铁强化厌氧氨氧化脱氮机理研究进展
王 倩,胡嘉源,李天皓,王彩霞,陈 洁,沈耀良,2,刘文如,2*
铁强化厌氧氨氧化脱氮机理研究进展
王 倩1,胡嘉源1,李天皓1,王彩霞1,陈 洁1,沈耀良1,2,刘文如1,2*
(1.苏州科技大学环境科学与工程学院,江苏 苏州 215000;2.苏州科技大学,城市生活污水资源化利用技术国家地方联合工程实验室,江苏 苏州 215000)
如何强化厌氧氨氧化细菌生长代谢,提高厌氧氨氧化工艺脱氮效能以及保障工艺长期稳定运行是有关厌氧氨氧化研究的热点之一.铁强化厌氧氨氧化是当前研究最为广泛且最具经济性和实用性的一种措施.本文简述了铁强化厌氧氨氧化技术及其强化脱氮效能;重点从优化厌氧氨氧菌的生长环境、促进胞外聚合物分泌、加速脱氮功能菌群富集、诱导细胞结构演变、调控关键酶和功能基因表达、促进信号分子合成以及强化非生物脱氮反应等方面总结了铁强化厌氧氨氧化菌生长代谢和厌氧氨氧化系统脱氮效能的机理.最后对铁强化厌氧氨氧化技术进行总结展望,并提出废铁屑强化厌氧氨氧化技术的构想.
厌氧氨氧化;铁;脱氮强化;机理
厌氧氨氧化(ANAMMOX)在厌氧环境下以亚硝酸盐(NO2-)为电子受体,将氨(NH3)转化为氮气[1].厌氧氨氧化技术相较于传统硝化反硝化脱氮技术,具有节省曝气、无需有机碳源、温室气体排放少等优点[2-3].然而,当前厌氧氨氧化技术的推广应用仍然面临如厌氧氨氧化菌倍增时间长且对环境因子(如温度)变化敏感性高、亚硝酸盐氧化菌(NOB)长期有效抑制(即NO2-稳定高效供给)难等诸多挑战[4-6].对于如何强化厌氧氨氧化细菌生长代谢、提高厌氧氨氧化工艺脱氮效能以及保障工艺长期稳定运行,研究者们进行了大量尝试.研究表明,投加Fe/Mn、氧化石墨烯等化学物质以及施加电场、磁场、超声波等均是实现厌氧氨氧化系统强化脱氮行之有效的方法[7-11],其中投加Fe方法(即铁强化厌氧氨氧化)是研究最为广泛的,也是最具经济性和实用性的措施.目前,有关铁强化厌氧氨氧化的研究中所投加的铁主要包括零价铁(包括nZVI、mZVI、海绵铁及铁碳材料等)、亚铁离子、铁离子以及其他铁化合物(如铁矿物、Fe3O4NPs)等.研究证实,尽管投加的铁种类不同,但它们均能不同程度地促进厌氧氨氧化菌生长代谢、强化厌氧氨氧化系统脱氮效能,从而形成了一系列基于不同铁材料的铁强化厌氧氨氧化技术.一般认为厌氧氨氧化菌只能摄取Fe2+/ Fe3+ [12],基于不同铁材料的铁强化厌氧氨氧化技术所涉及的作用机制可能有所不同.铁强化厌氧氨氧化脱氮的影响因素众多、作用机理十分复杂,这就使得铁强化厌氧氨氧化技术在铁投加量控制、运行条件优化、内在机制解释等方面难以形成共识.本文在总结当前众多文献报道的基础上,尝试对铁强化厌氧氨氧化脱氮机理进行梳理、评述,以期为铁强化厌氧氨氧化技术的发展与应用提供理论借鉴.
1 铁强化厌氧氨氧化脱氮技术
1.1 厌氧氨氧化菌对铁过量需求
铁的天然丰度及其氧化还原特性,使其成为微生物异养呼吸和自养生长的普遍底物[12].研究表明,厌氧氨氧化菌内铁含量远高于一般的微生物,铁不仅是厌氧氨氧化菌体内血红素合成的重要组分,还可以提高细胞膜传质能力[13].厌氧氨氧化菌的生理活动高度依赖铁结合蛋白,铁结合蛋白中铁价态的变化在厌氧氨氧化反应电子转移过程中发挥关键作用[12].此外,铁还是亚硝酸盐还原和氨氧化过程关键酶(例如硝酸盐还原酶、肼合成酶(HZS)和肼脱氢酶(HDH))的重要组成部分[14-16].总之,铁在厌氧氨氧化过程中扮演重要角色,因此铁的稳定供应十分重要[17].
1.2 铁强化厌氧氨氧工艺脱氮效能
投加铁对厌氧氨氧工艺的强化作用主要体现在缩短厌氧氨氧化工艺启动时间、提高厌氧氨氧化系统脱氮负荷和效率等方面.表1总结了部分文献报道的铁强化厌氧氨氧工艺的脱氮效能.不同铁材料对厌氧氨氧工艺脱氮效能的强化能力不同,例如相同条件下施加Fe2O3纳米颗粒(Fe2O3NPs)、Fe3+、Fe纳米颗粒(Fe NPs)、Fe3O4纳米颗粒(Fe3O4NPs)和Fe3+,厌氧氨氧化反应器脱氮效率分别为34.8%、49.4%、74.8%、84.9%和91.3%[28].与微米零价铁(mZVI)相比,纳米零价铁(nZVI)可以进一步缩短反应器启动时间约16.7%[27].

续表1
注:“*”表示反应器中铁均在试验初期一次性投加.UASB:上流式厌氧污泥床;SBR:序批式反应器;SBBR:序批式生物膜反应器;UABR:上流式厌氧耦合生物膜反应器;UFBR:上流式固定床反应器;ASBR:厌氧序批式反应器;IFFASR:综合浮膜活性污泥反应器;EPS:胞外分泌物;PN:蛋白质.
铁强化厌氧氨氧化系统的效果也与铁投加量密切相关.研究发现,在添加3.36,5.04mg/L Fe2+的条件下,厌氧氨氧化工艺的启动时间由未添加Fe2+时的70d分别缩短到58,50d[17].马娇等[26]实验结果指出,当nZVI浓度由0mg/L增加到100mg/L时,反应器总氮去除率从70.3%提高到90.1%,但当nZVI的浓度继续增加时,其总氮去除率降低.铁添加过量会降低系统内厌氧氨氧化菌丰度,减少血红素c浓度,抑制肼脱氢酶活性[18].一般而言,铁投加量对厌氧氨氧化工艺启动和脱氮效能的影响主要表现为低浓度促进,高浓度抑制.
2 铁辅助厌氧氨氧化工艺强化脱氮机理
如图1所示,铁强化厌氧氨氧化的作用机理主要表现在优化厌氧氨氧化菌的生长环境、促进厌氧氨氧化菌分泌胞外分泌物(EPS)、加速功能菌富集、改变细胞结构、调控功能基因表达、强化关键酶和辅因子活性、促进信号分子合成和强化非生物因素脱氮效能等方面.

图1 铁辅助厌氧氨氧化工艺强化脱氮机制
2.1 优化厌氧氨氧化菌的生长环境
2.1.1 氧化还原电位(ORP) 厌氧氨氧化系统适宜的ORP范围较广,为-107~375mV[31-33].厌氧氨氧化是一种复杂的生物反应,ORP影响厌氧氨氧化菌比活性(SAA)的机理较为复杂.SAA在一定ORP范围内(-100~110mV),随着ORP的增加而降低.向反应器中投加适量零价铁(ZVI)可以降低ORP,从而促进厌氧氨氧化菌代谢[31].Guo等[34]报道,添加1000mg/L ZVI的厌氧氨氧化系统ORP的波动范围和均值均小于对照组.相反地,Gao等[35]发现加入ZVI后的厌氧氨氧化系统中SAA与ORP的数值呈正相关,加入750mg/L的ZVI后,反应器的ORP上升到209.4mV,相较于对照组提高了82.1mV,而SAA则提高0.04kgN/kgVSS/d,即此研究表明较高的ORP值似乎有利于厌氧氨氧化菌的生长.ZVI通过改变ORP影响SAA和系统脱氮效能的结果不一,尚需要深入研究.
2.1.2 pH值碱度 适于厌氧氨氧化菌生存的pH值范围一般为6.4~8.3,最适pH值为7.5~8.0[36].不同形态铁的投加会改变厌氧氨氧化系统中pH值,从而影响厌氧氨氧化菌活性. Li等[37]研究发现,当厌氧氨氧化反应器中Fe3+投加量由2mg/L上升到6mg/L时,反应器中pH值由7.7上升到8.1,当 Fe3+浓度为10~ 120mg/L时,反应器中pH值在7.65~7.89范围内波动,但当Fe3+浓度进一步升高至120mg/L时pH值出现下降,这是由于反应器中生成Fe(OH)3沉淀,大量消耗OH-.Feng等[38]关于Fe2+对厌氧氨氧化系统pH值影响的研究与Li等[37]的研究结果基本类似,当Fe2+的浓度为1mg/L时,厌氧氨氧化系统内pH值为7.8~8.0,而当Fe2+的浓度上升到4~6mg/L时, pH值上升到8.1;但当Fe2+的浓度达到120mg/L时,pH值则下降至7.65~7.8.因此可以推测,低浓度Fe2+和Fe3+可以提高系统脱氮效能,促进厌氧氨氧化反应并产生大量OH-,致使反应器pH值上升.高浓度Fe2+和Fe3+不仅会抑制厌氧氨氧化菌活性,还会直接消耗大量OH-,生成Fe(OH)2和Fe(OH)3沉淀,导致反应器内pH值下降. Feng等[38]还提出了用pH值增量预测厌氧氨氧化系统氨氮去除率(NRE)的线性方程式:
NRE = 0.51ΔpH + 45.55 (1)
基于国内外关于厌氧氨氧化反应器运行的相关文献,对投加铁和不投加铁的反应器运行时的pH值数据进行分析[24,38-47].投加铁对厌氧氨氧化系统最适pH值无显著影响,最适pH值仍在7.5~8之间,但可以显著提高系统抵抗pH值冲击的能力,投加铁后厌氧氨氧化正常运行的pH值范围由原来的7.3~ 8.0拓宽到6.5~8.2.这一发现为铁强化厌氧氨氧化工艺处理酸性含氮废水提供了新的思路.
ZVI粉末的投加可以缓冲厌氧氨氧化系统内pH值的波动,使系统内pH值维持在相对稳定的范围,从而提高系统脱氮效能.Guo等[32]报道,在ZVI投入厌氧氨氧化体系的前期,系统pH值波动较大,而运行一段时间后,系统pH值逐渐升高(从6.5上升到7.8),即系统中H+被ZVI消耗致使pH值上升,相应地,系统氮去除率也随之提高.当然,厌氧氨氧化系统pH值不宜过高,过高的pH值会导致厌氧氨氧化菌失活.
2.2 促进厌氧氨氧化菌EPS分泌
铁可以刺激厌氧氨氧化系统中微生物分泌EPS,Ren等[27]报道,分别向厌氧氨氧化反应器中加入25g的mZVI和nZVI,运行一段时间后EPS含量相较于对照组分别提高了21.2,27.7mg/gVSS.EPS对微生物细胞的聚集具有重要作用.Tang等[23]指出,Fe2+可以刺激厌氧氨氧化污泥EPS的分泌,促进颗粒污泥的形成,投加Fe2+后系统中粒径大于2mm的污泥颗粒所占比例可达58.3%.同时,随着污泥直径的增大,EPS对Fe的吸附能力下降,更多的Fe转运到厌氧氨氧化菌细胞内,提高了系统的脱氮能力.反应器中PN/PS(蛋白质含量/多糖含量)也由8.51± 0.71逐渐下降至4.91±0.37,PN/PS比率反映了厌氧氨氧化颗粒污泥的结构稳定性[48],比率越高,稳定性越差[49].这表明,长期添加Fe2+可以增强厌氧氨氧化颗粒污泥的结构稳定性.
2.3 加速功能菌富集
2.3.1 促进厌氧氨氧化菌富集 厌氧氨氧化系统中ZVI的投加,为厌氧氨氧菌创造了更好的生存环境,有利于其生长繁殖.Yang等[50]发现,ZVI的投加能够加速优势菌群从异养反硝化细菌向自养厌氧氨氧化菌和Feammox(铁氨氧化)细菌的转变.需要注意的是,铁过量投加会对厌氧氨氧化菌生长繁殖产生抑制作用.随着Fe3+浓度从2.24mg/L增至5.60mg/ L,在厌氧氨氧化体系中比例从30.7%增加到35.3%,当Fe3+浓度继续增加到6.72mg/ L,比例下降到31.9%.高Fe3+浓度下会在厌氧氨氧化菌细胞内诱发类芬顿反应 ,导致厌氧氨氧化菌死亡[33].Wang等[51]发现,在投加50, 1000mg/LnZVI的厌氧氨氧化反应器中,相对丰度分别为52.5%和45.3%,优势厌氧氨氧化菌属的含量分别提高了46.5%和37.9%.因此,针对不同形态的铁,其最佳的投加量是铁强化厌氧氨氧化技术未来研究的重要内容之一.
2.3.2 促进其他功能菌富集 Yang等[50]指出,添加ZVI反应一段时间后,与接种污泥相比AOB(氨氧化细菌)的相对丰度(10.3%)显著高于对照组(3.0%).王海月等[52]报道,分别添加2.24,4.48,7.84mg/L Fe3+的厌氧氨氧化反应器中反硝化菌属的相对丰度均有所增加,且Fe3+投加量越多其相对丰度增加越高.此外, Zhang等[53]发现,当nZVI的添加量为5mg/L时,的相对丰度由6.6%增加到10.6%,和的相对丰度分别由31.6%下降到27.1%和10.4%下降到7.5%.而当nZVI的进水浓度增加到20mg/L时,的相对丰度下降至7.5%,和的相对丰度分别增加到31.1%和5.7%.、和等异养微生物常与厌氧氨氧化菌共存,它们可以分解厌氧氨氧化菌产生的胞外蛋白、多肽及细胞碎片等,并通过NO3-还原和交互共生作用促进厌氧氨氧化细菌生长代谢[54].例如厌氧氨氧化菌不具有合成叶酸的能力,而参与了叶酸合成.具有参与大部分磷酸碳水化合物合成的能力,但缺少糖核苷酸生物合成的能力,而厌氧氨氧化菌具有大量糖核苷酸生物合成途径相关功能基因.这些微生物在次级代谢物生物合成、碳水化合物代谢、细胞外蛋白质和肽降解以及部分反硝化方面的潜力与厌氧氨氧化菌的代谢潜力相辅相成[55].
此外,还可促进微生物细胞聚集与颗粒污泥完整.因此,铁的投加可以通过调节系统中、等共生细菌的丰度,促进厌氧氨氧化菌的生长代谢、厌氧氨氧化颗粒污泥的形成以及提高厌氧氨氧化系统的脱氮效能.外源添加铁还可以提高部分Feammox菌丰度,例如和.Zhu等[56]研究表明,在厌氧氨氧化系统中添加Fe3+化合物,可以促进显著富集.此外,Yang等[57]也有类似的发现,添加了高铁水合矿石的厌氧氨氧化系统中相对丰度由0.004%增长到0.05%,使系统脱氮效能得到提升.Yang等[50]研究报道添加ZVI一段时间后,异养微生物显著富集,其相对丰度与对照组相比增加12.4%.因此,铁对厌氧氨氧化反应体系的影响不应仅聚焦于铁对厌氧氨氧化菌的丰度的改变,还应重视铁对厌氧氨氧化反应器中其他功能微生物丰度的影响.
2.4 改变厌氧氨氧化菌细胞的结构
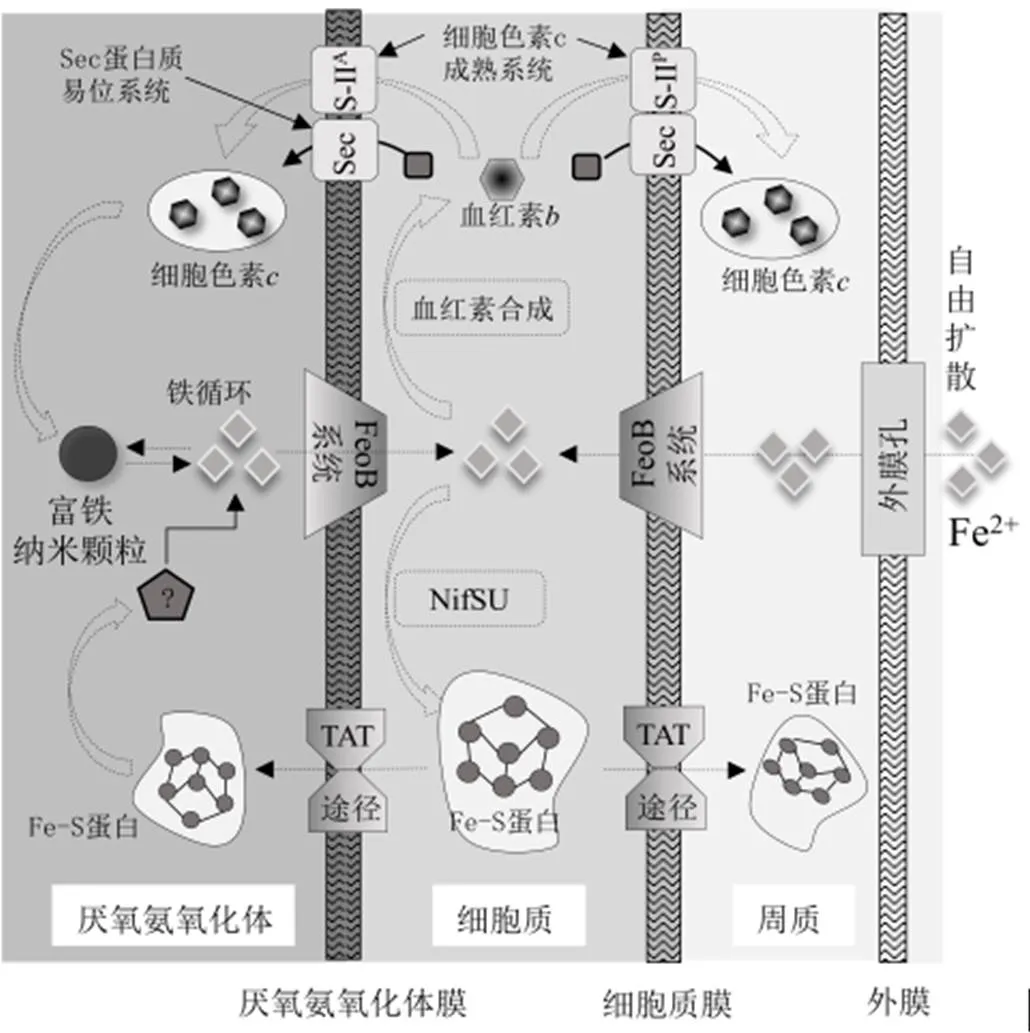
图2 厌氧氨氧化过程中Fe促进血红素c合成模型[12]
张蕾等[58]发现,添加铁离子可以改变厌氧氨氧化细菌的细胞结构,促使细胞内出现灰色区域.这一区域可能是储存有大量纳米铁颗粒的厌氧氨氧化体,在这里铁以球形中空蛋白质复合物的形式储存[59].如图2所示,厌氧氨氧化菌胞内铁浓度过低会抑制血红素b和Fe-S蛋白的合成,影响微生物正常生理活动,但铁浓度过高亦会导致微生物氧化损伤,因此厌氧氨氧化体内的纳米铁颗粒可能参与了厌氧氨氧化菌胞内铁浓度稳态的维持[16].Niftrik等[60]报道厌氧氨氧化细菌体内具有和这2个与铁贮存相关的基因,铁可以以无机矿物的形式储存于胞內.由于厌氧氨氧化菌缺乏将铁蛋白转运厌氧氨氧化体的通道,因此有学者提出厌氧氨氧化体可能与在细菌和古生菌体内发现的胶囊蛋白功能类似,即可利用蛋白质室对其生理代谢营养物质储存进行空间调控,并为特定的生理过程创造独特的微环境[61-62].目前,虽观察到了铁进入厌氧氨氧化体的途径,但是对于血红素降解和铁从厌氧氨氧化体中输出的机制尚不明确[12].
2.5 调控关键酶、功能基因等表达
2.5.1 调控系统脱氮基因表达 外源铁的添加对厌氧氨氧化系统中硝化反应(AOA-AOB-)、反硝化反应()、异化硝酸盐还原()等相关基因的表达有显著影响[53].Shu等[63]实验结果表明,低浓度Fe2+(1.12~3.36mg/L)可以提高厌氧氨氧化体系中AOA-AOB-基因的丰度和反硝化微生物活性.Fe对厌氧氨氧化系统中反硝化过程相关基因表达的影响较为复杂. Bi等[39]证实添加了Fe0的厌氧氨氧化系统中拷贝数明显增多,酶活性随Fe/NO3-比例升高而增强.同时,Nap的功能基因表达明显增强,而且在反应过程中始终可以检测到酶功能基因的表达,这表明Fe0的投加可以促进反硝化菌生长.但Bi等[19]的实验结果指出,施加铁后基因丰度下降,基因丰度显著上升,反硝化微生物活性减弱,反硝化作用可能不是引起系统中硝酸盐减少的主要原因.这与Chen等[64]观点一致,Chen等指出反应器中反硝化细菌的丰度未显著增加,因此可能是异化硝酸盐还原为氨(DNRA)反应利用Fe2+作为电子供体将硝酸盐转化为亚硝酸盐和氨.
2.5.2 强化厌氧氨氧化菌关键酶和辅因子活性 外源Fe的添加还可以提高厌氧氨氧化相关功能基因的丰度.肼合成酶是厌氧氨氧化菌必需的功能酶,其合成需要这3个功能基因参与,添加Fe可以显著提高的丰度,促进HZS的合成[65].厌氧氨氧化能量代谢极度依赖血红素c和Fe-S簇这两类辅因子[66].如图3所示,低浓度的Fe3+可以使三价铁还原酶(fhuF)和铁摄取(FeoA、FeoB和fur)相关的基因表达显著上调,从而导致更多的血红素c合成、HDH酶活性和SAA的提高[33].
Zhang等[53]研究表明加入低浓度的Fe3+(10mg/L)的反应器中血红素c含量与酶HDH活性显著增加,而加入高浓度的Fe3+(100mg/L)的反应器中血红素c含量与HDH活性显著降低.有研究表明,HDH氧化肼会生成氮气,为亚硝酸盐还原和肼合成提供电子,HDH还可能是负责将N2H4转化为最终N2的关键酶[67](图3).因此在实际运行过程中需要灵活变换添加Fe的形态,通过短期添加Fe2+与铁氧化物反应以恢复传质效果、强化电子传递过程、加速硝酸盐还原[68-70].最近,Shaw等[71]证实厌氧氨氧化菌具有电活性,可以将氨氮氧化过程中释放的电子传递至胞外不溶性电子受体,而铁基材料和铁氧化物可能可以成为充当电子受体的角色.该过程无需NO2-,对氨氮废水的节能(甚至产能)处理具有重要意义.

图3 厌氧氨氧化过程中Fe调控酶活性提高系统脱氮效率机理
2.6 强化厌氧氨氧化菌信号分子传递和群体感应
混合菌群中微生物之间依靠群体感应传递信号,以此调节微生物间的生理活动,抵抗外界不良因素的影响[72].环二鸟苷酸(c-di-GMP)是一种群体感应信号分子,如图4所示,厌氧氨氧化菌吸收的铁离子不仅使基因的丰度显著增加还使基因的丰度降低,因此添加nZVI可以提高c-di-GMP合成酶的丰度,降低c-di-GMP降解蛋白丰度从而促进c-di-GMP的合成.
c-di-GMP参与细菌分裂抑制蛋白的水解,加速厌氧氨氧化菌的分裂,从而增加厌氧氨氧化菌的丰度. Wang等[51]研究证实,添加50mg/L的nZVI后厌氧氨氧化菌()的相对丰度从42.1%增长到52.5%. c-di-GMP的富集还可以降低反应器内微生物的运动性,促进细菌分泌EPS,有利于厌氧氨氧化反应器内污泥颗粒的形成[73-74].
此外添加铁还可以使反应器上清液中含有群体感应信号C12-HSL,可以缩短厌氧氨氧化反应器启动时间17.5%,提高氮负荷率60%[75].

图4 铁通过促进c-di-GMP合成调控厌氧氨氧化菌生理活动示意[51]
2.7 强化反应器中非生物途径
Chen等[64]研究指出,在添加Fe2+的反应器中ΔNO3--N/ΔNH4+-N比低于理论值,且随Fe2+浓度的增加而降低.这一现象在其他报道中也有出现.然而,这些反应器中的反硝化细菌数量与Fe3+反应器相比没有明显变化.虽然Fe2+与硝酸盐的反应在热力学上是可行的,但不会自发进行.该反应受到pH值和Fe2+/NO2-物质的量比的影响,其反应机理和最终产物仍需研究[76].生物脱氮过程中N2O主要由硝化反硝化反应产生,N2O不参与厌氧氨氧化细菌的代谢,因此,在氧气和有机物缺乏的厌氧氨氧化系统中N2O的产率受到抑制[77-78].但是,从不同Fe2+浓度的反应器中检测到N2O释放,其产量与反应时间和Fe投加量呈正相关,这意味着Fe2+可以通过该化学反应(4Fe2++ 2NO2–+ 5H2O → 4FeOOH + N2O + 6H+)促进N2O的产生,提高反应器的脱氮效率[79].
铁的物理特性可以促进厌氧氨氧化菌生长和厌氧氨氧化污泥颗粒形成.李天皓等[80]在实验中观察到大量球状菌附着在废铁屑沟壑处,这表明废铁屑表面粗糙且存在大量沟壑,可为厌氧氨氧化菌提供栖息空间.可溶性Fe2+和Fe3+会通过盐桥效应使更多厌氧氨氧化菌聚集,从而促进厌氧氨氧化污泥颗粒化[35].
3 结论与展望
3.1 适量的铁的投加对厌氧氨氧化菌有明显的促进作用,投加铁可以优化厌氧氨氧化菌的生长环境,提高厌氧氨氧化体系中浮霉门菌的相对丰度和厌氧氨氧化菌的活性,促进厌氧氨氧化菌分泌EPS.但是铁辅助厌氧氨氧化工艺内氮转化途径极其复杂,优势厌氧氨氧化菌各有不同,再加上影响因素众多,目前尚无统一、明确的机理解释,有待深入研究.
3.2 厌氧氨氧化系统中铁的氧化还原也常有报道,铁氧化物是否可以替代氧化石墨烯或碳基电极作为胞外电子受体,辅助厌氧氨氧化细菌完成胞外呼吸并氧化NH3有待研究.
3.3 在实际运用过程中铁辅助厌氧氨氧化工艺中抑制现象经常发生,这与铁投加量、铁的种类、工艺运行条件等密切相关,投加不同的类型的铁,最适量也不同,过量添加铁会对厌氧氨氧化菌产生毒性,抑制厌氧氨氧化菌活性,甚至使厌氧氨氧化菌失活. 对此文献报道结论不一,有待进一步研究.
3.4 工程推广时铁离子、纳米零价铁及特殊的铁材料长期持续投加成本高,铁来源需要优化,废铁屑作为一种经济易得的工业副产物,相较于以往研究中直接添加零价铁及其衍生物,具有持续的、缓慢的释放铁离子的优点,实现废物资源化利用.
[1] Strous M, Heijnen J J, Kuenen J G, et al. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms [J]. Applied Microbiology and Biotechnology, 1998,50(5):589-596.
[2] Oshiki M, Satoh H, Okabe S. Ecology and physiology of anaerobic ammonium oxidizing bacteria [J]. Environmental Microbiology, 2016,18(9):2784-2796.
[3] Feng Y, Zhao Y P, Guo Y Z, et al. Microbial transcript and metabolome analysis uncover discrepant metabolic pathways in autotrophic and mixotrophic anammox consortia [J]. Water Research, 2018,128:402-411.
[4] Lackner S, Gilbert E M, Vlaeminck S E, et al. Full-scale partial nitritation/anammox experiences - An application survey [J]. Water Research, 2014,55:292-303.
[5] Cao Y S, Hong K B, van Loosdrecht M, et al. Mainstream partial nitritation and anammox in a 200,000m(3)/day activated sludge process in Singapore: Scale-down by using laboratory fed-batch reactor [J]. Water Science and Technology, 2016,74(1):48-56.
[6] 付昆明,付 巢,李 慧,等.主流厌氧氨氧化工艺的运行优化及其微生物的群落变迁[J]. 环境科学, 2018,39(12):5596-5604.
Fu K M, Fu C, Li H, et al. Optimization of the mainstream anaerobic ammonia oxidation process and its changes of the microbial community [J]. Environmental Science, 2018,39(12):5596-5604.
[7] Liu S T, Yang F L, Meng F A, et al. Enhanced anammox consortium activity for nitrogen removal: Impacts of static magnetic field [J]. Journal of Biotechnology, 2008,138(3/4):96-102.
[8] Qiao S, Yin X, Zhou J T, et al. Inhibition and recovery of continuous electric field application on the activity of anammox biomass [J]. Biodegradation, 2014,25(4):505-513.
[9] Cheng B N, Bao J G, Du J K, et al. Application of electric fields to mitigate inhibition on anammox consortia under long-term tetracycline stress [J]. Bioresource Technology, 2021,341:125730.
[10] Wang T, Zhang D D, Sun Y T, et al. Using low frequency and intensity ultrasound to enhance start-up and operation performance of anammox process inoculated with the conventional sludge [J]. Ultrasonics Sonochemistry, 2018,42:283-292.
[11] Yuan L Z, Wang T, Xing F H, et al. Enhancement of anammox performances in an ABR at normal temperature by the low-intensity ultrasonic irradiation [J]. Ultrasonics Sonochemistry, 2021,73:105468.
[12] Ferousi C, Lindhoud S, Baymann F, et al. Iron assimilation and utilization in anaerobic ammonium oxidizing bacteria [J]. Current Opinion in Chemical Biology, 2017,37:129-136.
[13] Mak C Y, Lin J G, Chen W H, et al. The short- and long-term inhibitory effects of Fe(II) on anaerobic ammonium oxidizing (anammox) process [J]. Water Science and Technology, 2019,79(10):1860-1867.
[14] Maalcke W J, Reimann J, de Vries S, et al. Characterization of anammox hydrazine dehydrogenase, a key N-2-producing enzyme in the global nitrogen cycle [J]. Journal of Biological Chemistry, 2016,291(33):17077-17092.
[15] Kartal B, Keltjens J T. Anammox biochemistry: a tale of heme c proteins [J]. Trends in Biochemical Sciences, 2016,41(12):998-1011.
[16] 董子阳,胡宝兰,韩佳慧.厌氧氨氧化细菌铁的吸收利用研究进展[J]. 微生物学通报, 2021, 48(5):1780-1787.
Dong Z Y, Hu B L, Han J H, et al. Research progress in the uptake and utilization of iron by the anaerobic ammonium-oxidizing bacterium[J]. Microbiology China, 2021, 48(5):1780-1787.
[17] Peng M W, Qi J, Yan P, et al. Insight into the structure and metabolic function of iron-rich nanoparticles in anammox bacteria [J]. Science of the Total Environment, 2022,806:150879.
[18] Qiao S, Bi Z, Zhou J T, et al. Long term effects of divalent ferrous ion on the activity of anammox biomass [J]. Bioresource Technology, 2013,142:490-497.
[19] Bi Z, Zhang W J, Song G, et al. Iron-dependent nitrate reduction by anammox consortia in continuous-flow reactors: A novel prospective scheme for autotrophic nitrogen removal [J]. Science of the Total Environment, 2019,692:582-588.
[20] Sindhu L, Niu K, Liu X, et al. Effect of Fe2+addition on anammox consortia, nitrogen removal performance and functional genes analysis during start-up of anammox process [J]. Journal of Water Process Engineering, 2021,43:102251.
[21] Huang X L, Gao D W, Peng S, et al. Effects of ferrous and manganese ions on anammox process in sequencing batch biofilm reactors [J]. Journal of Environmental Sciences, 2014,26(5):1034-1039.
[22] Yan Z C, Shen L, Jiao L Z, et al. Effect of Fe (II) on nitrogen removal of anammox under organic matter inhibition [J]. Journal of Water Process Engineering, 2022,46:102632.
[23] Tang S M, Xu Z H, Liu Y L, et al. Performance, kinetics characteristics and enhancement mechanisms in anammox process under Fe(II) enhanced conditions [J]. Biodegradation, 2020,31:221- 234.
[24] Mishra P, Burman I, Sinha A. Performance enhancement and optimization of the anammox process with the addition of iron [J]. Environmental Technology, 2021,42(26):4158-4169.
[25] Wang X, Shu D T, Yue H. Taxonomical and functional microbial community dynamics in an Anammox-ASBR system under different Fe (III) supplementation [J]. Applied Microbiology and Biotechnology, 2016,100(23):10147-10163.
[26] 马 娇,曾天续,宋 珺,等.纳米单质铁对厌氧氨氧化脱氮性能的影响[J]. 中国环境科学, 2022:42(6):2619-2627.
Ma J, Zeng T X, Song J, et al. Effect of nanoscale zero-valent iron on the denitrification performance of anaerobic ammonia oxidation [J]. China Environmental Science,2022:42(6):2619-2627.
[27] Ren L F, Ni S Q, Liu C, et al. Effect of zero-valent iron on the start-up performance of anaerobic ammonium oxidation (anammox) process [J]. Environmental Science and Pollution Research, 2015, 22(4):2925-2934.
[28] Zhang X J, Wei D H, Zhang H, et al. Comprehensive analysis of the impacts of iron-based nanoparticles and ions on anammox process [J]. Biochemical Engineering Journal, 2022,180:108371.
[29] Liu T, Tian R Q, Li Q, et al. Strengthened attachment of anammox bacteria on iron-based modified carrier and its effects on anammox performance in integrated floating-film activated sludge (IFFAS) process [J]. Science of The Total Environment, 2021,787:147679.
[30] Wang H, Fan Y F, Zhou M D, et al. Function of Fe(III)-minerals in the enhancement of anammox performance exploiting integrated network and metagenomics analyses [J]. Water Research, 2022,210: 117998.
[31] Viet T N, Behera S K, Ji W K, et al. Effects of oxidation reduction potential and organic compounds on anammox reaction in batch cultures [J]. Environmental Engineering Research, 2008,13(4):359- 360.
[32] Suneethi S, Joseph K. Batch culture enrichment of anammox populations from anaerobic and aerobic seed cultures [J]. Bioresource Technology, 2011,102(2):585-591.
[33] Daverey A, Chen Y C, Sung S W, et al. Effect of zinc on anammox activity and performance of simultaneous partial nitrification, anammox and denitrification (SNAD) process [J]. Bioresource Technology, 2014,165:105-110.
[34] Guo B B, Chen Y H, Lv L, et al. Transformation of the zero valent iron dosage effect on anammox after long-term culture: From inhibition to promotion [J]. Process Biochemistry, 2019,78:132-139.
[35] Gao F, Zhang H M, Yang F L, et al. The effects of zero-valent iron (ZVI) and ferroferric oxide (Fe3O4) on anammox activity and granulation in anaerobic continuously stirred tank reactors (CSTR) [J]. Process Biochemistry, 2014,49(11):1970-1978.
[36] Tomaszewski M, Cema G, Ziembinska-Buczynska A. Influence of temperature and pH on the anammox process: A review and meta- analysis [J]. Chemosphere, 2017,182:203-214.
[37] Li J, Feng L, Biswal B K, et al. Bioaugmentation of marine anammox bacteria (MAB)-based anaerobic ammonia oxidation by adding Fe(III) in saline wastewater treatment under low temperature [J]. Bioresource Technology, 2020,295:122292.
[38] Feng L, Li J, Ma H R, et al. Effect of Fe(II) on simultaneous marine anammox and Feammox treating nitrogen-laden saline wastewater under low temperature: Enhanced performance and kinetics [J]. Desalination, 2020,478:114287.
[39] Bi Z, Huang Y, Zhang W J, et al. Impacts of chosen parameters on fe-dependent nitrate reduction in anammox consortia: performance and bioactivity [J]. Water, 2020,12(5):1379.
[40] Li Z X, Peng Y Z, Gao H J. Enhanced long-term advanced denitrogenation from nitrate wastewater by anammox consortia: Dissimilatory nitrate reduction to ammonium (DNRA) coupling with anammox in an upflow biofilter reactor equipped with EDTA-2Na/ Fe(II) ratio and pH control [J]. Bioresource Technology, 2020,305: 123083.
[41] Oshiki M, Ishii S, Yoshida K, et al. Nitrate-dependent ferrous iron oxidation by anaerobic ammonium oxidation (anammox) bacteria [J]. Applied and Environmental Microbiology, 2013,79(13):4087-4093.
[42] Yan Y, Wang Y Y, Wang W G, et al. Comparison of short-term dosing ferrous ion and nanoscale zero-valent iron for rapid recovery of anammox activity from dissolved oxygen inhibition [J]. Water Research, 2019,153:284-294.
[43] Zhang D J, Ren L L, Yao Z B, et al. Removal of nitrogen oxide based on anammox through Fe(II)EDTA absorption [J]. Energy & Fuels, 2017,31(7):7247-7255.
[44] 李亚峰,马晨曦,张 驰.UASBB厌氧氨氧化反应器处理污泥脱水液的影响因素研究[J]. 环境科学, 2014,35(8):3044-3051.
Li Y F, Ma C X, Zhang C, et al. Influencing factors of sludge liquor treatment in UASBB [J]. Environmental Science, 2014,35(8):3044- 3051.
[45] 吕 娇,楼菊青,徐 帆.反硝化型甲烷厌氧氧化(DAMO)系统pH值耦合模型研究[J]. 中国环境科学, 2022,42(2):612-619.
Lv J, Lou J Q, Xu F, et al. pH coupling model of denitrifying anaerobic methane oxidation (DAMO) system [J]. China Environmental Science, 2022,42(2):612-619.
[46] 于德爽,李伟刚,李 津.ASBR反应器厌氧氨氧化脱氮Ⅰ:工艺特性与控制策略[J]. 中国环境科学, 2013,33(12):2176-2183.
Yu D S, Li W G, Li J, et al. Nitrogen removal in the Anammox sequencing batch reactor I: Performance and control strategies [J]. China Environmental Science, 2013,33(12):2176-2183.
[47] Gonzalez-Brambila M, Monroy O, Lopez-Isunza F. Experimental and theoretical study of membrane-aerated biofilm reactor behavior under different modes of oxygen supply for the treatment of synthetic wastewater [J]. Chemical Engineering Science, 2006,61(16):5268- 5281.
[48] Zhang Q, Zhang X, Bai Y H, et al. Exogenous extracellular polymeric substances as protective agents for the preservation of anammox granules [J]. Science of the Total Environment, 2020,747:141464.
[49] Miao L, Zhang Q, Wang S Y, et al. Characterization of EPS compositions and microbial community in an Anammox SBBR system treating landfill leachate [J]. Bioresource Technology, 2018,249:108- 116.
[50] Yang H, Deng L W, Xiao Y Q, et al. Construction of autotrophic nitrogen removal system based on zero-valent iron (ZVI): performance and mechanism [J]. Water Science and Technology, 2020,82(12):2990-3002.
[51] Wang Z B, Liu X L, Ni S Q, et al. Nano zero-valent iron improves anammox activity by promoting the activity of quorum sensing system [J]. Water Research, 2021,202:117491.
[52] 王海月,彭 玲,毛念佳,等.三价铁对有机物存在下厌氧氨氧化脱氮的影响[J]. 中国环境科学, 2021,41(4):1672-1680.
Wang H Y, Peng L, Mao N J, et al. Effect of Fe3+on nitrogen removal of anammox in the presence of organic matter [J]. China Environmental Science, 2021,41(4):1672-1680.
[53] Zhang S Q, Zhang L Q, Yao H N, et al. Responses of anammox process to elevated Fe(III) stress: Reactor performance, microbial community and functional genes [J]. Journal of Hazardous Materials, 2021,414:125051.
[54] Lawson C E, Wu S, Bhattacharjee A S, et al. Metabolic network analysis reveals microbial community interactions in anammox granules [J]. Nature Communications, 2017,8:15416.
[55] Zhao Y P, Liu S F, Jiang B, et al. Genome-centered metagenomics analysis reveals the symbiotic organisms possessing ability to cross- feed with anammox bacteria in anammox consortia [J]. Environmental Science & Technology, 2018,52(19):11285-11296.
[56] Zhu T T, Lai W X, Zhang Y B, et al. Feammox process driven anaerobic ammonium removal of wastewater treatment under supplementing Fe(III) compounds [J]. Science of the Total Environment, 2022,804:149965.
[57] Yang Y F, Jin Z, Quan X, et al. Transformation of nitrogen and iron species during nitrogen removal from wastewater via feammox by adding ferrihydrite [J]. ACS Sustainable Chemistry & Engineering, 2018,6(11):14394-14402.
[58] 张 蕾,郑 平,胡安辉.铁离子对厌氧氨氧化反应器性能的影响[J]. 环境科学学报, 2009,29(8):1629-1634.
Zhang L, Zheng P, Hu A H, et al. Effect of ferrous ion on the performance of an anammox reactor [J]. Acta Scientiae Circumstaniae, 2009,29(8):1629-1634.
[59] Harrison P M. Iron storage in bacteria [J]. Nature, 1979,279:15-16.
[60] van Niftrik L, Geerts W, van Donselaar E G, et al. Combined structural and chemical analysis of the anammoxosome: A membrane- bounded intracytoplasmic compartment in anammox bacteria [J]. Journal of Structural Biology, 2008,161(3):401-410.
[61] Giessen T W. Encapsulins: microbial nanocompartments with applications in biomedicine, nanobiotechnology and materials science [J]. Current Opinion in Chemical Biology, 2016,34:1-10.
[62] Martin W. Evolutionary origins of metabolic compartmentalization in eukaryotes [J]. Philosophical Transactions of the Royal Society B-Biological Sciences, 2010,365(1541):847-855.
[63] Shu D T, He Y L, Yue H, et al. Effects of Fe(II) on microbial communities, nitrogen transformation pathways and iron cycling in the anammox process: Kinetics, quantitative molecular mechanism and metagenomic analysis [J]. RSC Advances, 2016,6(72):68005-68016.
[64] Chen Y, Jia F X, Liu Y J, et al. The effects of Fe(III) and Fe(II) on anammox process and the Fe-N metabolism [J]. Chemosphere, 2021,285:131322.
[65] Wang H Y, Peng L, Mao N J, et al. Effects of Fe3+on microbial communities shifts, functional genes expression and nitrogen transformation during the start-up of Anammox process [J]. Bioresource Technology, 2021,320:124326.
[66] Kartal B, de Almeida N M, Maalcke W J, et al. How to make a living from anaerobic ammonium oxidation [J]. FEMS Microbiology Reviews, 2013,37(3):428-461.
[67] Kartal B, Maalcke W J, de Almeida N M, et al. Molecular mechanism of anaerobic ammonium oxidation [J]. Nature, 2011,479:127-159.
[68] Han L C, Yang L, Wang H B, et al. Sustaining reactivity of Fe-0for nitrate reduction via electron transfer between dissolved Fe2+and surface iron oxides [J]. Journal of Hazardous Materials, 2016,308: 208-215.
[69] 张文静,黄 勇,毕 贞,等.ANAMMOX菌铁自养反硝化工艺的稳定性[J]. 环境科学, 2019,40(7):3201-3207.
Zhang W J, Huang Y, Bi Z, et al. Stability of ZVI-dependent autotrophic denitrification by anammox bacteria [J]. Environmental Science, 2019,40(7):3201-3207.
[70] Li X, Yuan Y, Huang Y. Enhancing the nitrogen removal efficiency of a new autotrophic biological nitrogen-removal process based on the iron cycle: Feasibility, progress, and existing problems [J]. Journal of Cleaner Production, 2021,317:128499.
[71] Shaw D R, Ali M, Katuri K P, et al. Extracellular electron transfer-dependent anaerobic oxidation of ammonium by anammox bacteria [J]. Nature Communications, 2020,11(1):2058.
[72] Maddela N R, Sheng B B, Yuan S S, et al. Roles of quorum sensing in biological wastewater treatment: A critical review [J]. Chemosphere, 2019,221:616-629.
[73] Lorite G S, de Souza A A, Neubauer D, et al. On the role of extracellular polymeric substances during early stages of Xylella fastidiosa biofilm formation [J]. Colloids and Surfaces B- Biointerfaces, 2013,102:519-525.
[74] Voberkova S, Hermanova S, Hrubanova K, et al. Biofilm formation and extracellular polymeric substances (EPS) production by Bacillus subtilis depending on nutritional conditions in the presence of polyester film [J]. Folia Microbiologica, 2016,61(2):91-100.
[75] Zhao R, Zhang H M, Zhang F, et al. Fast start-up anammox process using Acyl-homoserine lactones (AHLs) containing supernatant [J]. Journal of Environmental Sciences, 2018,65:127-132.
[76] Wu D L, Shao B B, Fu M Y, et al. Denitrification of nitrite by ferrous hydroxy complex: Effects on nitrous oxide and ammonium formation [J]. Chemical Engineering Journal, 2015,279:149-155.
[77] Kartal B, Kuypers M, Lavik G, et al. Anammox bacteria disguised as denitrifiers: nitrate reduction to dinitrogen gas via nitrite and ammonium [J]. Environmental Microbiology, 2007,9(3):635-642.
[78] Duan H R, Ye L, Erler D, et al. Quantifying nitrous oxide production pathways in wastewater treatment systems using isotope technology - A critical review [J]. Water Research, 2017,122:96-113.
[79] Zhou B R, Chen G J, Dong C F, et al. The short-term and long-term effects of Fe(II) on the performance of anammox granules [J]. Water Environment Research, 2021,93(9):1651-1659.
[80] 李天皓,徐云翔,郭之晗,等.低温下废铁屑对厌氧氨氧化系统的影响[J]. 中国环境科学, 2022,42(4):1688-1695.
Li T H, Xu Y X, Guo Z H, et al. Effect of iron scraps on anammox systems at low temperature [J]. China Environmental Science,2022,42(4):1688-1695.
Research progress on the mechanisms of iron-assisted anaerobic ammonia oxidation process.
WANG Qian1, HU Jia-yuan1, LI Tian-hao1, WANG Cai-xia1, CHEN Jie1, SHEN Yao-liang1,2, LIU Wen-ru1,2*
(1.School of Environmental Science and Engineering, Suzhou University of Science and Technology, Suzhou 215000, China;2.National and Local Joint Engineering Laboratory for Municipal Sewage Resource Utilization Technology, Suzhou University of Science and Technology, Suzhou 215000, China)., 2022,42(11):5153~5162
How to promote the proliferation and metabolism of anammox bacteria, improve the nitrogen removal efficiency of anammox process, and ensure the long-term stable operation of the process are the hotspots of anammox research. Iron-assisted anammox is the most widely studied measure with high economical and practical potential. This paper briefly describes the iron-assisted anammox technology and its nitrogen removal performance. The mechanisms of iron-assisted anammox process in promoting anammox cells growth and enhancing systems nitrogen removal performance are summarized from the aspects of optimizing the habitats of anammox bacteria, stimulating the secretion of extracellular polymer, accelerating the accumulation of denitrification functional bacteria, inducing the evolution of cell microstructure, regulating the expression of key enzymes and functional genes, promoting signal molecular synthesis, and enhancing abiotic nitrogen removal. Finally, the iron-assisted anammox technology is summarized and prospected, and the concept of waste iron scrap-anammox process is proposed.
anaerobic ammonia oxidation;iron;enhanced nitrogen removal;mechanism
X703
A
1000-6923(2022)11-5153-10
王 倩(1998-),女,安徽芜湖人,苏州科技大学硕士研究生,研究方向为废水处理及其资源化.发表论文3篇.
2022-04-10
国家自然科学基金项目(51808367);城市生活污水资源化利用技术国家地方联合工程实验室开放课题项目(2018KF05);江苏省研究生科研创新计划(KYCX20_2778)
* 责任作者, 副教授, liuwenru1987@126.com

