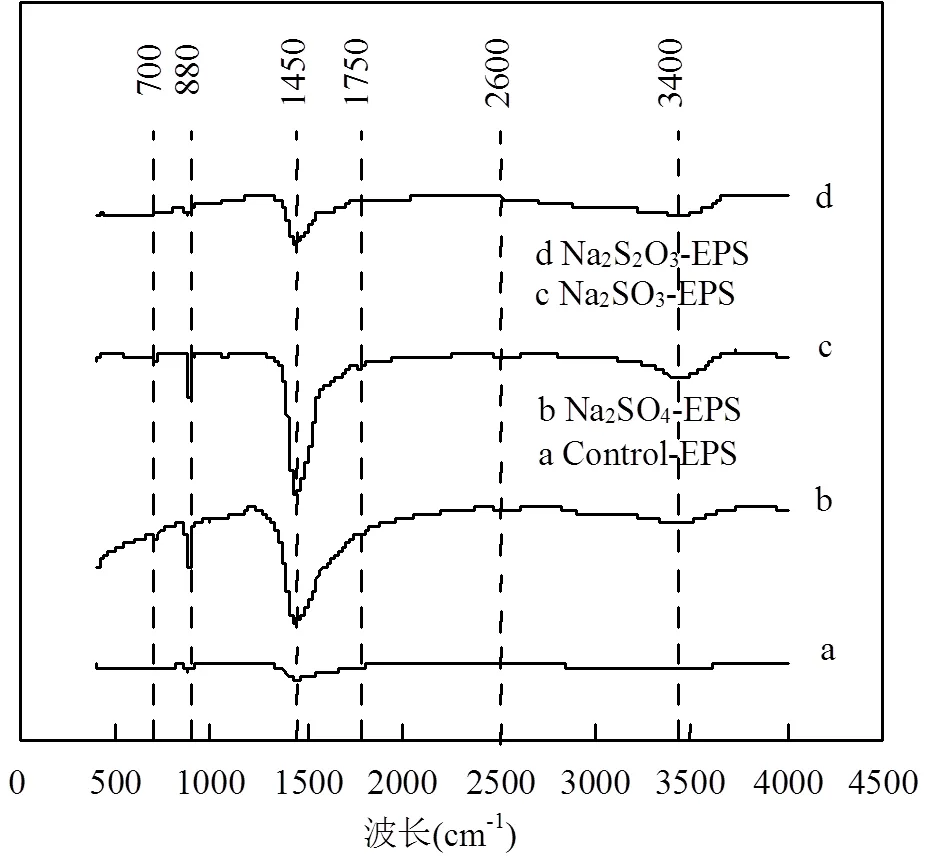
图5 外源硫胁迫/诱导前后EPS傅里叶红外光谱图
在3400cm-1处的特征峰对应于碳水化合物中-OH官能团的拉伸和弯曲[29],该官能团在Na2SO3-EPS中的相对峰值最大,Na2S2O3-EPS次之;1750cm-1处对应于酯类中的C=O[30],仅出现在Na2SO3-EPS中;1450cm-1处对应可取代的CO32-官能团,CO32-官能团的拉伸和弯曲可能与该官能团在有机相中连接着-OH和-NH有关[31],该官能团在Na2SO3-EPS中的峰值最大,Na2SO4-EPS次之; 880cm-1处为多糖中醚基(C-O-C)[32],该官能团在Na2SO3-EPS与Na2SO4-EPS中的峰值几乎一样;700cm-1处为碳骨架中的-CH2[33],2600cm-1处难以确定具体的官能团和振动种类[34].
上述官能团中的-OH、C=O、C-O-C在重金属吸附中能发挥重要作用[35],这些官能团在Na2SO3胁迫/诱导后增加幅度最大,说明Na2SO3-EPS具有最强的重金属吸附能力,这与前面的试验和测试结果一致.
值得指出的是,2.4中类酪氨酸的荧光强度变化规律与CO32-官能团红外光谱谱峰值变化规律一致,结合CO32-官能团在1450cm-1的振动规律,推断CO32-的出现极有可能是其连接着类酪氨酸中的-OH或-NH.因此,CO32-的峰值也能说明Na2SO3- EPS含有更多的类酪氨酸.
2.6 胁迫/诱导前后EPS X射线光电子能谱分析
2.6.1 XPS C谱分析 C是构成EPS的主要元素之一,胁迫/诱导前后EPS中C元素的存在形态及相对含量如图6和表2所示(XPS表征分析中EPS均未吸附重金属).

图6 外源硫胁迫/诱导前后EPS的X射线能谱分析(C谱)
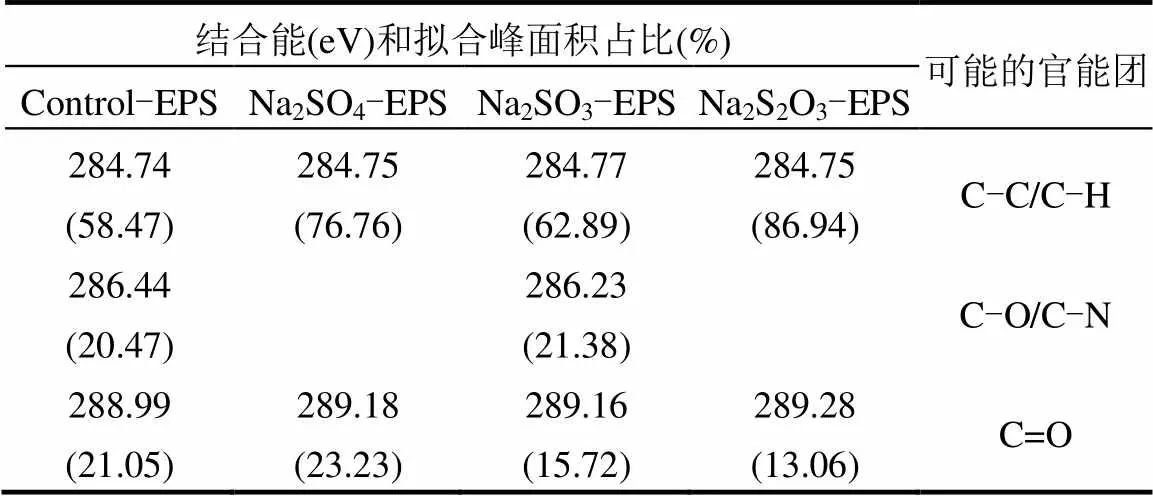
表2 胁迫/诱导前后EPS中C谱信息
通过图6和表2可知,EPS中C元素的存在形态要分为3种[36]:位于284.75eV的C-C/C-H键,存在于脂肪族或氨基酸侧链中[37];286.23-286.44eV处的C-O/C-N键;288.18-289.28处的C=O键.Na2SO4、Na2SO3和Na2S2O3·5H2O对C元素的胁迫/诱导效应整体上一致,都合成了更多的脂肪族或氨基酸侧链,生成更多的蛋白质.进一步分析,胁迫后Na2SO3- EPS脂肪族或氨基酸侧链占比最少,而C-O/C-N增加最多(其他两种情形未测出),C=O增加不是最多的.与其吸附能力相联系,整体上可以推断C=O与脂肪族或氨基酸侧链在吸附中很可能并不发挥主要作用,C-O/C-N才是吸附作用的主要官能团.官能团与重金属之间的作用还受到主链和相邻官能团的影响,还需要深入研究.
2.6.2 XPS O谱分析 O是构成EPS的主要元素之一,胁迫/诱导前后EPS中O元素的存在形态及相对含量如图7和表3所示.
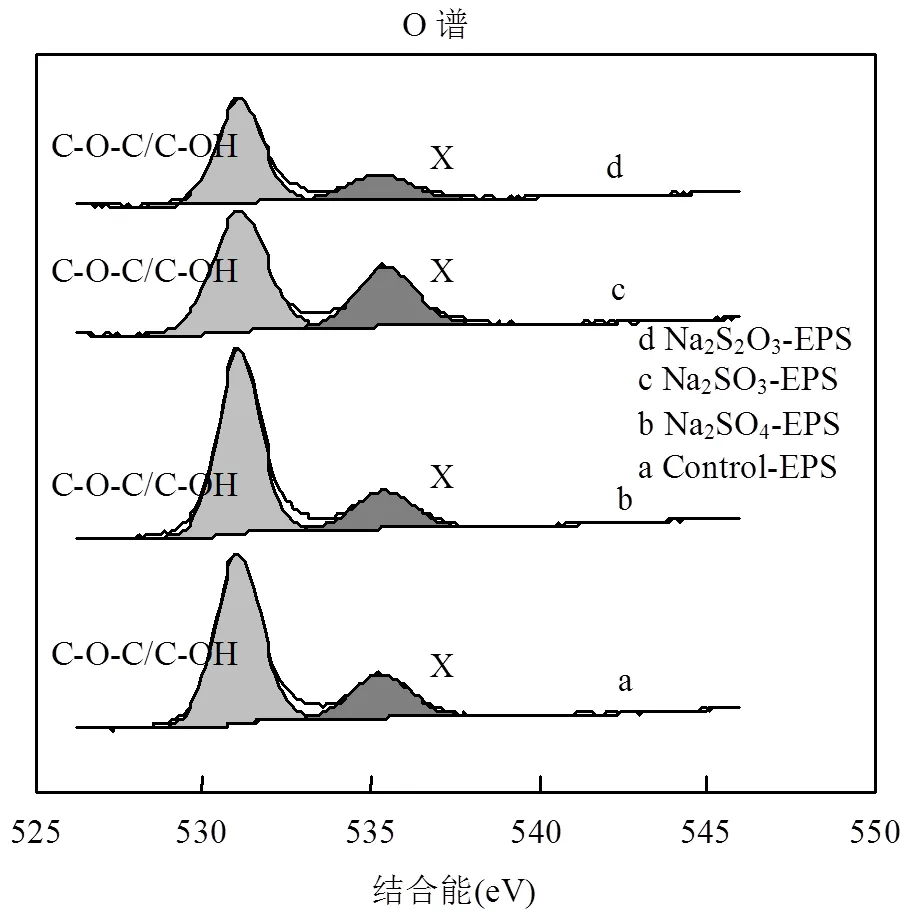
图7 外源硫胁迫/诱导前后EPS的X射线能谱分析(O谱)
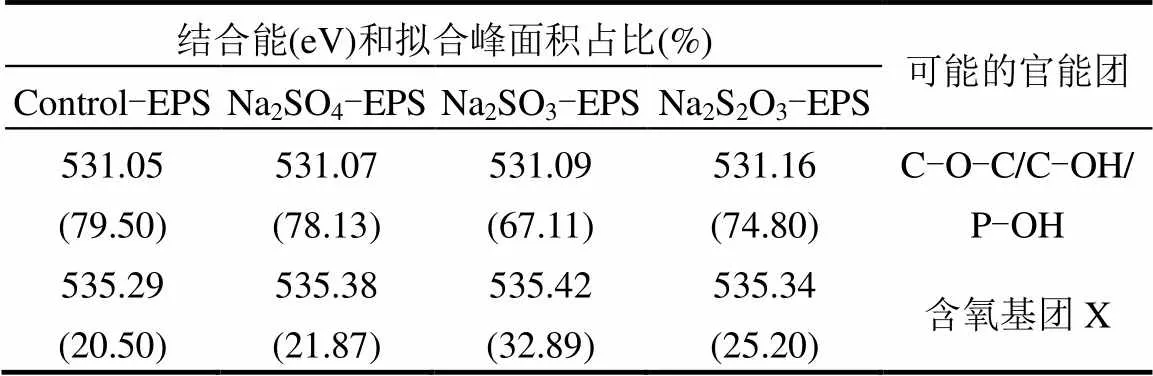
表3 胁迫/诱导前后EPS中O谱信息
文献认为,EPS中O的化学存在形态主要分为2种,分别对应约531eV(C-O-C/C-OH)和约532eV (C=O)[7,38-39],但在由上述图表中,未得到532eV处的峰,却意外得到了535.40eV处的峰,说明O还存在其他形态,但无法通过文献确定其名称,暂记为含氧基团X.由于其结合能较高,说明其比较稳定,且与重金属有良好的结合能力,该基团的名称、性质还需要进一步的研究.通过对比发现,C-O-C/C-OH键在Na2SO3和Na2S2O3·5H2O胁迫/诱导后出现不同程度的降低,而含氧基团X增加趋势与Zn(Ⅱ)吸附能力变化相一致,可能在吸附中发挥主要作用.
2.6.3 XPS N谱分析 N是构成EPS的重要元素,胁迫/诱导前后EPS中N元素的存在形态及相对含量如图8和表4所示.
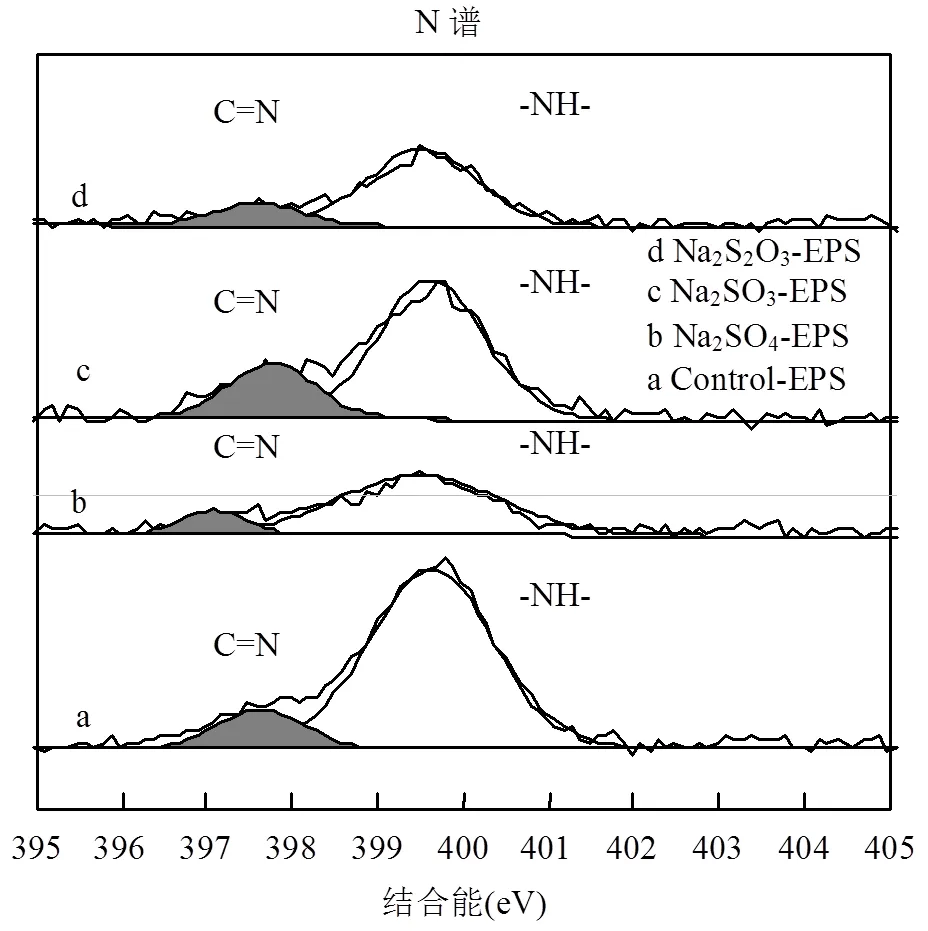
图8 外源硫胁迫/诱导前后EPS的X射线能谱分析(N谱)

表4 胁迫/诱导前后EPS中N谱信息
EPS中N的化学形态主要分为2种[36]:位于397.10~397.78eV的亚胺(C=N)键;399.48~399.72eV处的-NH-键.通过对比胁迫/诱导前后发现,在Na2SO3和Na2S2O3·5H2O的胁迫/诱导作用下C=N键的相对含量升高,而-NH-键数量降低.在Na2SO4胁迫/诱导作用下,两者相对含量变化轻微.
C=N键的形成一般认为是N取代了C=O键中的O,与重金属螯合的能力很强[40-41],使得EPS的重金属吸附能力增强;-NH-键的减少说明取代O的N可能来自-NH-键.Na2SO3-EPS中C=N键含量大幅增加很可能是重金属吸附能力提高的又一关键因素.
3 结论
3.1 在一定范围内,随着外源硫浓度的增大,.sp.EPS产量呈现出先增加后减少的趋势.0.50g/L Na2SO3的胁迫/诱导效果最好,单位质量微生物EPS和蛋白质产量比胁迫/诱导前提高了325.66%和318.33%,达到2104.39mg/g VSS和1888.52mg/g VSS.
3.2 胁迫/诱导下的EPS吸附Zn(Ⅱ)的性能也发生了重大变化.EPS对Zn(Ⅱ)的吸附量在整体上均体现出先增加后减少的趋势.在最佳Na2SO3胁迫/诱导强度下,吸附量从胁迫/诱导前的481.6mg/g EPS增加到954.4mg/g EPS,提高了98.17%.
3.3 3D-EEM和FTIR分析表明,胁迫/诱导后EPS中可溶性微生物产物和类酪氨酸的含量和与重金属离子结合的官能团(-OH、C=O、C-O-C等)大幅增加;XPS测试表明,Na2SO3-EPS具有丰富的C-O/ C-N、C=N和某种含氧基团,是吸附性能大幅增加的根本原因.
[1] Shi Y, Huang J, Zeng G, et al. Exploiting extracellular polymeric substances (EPS) controlling strategies for performance enhancement of biological wastewater treatments: An overview [J]. Chemosphere, 2017,180:396-411.
[2] Siddharth T, Sridhar P, Vinila V, et al. Environmental applications of microbial extracellular polymeric substance (EPS): A review [J]. Journal of Environmental Management, 2021,287:112307.
[3] Xie Q, Liu N, Lin D, et al. The complexation with proteins in extracellular polymeric substances alleviates the toxicity of Cd (II) to Chlorella vulgaris [J]. Environmental Pollution, 2020,263(Pt A):114102.
[4] Zhang P, Chen Y P, Peng M W, et al. Extracellular polymeric substances dependence of surface interactions of Bacillus subtilis with Cd(2+) and Pb(2+): An investigation combined with surface plasmon resonance and infrared spectra [J]. Colloids And Surfaces B -Biointerfaces, 2017,154:357-364.
[5] Cabral L, Giovanella P, Kerlleman A, et al. Impact of selected anions and metals on the growth and in vitro removal of methylmercury by Pseudomonas putida V1 [J]. International Biodeterioration & Biodegradation, 2014,91:29-36.
[6] 孙梦格,宋卫锋,杨佐毅,等.Cd(Ⅱ)胁迫/诱导下铜绿假单胞菌EPS组分变化及其吸附性能 [J]. 环境科学学报, 2021,41(9):3427-3436.
Sun M G, Song W F, Yang Z Y, et al.Effect of Cd(Ⅱ) stress on the variation in extracellular polymeric substances composition and adsorption performance of[J]. Journal of Environmental Science, 2021,41(9):3427-3436.
[7] Lian Z, Yang Z, Song W, et al. Effects of different exogenous cadmium compounds on the chemical composition and adsorption properties of two gram-negative bacterial EPS [J]. Science of Total Environment, 2021,806(Pt 1):150511.
[8] 曾峤婧,周 鑫,黄 超,等.白腐菌联合纳米零价铁强化去除水中Cd(Ⅱ) [J]. 中国环境科学, 2022,42(7):3174-3183.
Zeng Q J, Zhou X, Huang C, et al. Enhanced removal of Cd(Ⅱ) from aqueous solution by nanoscale zero-valent iron coupled with white rot fungus [J]. China Environmental Science, 2022,42(7):3174-3183.
[9] Li Y P, You L X, Yang X J, et al. Extrapolymeric substances (EPS) in Mucilaginibacter rubeus P2displayed efficient metal(loid) bio- adsorption and production was induced by copper and zinc [J]. Chemosphere, 2022,291(Pt 1):132712.
[10] Luo X, Zhou X, Peng C, et al. Bioreduction performance of Cr(VI) by microbial extracellular polymeric substances (EPS) and the overlooked role of tryptophan [J]. Journal of Hazardous Materials, 2022,433.
[11] Yue Z B, Li Q, Li C C, et al. Component analysis and heavy metal adsorption ability of extracellular polymeric substances (EPS) from sulfate reducing bacteria [J]. Bioresource Technology, 2015,194:399- 402.
[12] 李明明.硫酸盐还原菌胞外聚合物与金属离子的交互作用 [D]. 合肥:合肥工业大学, 2014.
Li M M. Analysis of the interaction between heavy metal and EPS isolated from sulfate reducing bacteria [D]. Hefei: Hefei University of Technology, 2014.
[13] Miqueleto A P, Dolosic C C, Pozzi E, et al. Influence of carbon sources and C/N ratio on EPS production in anaerobic sequencing batch biofilm reactors for wastewater treatment [J]. Bioresource Technology, 2010,101(4):1324-1330.
[14] Qian L, Ye X, Xiao J, et al. Nitrogen concentration acting as an environmental signal regulates cyanobacterial EPS excretion [J]. Chemosphere, 2022,291(Pt 2):132878.
[15] Li Q, Song W, Sun M, et al. Response of Bacillus vallismortis sp. EPS to exogenous sulfur stress/ induction and its adsorption performance on Cu(II) [J]. Chemosphere, 2020,251:126343.
[16] Han X, Wang Z, Chen M, et al. Acute Responses of Microorganisms from Membrane Bioreactors in the Presence of NaOCl: Protective Mechanisms of Extracellular Polymeric Substances [J]. Environmental Science & Technology, 2017,51(6):3233-3241.
[17] Dai M, Zhou G, Ng H Y, et al. Diversity evolution of functional bacteria and resistance genes (CzcA) in aerobic activated sludge under Cd(II) stress [J]. Journal of Environmental Management, 2019,250: 109519.
[18] Xu R, Fu Y, Xu Y, et al. Comparing biotransformation of extracellular polymeric substances (EPS) under aerobic and anoxic conditions: Reactivities, components, and bacterial responses [J]. Chemosphere, 2022,296:133996.
[19] Zheng Y, Bu N-S, Long X-E, et al. Sulfate reducer and sulfur oxidizer respond differentially to the invasion of Spartina alterniflora in estuarine salt marsh of China [J]. Ecological Engineering, 2017,99: 182-190.
[20] 王 原.珠江沉积物中SRB的群落结构、分离筛选和生理生化特性鉴定 [D]. 华南理工大学, 2013.
Wang Y. Community Structure, Isolation, Biochemical and Physiological Identification of Sulfate-Reducing Bacteria from Pearl River Sediments [D]. Guangzhou: South China University of Technology, 2013.
[21] Su Z, Li X, Xi Y, et al. Microbe-mediated transformation of metal sulfides: Mechanisms and environmental significance [J]. Science of Total Environment, 2022,825:153767.
[22] Wang J, Li Q, Li M M, et al. Competitive adsorption of heavy metal by extracellular polymeric substances (EPS) extracted from sulfate reducing bacteria [J]. Bioresource Technology, 2014,163:374-6.
[23] Qi S, Zhang M, Guo X, et al. Controlled extracellular biosynthesis of ZnS quantum dots by sulphate reduction bacteria in the presence of hydroxypropyl starch as a mediator [J]. Journal of Nanoparticle Research, 2017,19(6).
[24] 万正强.EPS在硫酸盐脱硫弧菌(Desulfovibrio desulfuricans)去除重金属Cd2+过程作用研究 [D]. 合肥:合肥工业大学, 2013.
Wan Z Q. Study on the role of EPS in the removal of heavy metal Cd2+by Desulfovibrio desulfuricans [D]. Hefei: Hefei University of Technology, 2013.
[25] Zan F, Huang H, Guo G, et al. Sulfite pretreatment enhances the biodegradability of primary sludge and waste activated sludge towards cost-effective and carbon-neutral sludge treatment [J]. Science of Total Environment, 2021,780:146634.
[26] Yu B, Lou Z, Zhang D, et al. Variations of organic matters and microbial community in thermophilic anaerobic digestion of waste activated sludge with the addition of ferric salts [J]. Bioresource Technology, 2015,179:291-298.
[27] Ma B, Li S, Wang S, et al. Effect of Fe3O4nanoparticles on composition and spectroscopic characteristics of extracellular polymeric substances from activated sludge [J]. Process Biochemistry, 2018,75:212-220.
[28] Palencia M. Functional transformation of Fourier-transform mid-infrared spectrum for improving spectral specificity by simple algorithm based on wavelet-like functions [J]. Journal of Advanced Research, 2018,14: 53-62.
[29] Xu Y, Liu P, Zhang Y. Mid-infrared spectroscopy of hemispherical water droplets [J]. Spectrochimica Acta Parta A-Molecular And Biomolecular Spectroscopy, 2022,264:120256.
[30] Kamnev A A, Tugarova A V, Dyatlova Y A, et al. Methodological effects in Fourier transform infrared (FTIR) spectroscopy: Implications for structural analyses of biomacromolecular samples [J]. Spectrochimica Acta Parta A-Molecular And Biomolecular Spectroscopy, 2018,193:558-564.
[31] Waheed S, Sultan M, Jamil T, et al. Comparative Analysis of Hydroxyapatite Synthesized by Sol-gel, Ultrasonication and Microwave Assisted Technique [J]. Materials Today: Proceedings, 2015,2(10):5477-5484.
[32] Ali H U, Iqbal D N, Iqbal M, et al. HPMC crosslinked chitosan/ hydroxyapatite scaffolds containing Lemongrass oil for potential bone tissue engineering applications [J]. Arabian Journal of Chemistry, 2022,103850.
[33] Khare N, Bajpai J, Bajpai A K. Graphene coated iron oxide (GCIO) nanoparticles as efficient adsorbent for removal of chromium ions: Preparation, characterization and batch adsorption studies [J]. Environmental Nanotechnology, Monitoring & Management, 2018, 10:148-162.
[34] Bao F, Zong L, Li N, et al. Synthesis of novel poly(phthalazinone fluorenyl ether ketone ketone)s with improved thermal stability and processability [J]. Thermochimica Acta, 2020,683.
[35] Zhang M, Hou S, Li Y, et al. Single evaluation and selection of functional groups containing N or O atoms to heavy metal adsorption: Law of electric neutrality [J]. Chemosphere, 2022,287(Pt 2):132207.
[36] Maaza L, Djafri F, Belmokhtar A, et al. Evaluation of the influence of Al2O3nanoparticles on the thermal stability and optical and electrochemical properties of PANI-derived matrix reinforced conducting polymer composites [J]. Journal of Physics and Chemistry of Solids, 2021,152.
[37] 连泽阳,杨佐毅,宋卫锋,等.外源Cd(Ⅱ)胁迫Alcaligenes faecalis过程中阴离子对EPS产量及其特性的影响 [J]. 环境科学学报, 2022,42(4):81-90.
Lian Z Y, Yang Z Y, Song W F, et al. The effect of anions on the yield and characteristics of EPS during the process of exogenous Cd(Ⅱ) stress/induction of Alcaligenes faecalis [J]. Journal of Environmental Science: 2022,42(4):81-90.
[38] Szcześ A, Czemierska M, Jarosz-Wilkołazka A. Calcium carbonate formation on mica supported extracellular polymeric substance produced by Rhodococcus opacus [J]. Journal of Solid State Chemistry, 2016,242:212-221.
[39] Lin D, Ma W, Jin Z, et al. Interactions of EPS with soil minerals: A combination study by ITC and CLSM [J]. Colloids And Surfaces B -Biointerfaces, 2016,138:10-6.
[40] Han J, Pei L, Du Y, et al. Tripolycyanamide-2,4,6-triformyl pyrogallol covalent organic frameworks with many coordination sites for detection and removal of heavy metal ions [J]. Journal of Industrial and Engineering Chemistry, 2022,107:53-60.
[41] Qin L, Ge Y, Deng B, et al. Poly (ethylene imine) anchored lignin composite for heavy metals capturing in water [J]. Journal of the Taiwan Institute of Chemical Engineers, 2017,71:84-90.
The EPS characteristics ofsp. and its adsorption performance for Zn(Ⅱ) under exogenous sulfur induction.
GAN Yu, SONG Wei-feng*, YANG Zuo-yi, LIAN Ze-yang, MA Shuang-nian, HUANG Xiang-wu, YANG Ren-gao, WEN Yan-biao
(School of Environmental Science and Engineering, Guangdong University of Technology, Guangzhou 510006, China)., 2022,42(11):5144~5152
In this paper, the effect of exogenous sulfur (Na2SO4, Na2SO3, Na2S2O3·5H2O) stress/induction on extracellular polymeric substances(EPS) ofsp. (.sp.)was studied. The results showed that addition of 0.50g/L Na2SO3led to the highest EPS yield of 2104.39mg/g VSS (1888.52mg/g VSS of protein content), which was 300% higher than that without Na2SO3addition, and as a result, 98.17% increase in Zn(II) adsorption capacity (954.4mg/g EPS) achieved. Three-dimensional fluorescence (3D-EEM) spectral results showed that tyrosine-like substances in EPS were greatly increased aftersulfurstress/induction; Fourier transform infrared spectroscopy (FTIR) results showed that significant increases in functional groups such as —OH, C=O, C—O—C EPS were mainly responsible for the enhance adsorption of Zn(II); The results of X-ray photoelectron spectroscopy (XPS) results showed that the content of C—O/C—N, C=N and oxygen-containing functional group (X) in EPS increased after sulfur stress/induction (Na2SO3and Na2S2O3·5H2O).
exogenous sulfur;stress/induction;EPS;Zn(Ⅱ)
X172
A
1000-6923(2022)11-5144-09
甘 雨(1997-),男,广东惠州人,广东工业大学硕士研究生,主要从事EPS吸附重金属及介导合成金属硫化物.发表论文2篇.
2022-04-18
广东省自然科学基金资助项目(2021A1515010558)
* 责任作者, 教授, weifengsong@gdut.edu.cn

