pH值调控柠檬酸污泥厌氧发酵产酸及碳源潜力研究
孙东霞,周子安,冯志合,胡修玉,祁光霞,董黎明*
pH值调控柠檬酸污泥厌氧发酵产酸及碳源潜力研究
孙东霞1,周子安1,冯志合2,胡修玉2,祁光霞1,董黎明1*
(1.北京工商大学,中国轻工业清洁生产和资源综合利用重点实验室,国家环境保护食品链污染防治重点实验室,北京 100048;2.中国生物发酵产业协会,北京 100083)
以柠檬酸废水厌氧颗粒污泥为接种物,在不同pH值调控条件下开展柠檬酸生产废水剩余活性污泥厌氧发酵产酸研究.通过对发酵液挥发性脂肪酸(VFAs)、有机质、氮磷和污泥脱水性能的分析,探讨了柠檬酸污泥厌氧产酸机制.结果表明,pH³10的碱性条件更有利于有机质的溶出从而促进VFAs的产生.三维荧光光谱分析发现在恒定pH值下腐殖酸(HA)和富里酸(FA)会大量溶出降低VFAs的产量.初始pH=10是柠檬酸污泥厌氧产酸的最佳pH值,发酵4d的VFAs浓度最高达(6681.47±126.82) mg COD/L,是文献报道中市政污泥产酸量的近2倍,其中乙酸占比49.8%,发酵后产酸功能菌Chloroflexi、Bacteroidota的相对丰度分别由初始的9.52%、10.87%增至16.84%、14.39%,污泥归一化毛细吸水时间(CST)为(11.34±0.27) s×L/g,脱水性能良好,发酵液TP浓度为(20.45±0.33) mg/L.研究表明,利用柠檬酸剩余活性污泥碱性厌氧发酵产酸作为污水处理过程中的外加碳源具有较大潜力.
pH值调控;柠檬酸污泥;碱性厌氧发酵;挥发性脂肪酸;污泥脱水性能
城市污水处理厂通常采用生物处理技术去除废水中的营养物质以缓解水体富营养化,然而目前国内污水进水碳源不足极大地限制了氮、磷的去除效率,因此在废水处理过程中通常使用甲醇、乙醇和乙酸作为有机碳源,但化学药品的添加不仅增加了运营成本也会造成二次污染[1-2].污水好氧处理的剩余活性污泥富含丰富的有机物,通过厌氧发酵可产生挥发性脂肪酸(VFAs),将其作为污水处理的有机碳源,可实现对剩余活性污泥的资源化利用[3].
影响污泥厌氧发酵的因素包括温度、pH值、微生物、水力停留时间等[4],其中pH值不仅影响污泥水解和产物组成,还影响微生物群落变化,是污泥厌氧发酵产生VFAs的最重要因素之一[5].然而产酸发酵细菌对pH值的适应性较强[6-7],有研究表明酸性启动(pH=6.0)VFAs最高累积质量浓度为1683.5mg/L,比碱性启动模式(pH=10.0)提高了37.5%[8].但碱性条件有利于促进有机物水解,提供高浓度的可溶性底物(SCOD),增加VFAs的产生[5].而通过不断调控pH值,碱性发酵(pH=10.0)VFAs产量为2901.33mg COD/L,是酸性发酵的2.7倍[9].由于污泥种类以及实验条件的不同,产酸条件所需的最优pH值不同,但高浓度的SCOD更有利于VFAs的产生结论一致[4].有研究者[10-12]已建立完整的污水污泥碱性厌氧发酵产VFAs和作为外部碳源提高污水厂的生物脱氮除磷的工艺系统,长期运行结果表明,该系统可实现污泥减量和碳源回收,减少约54%的污泥量,平均VFAs产量达到261.32mg COD/g VSS,该系统净利润为9.12美元/m3,比污泥厌氧消化产沼气(3.71美元/m3)有更大的经济优势.但目前基本是针对市政污泥产酸条件与机制的研究,鲜见对工业污泥的发酵产酸研究.而我国是柠檬酸生产大国,占世界柠檬酸产量的70%以上,产量每年增长7%,其主要利用玉米进行发酵生产,产生的废水可生化性高,经厌氧处理产生的颗粒污泥是重要的微生物源,再经好氧处理会产生大量有机质含量较高的剩余活性污泥[13],其处理处置成本约占污水处理厂运营成本的60%[14].相比市政污泥,其有机质含量较高,可为发酵提供充足的底物,因此本研究通过分析不同pH值对柠檬酸剩余活性污泥厌氧发酵产VFAs的影响,探讨发酵过程VFAs积累的机制与发酵液作污水处理过程中外源碳源的潜力,为发酵工业剩余活性污泥的资源化利用提供参考.
1 材料与方法
1.1 实验材料
选取山东省某柠檬酸生产企业好氧生化处理的剩余污泥和厌氧颗粒污泥,其中厌氧颗粒污泥作为剩余污泥厌氧发酵的初始菌种,经自然沉降弃去上清液,保存在4℃冰箱中备用.实验时剩余污泥过60目网筛去除沙砾,其含固率(TS)为(4.24±0.64)%,有机物含量(VS)为(50.10±1.21)%,溶解性化学总需氧量(SCOD)为(687.50±51.03) mg/L,可溶性蛋白质(PN)为(253.33±11.11) mg/L,可溶性多糖(PS)为(19.13±2.37) mg/L,总磷(TP)为(23.17±1.31) mg/L,氨氮(NH3-N)为(294.91±12.02) mg/L.
1.2 实验方法
采用序批式实验,将剩余污泥和厌氧颗粒污泥按照质量比TS=4:1的比例混合均匀,测得pH= (7.12±0.22),以此为空白对照组(Control).将300g混合污泥加入500mL厌氧发酵瓶中,通入氮气,保证厌氧密闭环境,在(36±2)℃,(120±10) r/min的水浴摇床中进行厌氧发酵.
使用6mol/L的HCl或NaOH,将发酵罐中混合污泥分别调节pH值为5、6、8、9、10、11、12,此后不再调控pH值,记为初始pH值调控组(pH),同时对产生VFAs的实验组再次进行维持整个发酵过程恒定pH值的实验,记为恒定pH值调控组(C-pH).
所有发酵罐均设置平行实验,在VFAs连续下降3d后停止实验.取调节pH值后的混合污泥样品记为0d,间隔24h取样,样品经9000r/min离心10min,上清液过0.45μm滤膜后用于指标测定,沉淀测定微生物.
1.3 指标测定
参照《城市污水处理厂污泥检测方法》(CTJ221-2005)测定样品的TS和VS,TP和NH3-N分别采用钼酸铵分光光度法和纳氏试剂分光光度法测定[15],用Lowry-Folin法和蒽酮-硫酸法分别测定PN和PS[16],毛细吸水时间(CST)使用CST测定仪(TR04-304M, Triton,英国)测定,结果归一化[9],见式(1).
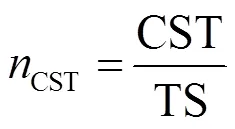
式中:CST为归一化结果,s·L/g; CST为仪器测定的毛细吸水时间,s; TS为污泥含固率,g/L.
Zeta电位使用激光Zeta粒度分析仪(Zetasizer Nano ZS,马尔文,英国)测定;溶解性总有机碳(DOC)使用DOC分析仪(VarioEL III, Elementar,德国)测定;使用哈克旋转流变仪(HAAKE MARS III, Thermo Scientific,美国),选择速率与黏度模型,CC25DIN Ti转子,剪切率10~300s-1,在25℃下对污泥样品流变特性进行测定[17];VFAs采用气相色谱仪(GC-2014,岛津,日本)检测,换算关系为:1.07g COD/g乙酸, 1.51g COD/g丙酸,1.82g COD/g丁酸和2.04g COD/g戊酸[2].样品经处理后(UV254<0.3),使用三维荧光光谱仪(Spectrofluorometer FS5,爱丁堡,英国)在x/m= 220~550nm/240~600nm,间隔5nm,设置中扣除空白散射,测其三维荧光(3D-EEM)谱图,结果采用MATLAB 2018b进行平行因子(PARAFAC)分析[18-19].微生物由上海美吉生物公司测定,样品经DNA提取后,使用引物(338F和806R)进行PCR扩增后,对16S rDNA的V3-V4可变区基因进行测序分析[1].所有数据使用origin 2018作图.样品进行了3次平行测定.
2 结果分析
2.1 初始pH值对污泥发酵性能的影响
SCOD是反映污泥水解和酸化程度的重要指标,如图1(a)所示,在0d时酸碱的加入都促进污泥水解,但pH³10的条件下SCOD浓度更高,污泥水解的效果更佳.根据厌氧发酵的主要产物甲烷和VFAs的变化情况(图1(b)),初始pH=5~9的条件有利于甲烷的产生,其中Control组累计最大甲烷产量为(40.25±2.86)mL/g VS,其他条件下甲烷产量降低甚至完全消失,是产甲烷菌的活性受到抑制或丧失所致,甲烷的产生消耗有机物,与发酵后SCOD下降结果一致.初始pH=10~12的实验组厌氧发酵后(8d)产生了大量VFAs,导致SCOD浓度增加,其中pH=10的实验组在8d时VFAs含量最高为(3149.45±202.53) mg COD/L.因此初始pH=10~12有利于柠檬酸剩余污泥厌氧发酵VFAs的积累,这与Wu等[2]和Ma等[20]对不同pH值下污泥厌氧发酵得出碱性条件更利于污泥厌氧产VFAs的结论相一致.
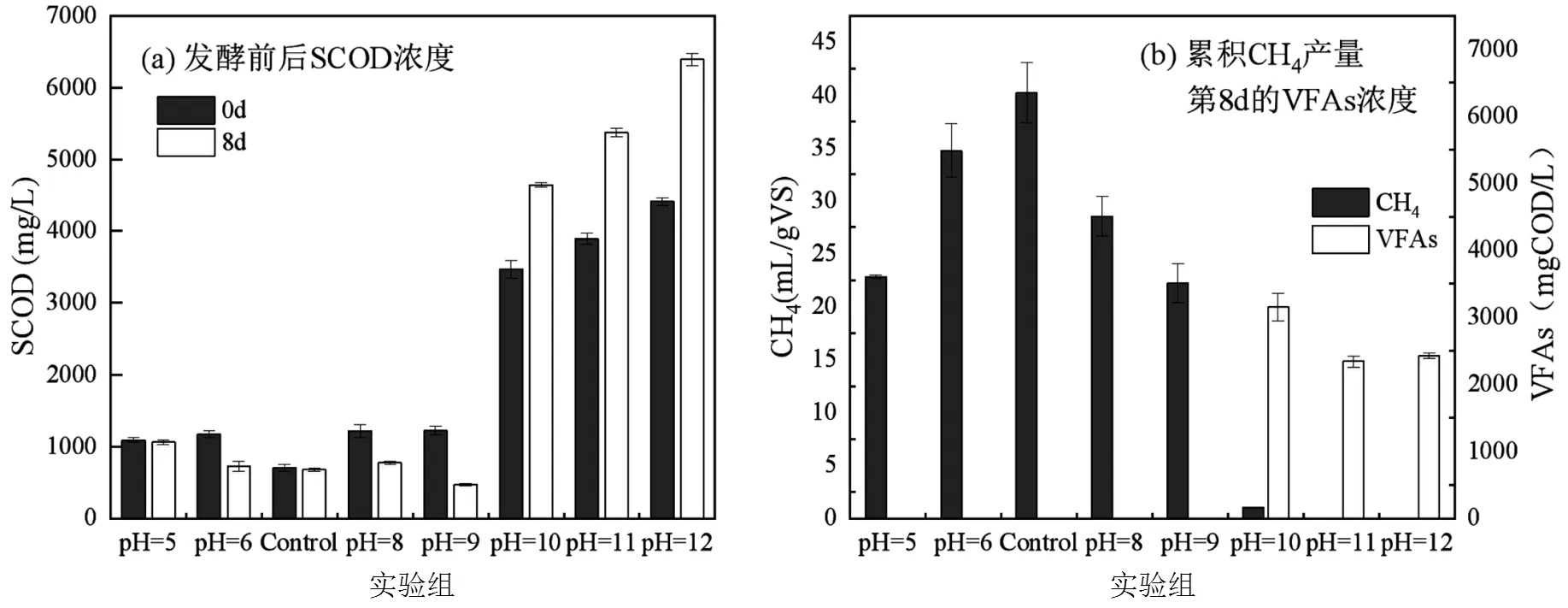
图1 不同初始pH值厌氧发酵前后SCOD浓度与发酵过程累积CH4产量和第8d的VFAs浓度变化
2.2 恒定和初始碱性条件对柠檬酸污泥厌氧产酸的影响
2.2.1 恒定和初始碱性条件对VFAs产量的影响 对产生VFAs的实验组(pH=10、11、12)进行维持恒定pH值的厌氧发酵实验,如图2所示.不同条件总VFAs的最大浓度不同,其顺序为:pH=10 ((6681.47± 126.82)mg COD/L)>pH=11((5964.85±524.72) mg COD/L)> C-pH=11((4902.85±596.79)mg COD/L) >C-pH=10 ((4427.41±111.48)mg COD/L)>C-pH=12 ((3321.91±461.07)mg COD/L)>pH=12((2746.54± 55.82) mg COD/ L),pH值为12的两组VFAs浓度低,是因为大多数产酸菌不易在pH³12条件下存活[3].此外到达总VFAs最大浓度的时间亦不同,pH=10时间最短仅为4d,其次是pH=11和C-pH=10为5d,时间延长VFAs出现下降趋势,可能是底物不足或被产酸菌利用的结果[2].因此pH=10是柠檬酸剩余污泥厌氧产酸较佳的条件,约为相似条件下的市政污泥厌氧发酵产VFAs浓度的2倍(最大VFAs浓度为2500~ 3000mg COD/L,时间为5~6d)[9,21].不同条件对VFAs的组成有不同影响,其中乙酸占VFAs总量的45%~ 66%,决定VFAs变化总趋势,因为大多数微生物都能产生乙酸[22],同时它是污水处理过程中受欢迎的碳源,含量越高碳源利用潜力越大[22].其次是异戊酸和丙酸占比为8%~25%,异丁酸、正丁酸和正戊酸由于分解较快[23]仅占2%~13%.
2.2.2 碱性厌氧产酸发酵过程中有机质的变化 如图3所示,SCOD与DOC变化趋势基本与PN、PS和VFAs浓度变化相一致.在0d时PN、PS的水解程度与碱性pH值呈正相关,但PN的水解浓度是Control组的2.66~4.90倍,高于PS(1.10~2.29倍),碱性条件更有利于PN的析出[2,20].随着发酵时间的延长Control组PN浓度基本不变,而PS有明显的先升后降趋势,可能是中性条件下更有利于产甲烷菌对PS的水解和利用.相反在恒定pH值的厌氧发酵过程中PN、PS浓度逐渐升高,是碱性环境促进污泥絮体的破坏所致[3,24],在5~8d时PN快速下降,而此时VFAs浓度没有明显上升,可能是因为强碱与氨基、羧基反应生成盐导致蛋白质变性.pH=11、12的实验组发酵过程中PN和PS呈不明显上升趋势,在pH=10的实验组PN和PS变化趋势显著,0~4d时VFAs浓度迅速上升,此时PN浓度下降而PS上升,可能是产酸菌对PN的利用率高于PN的水解率和PS的利用率,在4~5d时PN和PS可能达到此条件下最大水解程度,时间延长产酸底物不断减少VFAs浓度下降.

图2 不同碱性条件对VFAs产量及组成的影响
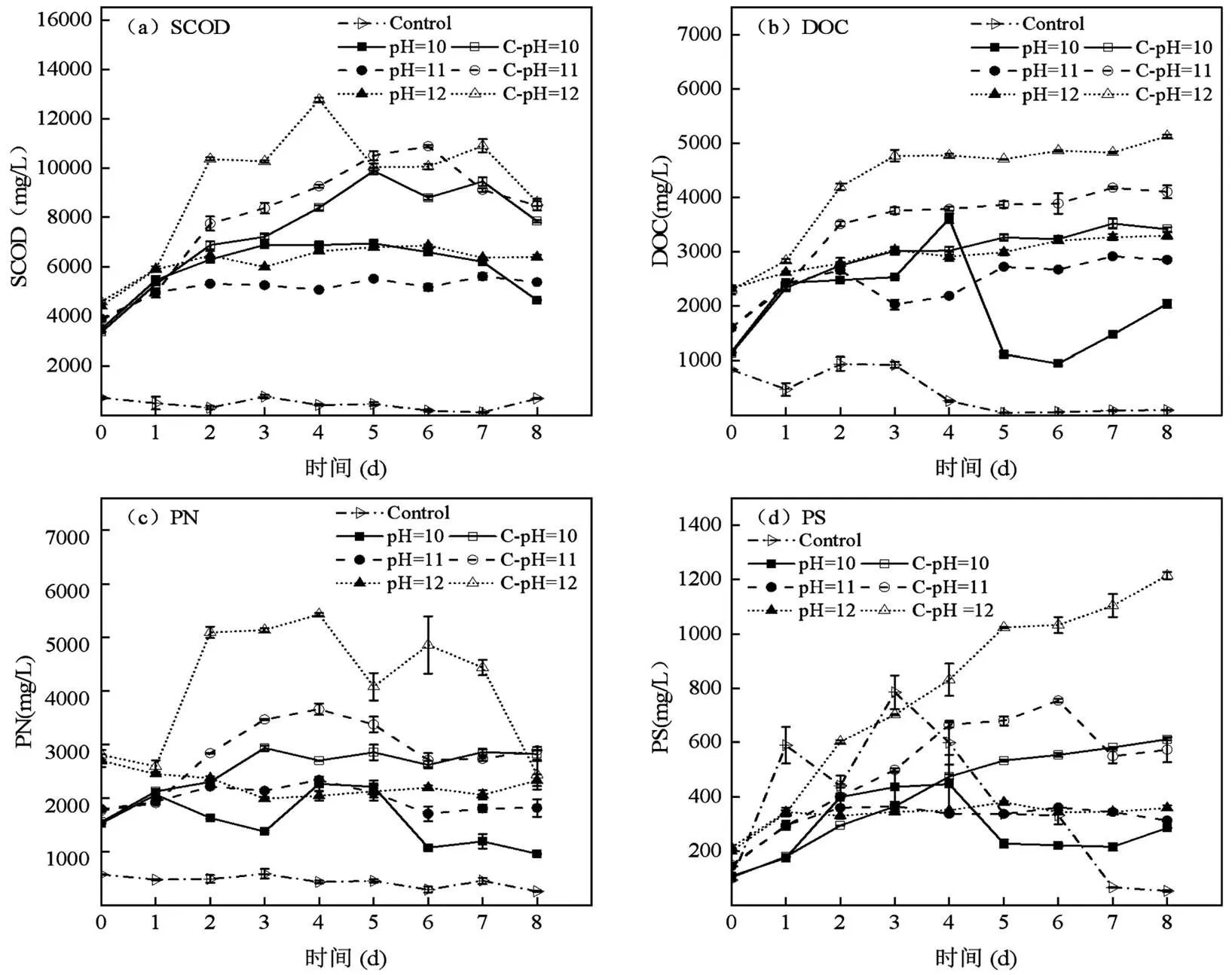
图3 碱性厌氧产酸发酵过程中SCOD、DOC、PN和PS的变化
2.2.3 荧光组分的变化 通过PARAFAC分析对上清液3D-EEM光谱进行拆分发现,3种主要荧光物质[25](图4),分别为色氨酸类蛋白质(TPN):x/m= 275nm/360nm,腐殖酸类物质(HA):x/m=360(415) nm/470nm和富里酸类物质(FA):x/m=320nm/ 400nm,同时得到最大荧光max图5,通常TPN、FA和HA都被认为是难生物降解的化合物[25].根据图5可知,TPN的max值最高是主要的荧光物质,且在初始pH值实验组的变化趋势与PN浓度变化几乎一致,因此TPN可以被柠檬酸污泥碱性厌氧发酵产酸过程利用.而在恒定pH值的实验组尤其是C-pH=11和C-pH=12实验组的TPN变化趋势与PN浓度变化不同,可能因为发酵过程中HA和FA的大量溶出对PN测定产生干扰,同时FA和HA已被证实无法通过微生物分解产生VFAs[26],因此FA和HA的大量溶出会降低产酸效率[27-28],与VFAs的浓度降低相符.
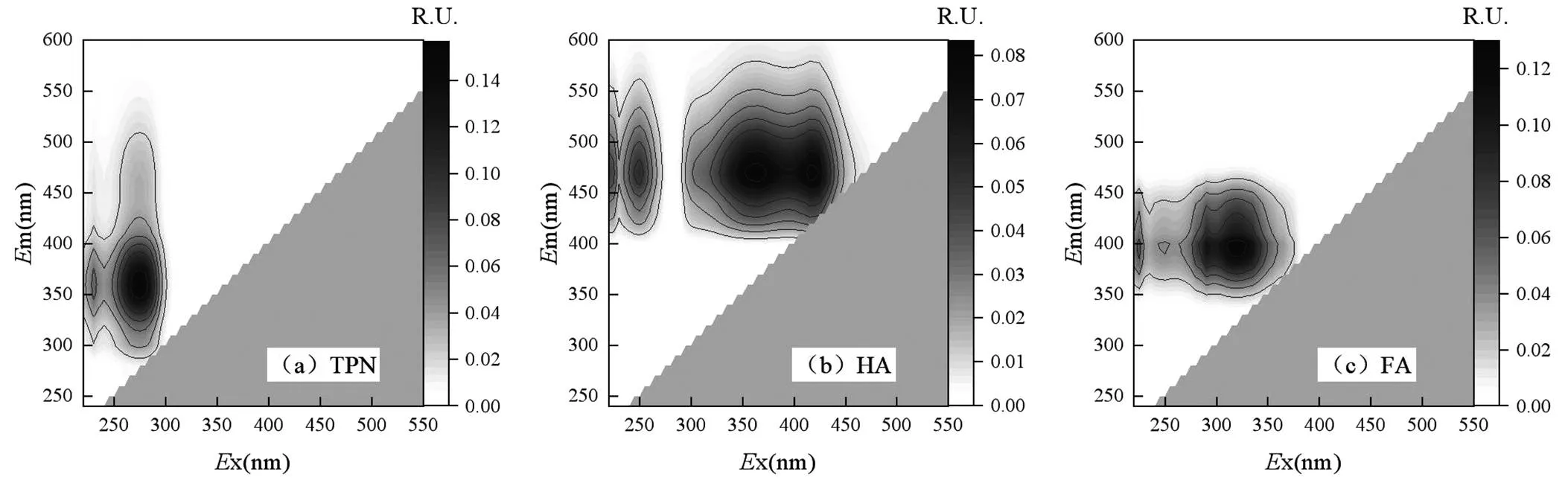
图4 碱性厌氧发酵液的荧光组分
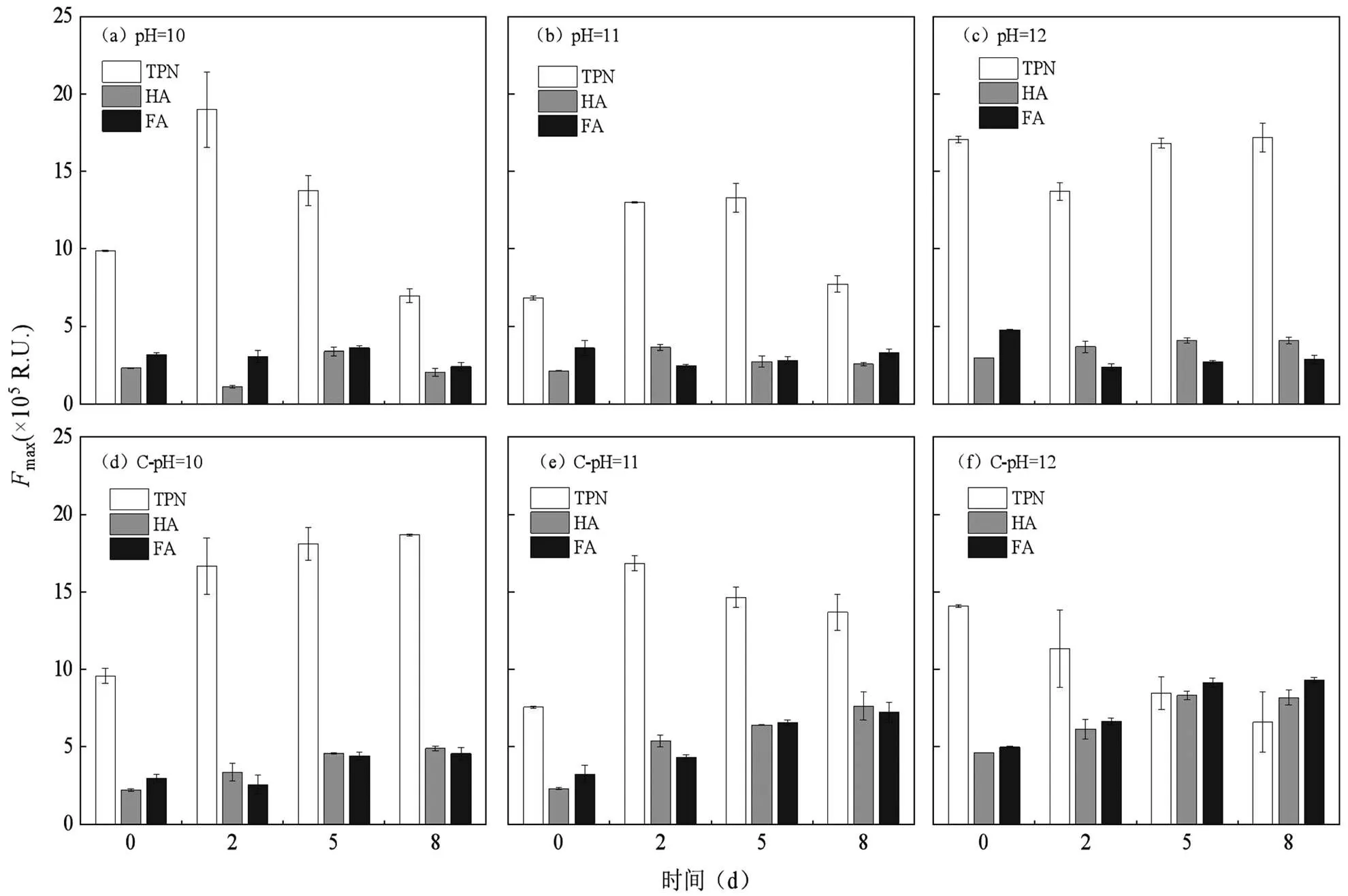
图5 碱性厌氧发酵液荧光组分Fmax的变化
2.2.4 厌氧发酵前后微生物群落的变化 由pH=10发酵前后(0和8d)微生物丰度和多样性的变化结果(表1)可知,厌氧发酵后OUT指数、ACE指数、Chao指数和Shannon指数都明显降低,表明碱性厌氧发酵产酸的菌群多样性明显低于初始污泥的多样性.这一现象在属(图6b)水平上尤为明显,如菌属消失,以及vadinHA17和SBR1031*等菌属的大量增加.其中是蛋白质降解厌氧菌[29],其消失可能与发酵后其PN含量降低有关;而HA17*菌属[30]能够利用葡萄糖产生乙酸盐、丙酸盐和氢气[30]属于产乙酸菌,有利于增加乙酸含量;菌属[31]具有长链脂肪酸(C4及以上)降解功能,降低丁酸、戊酸等长链脂肪酸在总VFAs中占比;SBR1031*菌属可代谢NH3-N[32],与发酵罐中的NH3-N含量变化有关.

表1 微生物群落丰度和多样性变化
注:OTU是操作分类单位,Coverage反应测序深度指数,数值高于0.99表明测序深度足够,结果可靠;ACE和Chao指数代表微生物丰度,数值越高丰度越高;Shannon和Simpson指数为香浓指数和辛普森指数,代表微生物多样性,Shannon指数越高,多样性越高,Simpson指数则相反.
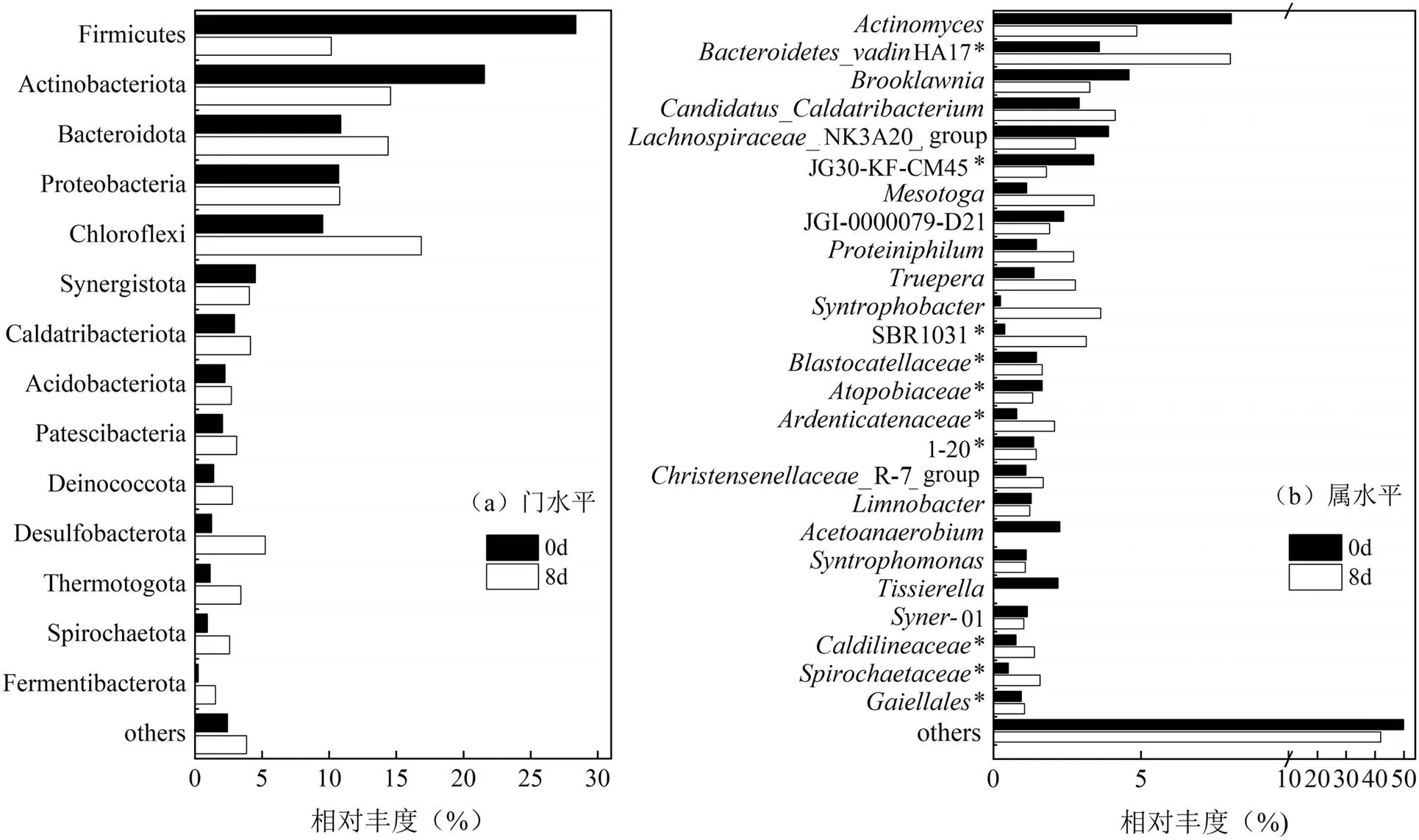
图6 门、属水平上的物种相对丰度
*表示没有明确的分类信息或分类名称
基于样品OTUs的注释结果,门水平和属水平微生物相对丰度如图6所示,主要优势菌门为Firmicutes, Actinobacteriota, Bacteroidota, Proteobacteria, Chloroflexi,属于污泥碱性发酵的优势菌群[33],但柠檬酸污泥碱性厌氧发酵过程改变了初始环境特征菌群的相对丰度.Firmicutes具有厚厚的细胞壁能够在不同的污泥处理(例如加热、碱化、酸化)中存活,含有多种产乙酸菌,可将多种VFAs代谢成乙酸、H2和CO2[34-35], Actinobacteriota中的细菌能降解多糖生成单糖和VFAs[36],然而发酵后Firmicutes和Actinobacteriota相对丰度分别由28.37%、21.58%降至10.15%、14.56%,可能是两者菌门中不适于碱性厌氧环境下的劣势菌种被淘汰所致[37-38].而Chloroflexi和Bacteroidota相对丰度分别由9.52%、10.87%增加至16.84%、14.39%,这是因为Chloroflexi菌门的微生物主要代谢碳水化合物,促进VFAs底物降解[39], Bacteroidota的微生物可分泌多种细胞外水解酶,将葡萄糖、纤维二糖、淀粉等物质转化为乙酸、丁酸、异戊酸、H2和CO2[37,40],这两种菌门中多种微生物适应碱性厌氧环境,有助于促进有机物的水解和VFAs的产生.同时发现部分非优势菌种Desulfobacterota、Thermotogota等相对丰度增加,据报道,Thermotogae菌群可以降解复杂的有机物,如木糖和纤维素等[41]. Desulfobacterota的部分菌群在厌氧条件下还原硫酸盐,竞争NO2-电子供体,抑制NO2-还原产生N2O的反硝化过程[42],与氮含量变化有关.
2.3 碱性厌氧产酸发酵液外源碳源利用潜力分析
2.3.1 污泥脱水性能分析 如图7(a)所示,碱处理和厌氧发酵导致CST增大,是因为OH-与金属盐离子聚集、发酵过程释放的磷形成的化合物[43]以及上清液有机质含量增加等因素使大量水分被聚合,导致污泥过滤性能变差,这与Chen等[9]得出的酸性厌氧发酵可提高污泥的脱水能力结论一致.但OH-与盐离子聚集以及VFAs产生的H+中和负电离子会降低Zeta电位绝对值(图7b),甚至在pH=10的实验组出现正电位,为维持强碱性环境的C-pH=11和C-pH=12实验组,不断引入OH-,与盐离子和H+全部反应后仍有大量OH-游离,导致Zeta电位绝对值进一步增大,干扰污泥絮体聚集进一步增加CST.因而pH=10的实验组脱水性能相对较好.由图7(c)可以看出,碱性厌氧发酵可以降低污泥表观黏度,因为在发酵过程中大分子有机质被降解为小分子物质,网络结构被破坏内部阻力降低[44],这与Zhang等[45]和Zhang等[46]对市政污泥厌氧发酵对污泥脱水性能的影响研究得出的碱性厌氧发酵可以增强污泥流动性,降低污泥表观黏度结论一致.因此可以考虑从流变方面对发酵后污泥进行脱水研究.
2.3.2 发酵液N、P的变化 使用厌氧产酸发酵液作为碳源时,还需要考虑发酵液中氮磷含量的影响.由图8(a)可知,pH值不影响NH3-N的变化(0d),在发酵过程PN水解生成的氨基酸分子被厌氧菌利用时会生成游离态的NH3-N[1],使发酵后NH3-N浓度升高,因而代谢NH3-N的SBR1031*菌属相对丰度升高.由于厌氧发酵无法完成硝化反硝化作用[47],且抑制反硝化过程相关的Desulfobacterota菌门相对丰度升高,使得NH3-N含量不断升高.然而从图8(b)可知pH值会影响TP的变化(0d),当pH³11时TP浓度明显升高,因为无机磷酸盐类在pH³11时不能稳定存在[43],而这种高pH值导致的磷的释放是可逆[48],在初始pH值实验组中由于VFAs的产生降低pH值使无机磷酸盐类重新沉淀,因此初始pH值的实验组在8d时的TP浓度小于恒定pH实验组,尤其是pH=10的处理组TP几乎无明显变化.

总体而言,污泥碱性厌氧发酵在增大污泥脱水难度的同时使得大量氨氮和可溶性磷释放到发酵上清液中,已有研究表明[9,49],同时添加KH2PO4和MgCl2,可以在去除N、P的同时(NH3-N去除率>75%,TP去除率>80%)提高发酵后污泥的脱水能力,但药剂添加会导致成本增加,影响污泥发酵产酸再利用的经济性.
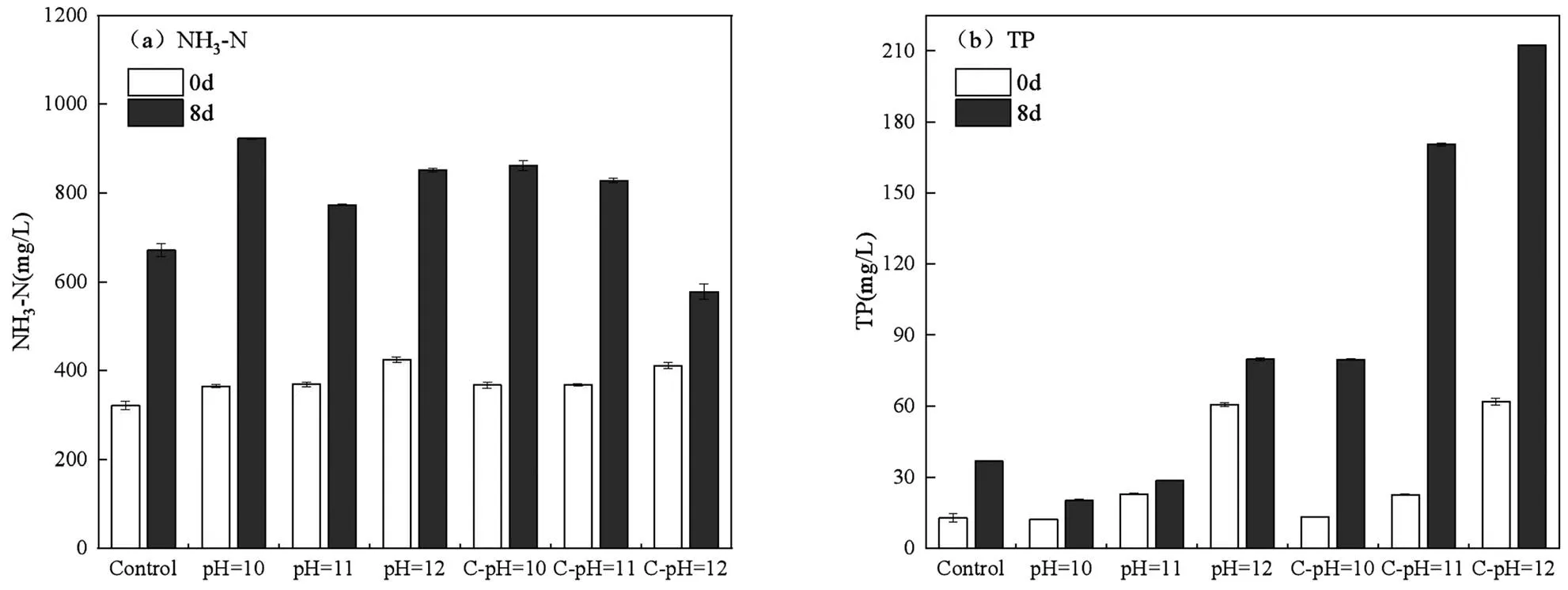
图8 厌氧发酵前后发酵液NH3-N、TP的变化
3 结论
3.1 在初始pH=5~9的条件有利于柠檬酸剩余污泥厌氧发酵产甲烷,其中Control组累计甲烷产量最大为(40.25±2.86) mL/g VS,在初始和恒定pH³10的碱性条件下,厌氧发酵易产生VFAs,同时更易于PN、PS的释放.在恒定pH值实验组,难分解的HA和FA会大量溶出不利于VFAs的产生.
3.2 初始pH=10的条件是柠檬酸剩余污泥厌氧产酸最佳的条件,发酵后产酸功能菌Chloroflexi、Bacteroidota的相对丰度分别由初始的9.52%、10.87%增至16.84%、14.39%,发酵4d的VFAs= (6681.47±126.82) mg COD/L浓度最高,是文献报道中市政污泥产酸量的近2倍,此时乙酸为总VFAs的49.8%,有很大的碳源利用潜力.
3.3 碱性厌氧发酵过程中盐离子的聚集和有机质的增加恶化污泥脱水性能,同时还增加N、P等物质的溶出,不利于发酵液作为外源碳源,因此针对发酵液用于污水处理的外部碳源,需要进一步了解污泥碱性发酵过程的SCOD、N、P变化规律和发酵液的性质,以便发现在提高N、P去除率的同时,还能改善发酵后污泥脱水性能的成本低、操作简单的方法.
[1] 黄 潇,董文艺,赵福祥,等.发酵周期对初沉污泥厌氧发酵产酸影响及微生物机制研究[J]. 环境科技, 2019,32(2):33-39.
Huang X, Dong W Y, Zhao F X, et al. Effect of fermentation period on acid production by primary sludge anaerobic fermentation and microbial mechanisms [J]. Environmental Science and Technology, 2019,32(2):33-39.
[2] Wu H, Gao J, Yang D, et al. Alkaline fermentation of primary sludge for short-chain fatty acids accumulation and mechanism [J]. Chemical Engineering Journal, 2010,160(1):1-7.
[3] Fang W, Zhang X, Zhang P, et al. Overview of key operation factors and strategies for improving fermentative volatile fatty acid production and product regulation from sewage sludge [J]. Journal of Environmental Sciences, 2020,87:93-111.
[4] 宋青青,任宏宇,孔凡英,等.不同预处理方法促进剩余污泥发酵制氢研究进展 [J]. 中国环境科学, 2021,41(10):9.
Song Q Q, Ren H Y, Kong F Y, et al. Progress of different pretreatment methods to promote residual sludge fermentation for hydrogen production [J]. China Environmental Science, 2021,41(10): 9.
[5] Ma H, Chen X, Liu H, et al. Improved volatile fatty acids anaerobic production from waste activated sludge by pH regulation: Alkaline or neutral pH? [J]. Waste Management, 2016,48:397-403.
[6] Latif M A, Mehta C M, Batstone D J. Influence of low pH on continuous anaerobic digestion of waste activated sludge [J]. Water Research, 2017,113:42-49.
[7] Zhang W, Li X, Zhang T, et al. High-rate lactic acid production from food waste and waste activated sludge via interactive control of pH adjustment and fermentation temperature [J]. Chemical Engineering Journal, 2017,328:197-206.
[8] 朱凤霞,李 平,冯 涛,等.酸性/碱性启动模式下SRT对剩余污泥水解酸化的影响[J]. 现代化工, 2017,37(7):128-132.
Zhu F X, Li P, Feng T, et al. Influences of SRT on hydrolytic acidification of excess sludge in acid /alkaline startup modes [J]. Modern Chemical Industry, 2017,37(7):128-132.
[9] Chen Y, Ruhyadi R, Huang J, et al. Comprehensive comparison of acidic and alkaline anaerobic fermentations of waste activated sludge [J]. Bioresource Technology, 2021,323:124613.
[10] Liu H, Han P, Liu H, et al. Full-scale production of VFAs from sewage sludge by anaerobic alkaline fermentation to improve biological nutrients removal in domestic wastewater [J]. Bioresource Technology, 2018,260:105-114.
[11] 王率率,陆小游,姜 谦,等.城镇污水厂剩余污泥厌氧发酵产酸工程示范研究[J]. 环境科学研究, 2020,33(12):2829-2837.
Wang S S, Lu X Y, Jiang Q, et al. Full-scale volatile fatty acid production from excess sludge of municipal wastewater treatment plant by anaerobic fermentation [J]. Research of Environmental Sciences, 2020,33(12):2829-2837.
[12] 张闻多.示范工程规模下高负荷污泥厌氧发酵产酸工艺的研究[D]. 江苏:江南大学, 2018.
Zhang W D. Demonstration project research on sewage sludge anaerobic fermentation for acids production under high loading rate [D]. Jiangsu:Jiangnan University, 2018.
[13] 张 晨,李杨杨,董黎明,等.预加热对柠檬酸脱水污泥冬季生物干化的影响[J]. 中国环境科学, 2019,39(7):2928-2937.
Zhang C, Li Y Y, Dong L M, et al. Effect of pre-heating on bio-drying of citric acid dewatered sludge in winter [J]. China Environmental Science, 2019,39(7):2928-2937.
[14] Wei W, Wang Q, Zhang L, et al. Free nitrous acid pre-treatment of waste activated sludge enhances volatile solids destruction and improves sludge dewaterability in continuous anaerobic digestion [J]. Water Research, 2018,130:13-19.
[15] 国家环境保护总局.水和废水监测分析方法(4版) [M]. 北京:中国环境科学出版社, 2022.
The State Environmental Protection Administration. Standard methods for the examination of water and wastewater (4th) [M]. Beijing: China Environmental Science Press, 2002.
[16] 张心钰,陈 瑶,董黎明,等.柠檬酸废水污泥脱水中蛋白质和多糖变化特征[J]. 环境科学与技术, 2017,40(S2):76-80.
Zhang X Y, Chen Y, Dong L M, et al. Changes of protein and polysaccharide during dehydration of citric acid wastewater sludge [J]. Environmental Science & Technology, 2017,40(S2):76-80.
[17] Baroutian S, Eshtiaghi N, Gapes D J. Rheology of a primary and secondary sewage sludge mixture: Dependency on temperature and solid concentration [J]. Bioresource Technology, 2013,140:227-233.
[18] Zhu Y, Cao L, Ni L, et al. Insights into fouling behavior in a novel anammox self-forming dynamic membrane bioreactor by the fluorescence EEM-PARAFAC analysis [J]. Environmental Science and Pollution Research, 2020,27(32):40041-40053.
[19] Stedmon C A, Bro R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: a tutorial [J]. Limnology and Oceanography: Methods, 2008,6(11):572-579.
[20] Ma S, Hu H, Wang J, et al. The characterization of dissolved organic matter in alkaline fermentation of sewage sludge with different pH for volatile fatty acids production [J]. Water Research, 2019,164:114924.
[21] Jiang X, Qin Z, Feng L, et al. Volatile fatty acids production from waste activated sludge during anaerobic fermentation: The effect of superfine sand [J]. Bioresource Technology, 2021,319:124249.
[22] Li X, Liu G, Liu S, et al. The relationship between volatile fatty acids accumulation and microbial community succession triggered by excess sludge alkaline fermentation [J]. Journal of Environmental Management, 2018,223:85-91.
[23] Chen S, Dai X, Yang D, et al. Effects of sludge age on anaerobic acidification of waste activated sludge: Volatile fatty acids production and phosphorus release [J]. Journal of Environmental Sciences, 2021, 105:11-21.
[24] Zhao J, Wang D, Liu Y, et al. Novel stepwise pH control strategy to improve short chain fatty acid production from sludge anaerobic fermentation [J]. Bioresource Technology, 2018,249:431-438.
[25] Chen H, Rao Y, Cao L, et al. Hydrothermal conversion of sewage sludge: Focusing on the characterization of liquid products and their methane yields [J]. Chemical Engineering Journal, 2019,357:367-375.
[26] Wang Y, Sun P, Guo H, et al. Performance and mechanism of sodium percarbonate (SPC) enhancing short-chain fatty acids production from anaerobic waste activated sludge fermentation [J]. Journal of Environmental Management, 2022,313:115025.
[27] Wu Y, Song K, Sun X, et al. Effects of free nitrous acid and freezing co-pretreatment on sludge short-chain fatty acids production and dewaterability [J]. Science of The Total Environment, 2019,669:600- 607.
[28] Yu L, Zhang W, Liu H, et al. Evaluation of volatile fatty acids production and dewaterability of waste activated sludge with different thermo-chemical pretreatments [J]. International Biodeterioration & Biodegradation, 2018,129:170-178.
[29] Chen S, Dong B, Dai X, et al. Effects of thermal hydrolysis on the metabolism of amino acids in sewage sludge in anaerobic digestion [J]. Waste Management, 2019,88:309-318.
[30] Wang R, Li C, Lv N, et al. Deeper insights into effect of activated carbon and nano-zero-valent iron addition on acidogenesis and whole anaerobic digestion [J]. Bioresource Technology, 2021,324:124671.
[31] 张 雪,张 辉,承 磊.获取有机物厌氧降解产甲烷过程中关键功能类群——互营细菌培养物[J]. 微生物学报, 2019,59(2):211-223.
Zhang X, Zhang H, Cheng L. Key players involved in methanogenic degradation of organic compounds: progress on the cultivation of syntrophic bacteria [J]. Acta Microbiologica Sinica, 2019,59(2):211- 223.
[32] Tian X, Shen Z, Zhou Y, et al. Inhibition on biological acidification and microbial community by high-strength acetaldehyde [J]. Process Safety and Environmental Protection, 2020,143:231-238.
[33] Huang X, Dong W, Wang H, et al. Role of acid/alkali-treatment in primary sludge anaerobic fermentation: Insights into microbial community structure, functional shifts and metabolic output by high-throughput sequencing [J]. Bioresource Technology, 2018,249: 943-952.
[34] Ren S, Usman M, Tsang D C W, et al. Hydrochar-Facilitated Anaerobic Digestion: Evidence for Direct Interspecies Electron Transfer Mediated through Surface Oxygen-Containing Functional Groups [J]. Environmental Science & Technology, 2020,54(9):5755- 5766.
[35] Zhao Z, Li Y, Zhao Z, et al. Effects of dissimilatory iron reduction on acetate production from the anaerobic fermentation of waste activated sludge under alkaline conditions [J]. Environmental Research, 2020,182:109045.
[36] Jin Y, Lin Y, Wang P, et al. Volatile fatty acids production from saccharification residue from food waste ethanol fermentation: Effect of pH and microbial community [J]. Bioresource Technology, 2019, 292:121957.
[37] Wang R, Li C, Lv N, et al. Deeper insights into effect of activated carbon and nano-zero-valent iron addition on acidogenesis and whole anaerobic digestion [J]. Bioresource Technology, 2021,324:124671.
[38] Chen S, Cheng H, Wyckoff K N, et al. Linkages of Firmicute and Bacteroidetes populations to methanogenic process performance [J]. Journal of Industrial Microbiology and Biotechnology, 2016,43(6): 771-781.
[39] van Vliet D M, Palakawong Na Ayudthaya S, Diop S, et al. Anaerobic degradation of sulfated polysaccharides by two novel kiritimatiellales strains isolated from black sea sediment [J]. Frontiers in Microbiology, 2019,10:253.
[40] Alalawy A I, Guo Z, Almutairi F M, et al. Explication of structural variations in the bacterial and archaeal community of anaerobic digestion sludges: An insight through metagenomics [J]. Journal of Environmental Chemical Engineering, 2021,9(5):105910.
[41] 常 城,明磊强,牟云飞,等.厨余垃圾与污泥厌氧发酵产甲烷的协同作用[J]. 中国环境科学, 2022,42(3):1259-1266.
Chang C, Ming L Q, Mou Y F, et al. Synergistic effect of kitchen waste and sludge anaerobic fermentation for methane production [J]. China Environmental Science, 2022,42(3):1259-1266.
[42] Wang S, Zhu G, Zhuang L, et al. Anaerobic ammonium oxidation is a major N-sink in aquifer systems around the world [J]. The ISME Journal, 2020,14(1):151-163.
[43] Liu J, Deng S, Qiu B, et al. Comparison of pretreatment methods for phosphorus release from waste activated sludge [J]. Chemical Engineering Journal, 2019,368:754-763.
[44] Hong E, Yeneneh A M, Sen T K, et al. A comprehensive review on rheological studies of sludge from various sections of municipal wastewater treatment plants for enhancement of process performance [J]. Advances in Colloid and Interface Science, 2018,257:19-30.
[45] Zhang J, Li N, Dai X, et al. Enhanced dewaterability of sludge during anaerobic digestion with thermal hydrolysis pretreatment: New insights through structure evolution [J]. Water Research, 2018,131: 177-185.
[46] Zhang W, Dong B, Dai X. Mechanism analysis to improve sludge dewaterability during anaerobic digestion based on moisture distribution [J]. Chemosphere, 2019,227:247-255.
[47] 周 倩,张 林,唐 溪,等.基于DGAOs富集的内碳源短程硝化反硝化工艺特性 [J]. 中国环境科学, 2021,41(12):5673-5679.
Zhou Q, Zhang L, Tang X, et al. Short-cut nitrification and denitrification process characteristics of internal carbon source based on DGAOs enrichment [J]. China Environmental Science, 2021,41(12): 5673-5679.
[48] Bashir A, Wang L, Deng S, et al. Phosphorus release during alkaline treatment of waste activated sludge from wastewater treatment plants with Al salt enhanced phosphorus removal: Speciation and mechanism clarification [J]. Science of The Total Environment, 2019,688:87-93.
[49] Liu W, Yang H, Ye J, et al. Short-chain fatty acids recovery from sewage sludge via acidogenic fermentation as a carbon source for denitrification: A review [J]. Bioresource Technology, 2020,311: 123446.
Effect of pH on acid production by anaerobic fermentation of citric acid sludge and carbon source potential of fermentation broth.
SUN Dong-xia1, ZHOU Zi-an1, FENG Zhi-he2, HU Xiu-yu2, QI Guang-xia1, DONG Li-ming1*
(1.Key Laboratory of Cleaner Production and Integrated Resource Utilization of China National Light Industry, State Environmental Protection Key Laboratory of Food Chain Pollution Control, Beijing Technology and Business University, Beijing 100048, China;2.China Biotech Fermentation Industry Association, Beijing 100083, China)., 2022,42(11):5198~5207
The research of acid production by anaerobic fermentation with different pH control conditions was carried out for the treatment of waste activated sludge from citric acid wastewater, using anaerobic granular sludge of citric acid wastewater as inoculum. The mechanism of anaerobic acid production of citric acid sludge was evaluated by the analysis of volatile fatty acids (VFAs), organic matter, nitrogen and phosphorus contents and sludge dewatering performance. The results showed that the alkaline conditions with pH³10 were more conducive to the dissolution of organic matter to promote the production of VFAs. It was obvious that humic acid (HA) and fulvic acid (FA) at constant pH conditions would be dissolved in large quantities with Three-dimensional Excitation-Emission-Matrix Spectra analysis, thus reducing the yield of VFAs. The initial pH=10 was the optimum pH value for anaerobic acid production of citric acid sludge, and the VFAs concentration of (6681.47±126.82) mg COD/L for 4 days was the highest, which was nearly 2 times that of municipal sludge acid production reported in the literature, among which acetic acid was 49.8%. After fermentation, the relative abundances of acid-producing functional bacteria Chloroflexi and Bacteroidota increased from initial 9.52% and 10.87% to 16.84% and 14.39%, respectively. The normalized capillary suction time (CST) value of the final sludge was (11.34±0.27) s·L/g with good dewatering performance, and the TP concentration of fermentation broth was (20.45±0.33) mg/L. Studies have shown that the alkaline anaerobic fermentation of citric acid waste activated sludge to produce acid fermentation broth has a good development potential as an exogenous carbon source in the sewage treatment process.
pH value;citric acid waste activated sludge;alkaline anaerobic fermentation;volatile fatty acids;sludge dewatering performance
X705
A
1000-6923(2022)11-5198-10
孙东霞(1996-),女,山东德州人,北京工商大学硕士研究生,主要从事清洁生产与资源综合利用研究.发表论文1篇.
2022-04-06
国家自然科学基金资助项目(41861124004)
* 责任作者, 教授, donglm@btbu.edu.cn

