水位波动和氮浓度变化对氮转化功能基因丰度的影响
崔荣阳,刘刚才,胡万里,付 斌,陈安强*
水位波动和氮浓度变化对氮转化功能基因丰度的影响
崔荣阳1,2,刘刚才1,胡万里3,付 斌3,陈安强3*
(1.中国科学院水利部成都山地灾害与环境研究所,中国科学院山地表生过程与生态调控重点实验室,四川 成都 610041;2.中国科学院大学,北京 100049;3.云南省农业科学院农业环境资源研究所,云南 昆明 650201)
为探索浅层地下水氮浓度及水位波动对土壤剖面中氮转化功能基因丰度的影响,以洱海近岸农田原状土壤剖面为对象,研究了模拟常规氮浓度的浅层地下水进行水位波动(SND)和持续淹水(SNF),以及无氮浓度的浅层地下水位波动(0ND)后土壤剖面氮浓度和氮转化功能基因丰度的变化,探讨了土壤因子与功能基因丰度的关系.结果表明:SNF、SND和0ND处理较试验前土壤剖面中溶解性总氮(TDN)浓度分别降低了44%、21%和30%,NO3−-N浓度分别降低了55%、28%和38%.同时,0ND和SNF处理较SND处理土壤剖面中反硝化功能基因丰度分别降低20%和1%,厌氧氨氧化功能基因丰度则分别增加68%和7%,硝化功能基因丰度分别降低34%和增加23%,土壤含水率(MC)、NH4+-N、NO3−-N和TDN均为功能基因丰度变化的重要驱动因子.土壤剖面持续淹水会显著降低溶解性氮浓度,浅层地下水波动及水中氮浓度引起的土壤剖面干湿交替和氮浓度变化是氮转化功能基因丰度变化的主要驱动力.
氮转化功能基因;土壤剖面;干湿交替;浅层地下水位波动
土壤氮循环是生物地球化学循环最重要的过程之一,其循环过程受土壤微生物驱动[1].氮在微生物作用下进行着复杂的转化,保持着土壤中氮素的动态平衡.土壤中氮素形态转化决定了氮素的植物利用效率[2],影响着氮素向水-气环境中的排放量,从而造成了温室气体排放、水体富营养化、地下水硝酸盐污染等环境问题[3-4].土壤中氮形态间的转化过程受微生物代谢产生的酶控制,每个代谢过程中产生的酶均有标志性的基因编码[1],如AOA-和AOB-是参与NH4+-N转化为NO3−-N的关键功能基因[5];NO2−-N转化为N2O的关键编码基因为、和[1].
浅层地下水位波动是造成土壤剖面氮素流失的重要途径,该流失路径与土壤微生物调控的硝化、反硝化、厌氧氨氧化、硝酸盐异化成铵等氮转化过程密切相关[6-7].由于水位升降造成土壤剖面环境变化,改变了土壤微生物群落结构及功能基因丰度,影响着土壤氮素形态转化,驱动着土壤剖面中氮素的累积和流失[7-8].明确浅层地下水位波动下土壤微生物功能基因变化及其主要驱动因子对于预测氮素转化和流失至关重要.一般来说,水位的周期性升降常发生在消落带、湿地、河湖岸带等区域,这导致溶解氧、pH值、土壤含水量、温度、碳源等众多影响土壤微生物的因子也发生周期性变化,而水位滞留时间、流速等同样影响着土壤微生物及氮转化功能基因丰度变化[9],加剧了这些区域氮素转化过程及微生物变化的复杂性.前期研究发现,随着水位降低和土壤剖面持续干旱,参与反硝化过程的、、和基因丰度逐渐降低,而AOA-和AOB-基因丰度则逐渐增加,且基因丰度显著高于[10-12].但较多研究主要关注表层土壤功能基因丰度变化,而对于地下水位周期性波动引起的土壤剖面干湿交替和底物浓度变化对氮转化功能基因丰度的影响研究较少.
浅层地下水位波动引起的农田土壤剖面-地下水界面变化是氮素迁移转化活跃的关键地带,地下水位波动影响着氮素在土壤剖面中的滞留时间[13]、对流弥散、吸附解析、有机氮矿化、硝化和反硝化等过程[14-15],促进了水土界面间的氮素交换,使得土壤剖面与浅层地下水之间氮浓度呈显著正相关[16-17].浅层地下水位波动改变了氮形态及其浓度在土壤剖面中的空间分布,然而,不同地下水氮浓度及其水位波动是否会造成土壤剖面中氮转化功能基因丰度呈现出差异性变化仍不清楚.本文以洱海湖周农田土壤剖面为研究对象,通过微宇宙试验和PCR技术,研究了高、低氮浓度的浅层地下水,长期淹水与周期升降两种水位波动模式下土壤剖面氮浓度和氮转化功能基因丰度的变化,探究地下水氮浓度和水位波动模式对土壤氮转化功能基因丰度变化的主要驱动,以期为认识农田土壤剖面-地下水界面氮素生物地球化学循环过程提供科学支撑.
1 材料与方法
1.1 原状土柱取样
试验土柱取于洱海西岸湖滨区大庄蔬菜地(100°12′31″E,25°40′14″N),海拔1966m.气候类型为低纬亚热带高原季风气候,年均降雨量约为1100mm,降雨主要集中在6~11月(占年降雨量85%~90%),年均气温为15.7℃.同时,该区域属于典型集约化露地蔬菜种植区,一年平均种植蔬菜3茬,每茬蔬菜种植的肥料施用量为375kg N/hm2、165kg P2O5/hm2和1200kg/hm2有机肥料.农田土壤类型为水稻土,80年代后期开始种植蔬菜,土壤剖面按发生层分为4层:耕作层(A层,0~30cm)、犁底层(B层,30~45cm)、潴育层(C层,45~70cm)和潜育层(D层,>70cm),各层土壤特性见表1[7].前期调查发现,该区域浅层地下水平均总氮浓度为33.20mg/L[7],雨、旱季降雨差异造成的地下水位波动范围约为101cm[18].

表1 土壤剖面理化性质
使用直径30cm、高110cm的PVC管采集100cm深的原状土柱.首先,挖大约20cm深、直径略大于30cm的圆形土柱;然后将PVC管底部置于圆形土柱上,用橡皮锤敲击PVC管顶部,直至挖出的圆形土柱楔入PVC管中,依次重复上述过程,直到PVC管中土壤剖面达到100cm;最后在土柱底部放置一块直径29.5cm、厚1cm的透水石和孔径为2mm的尼龙网,并用盖子将PVC管底部和顶部密封,将土柱运至实验室,置于钢架上静置1周,使土体逐渐稳定.
1.2 试验处理与采样
微宇宙试验装置主要由原状土柱、进水口、出水口、供水桶、溶液收集桶和蠕动泵构成,进水口和出水口分别位于原状土柱底部的盖子和PVC管顶部的管壁,并通过硅胶管分别与蠕动泵和溶液收集桶连接;蠕动泵另一端通过硅胶管与供水桶连接.试验设3个处理:模拟浅层地下水氮浓度+水位升降处理(SND)、模拟浅层地下水氮浓度+持续淹水处理(SNF)、无氮添加+水位升降处理(0ND).模拟浅层地下水氮溶液(NH4+-N 0.5mg/L+NO3−-N 30mg/L)由KNO3、(NH4)SO4和蒸馏水配置.
试验开始时,在供水桶中加入配好的模拟浅层地下水溶液,将蠕动泵流速调节为7mL/min,之后打开蠕动泵将溶液通过进水口泵入土柱中,在整个淹水阶段,3个处理的土壤表层均保持薄薄的水层,超过该水层的溶液经管壁出水口排至溶液收集桶.整个试验周期为120d,SNF处理持续淹水120d,SND和0ND处理分两次干湿交替,每次干湿交替的试验周期为60d,其中前30d为淹水阶段,后30d为落干阶段.SND与0ND处理在落干阶段停止蠕动泵输送溶液,打开土柱底部入水口,使土柱内溶液慢慢渗出直至落干.每隔30d使用直径2cm的小型土钻对A、B、C和D层土壤进行取样(第1次记为FІ、第2次记为DІ、第3次记为FⅡ、第4次记为DⅡ),一份存储于4℃冰箱中用于测定土壤含水率(MC)、NH4+-N、NO3−-N和溶解性总氮(TDN),一份土样冻干后,存储于-80℃超低温冰箱中用于测定氮转化功能基因丰度.取完土壤剖面样后,用直径2cm的PVC管插入取样留下的洞中,防止土体破坏.
1.3 指标测定
土壤中NH4+-N、NO3−-N采用CaCI2溶液浸提-AA3连续流动分析仪测定(Bran+Luebbe,德国),TDN采用CaCI2溶液浸提,碱性过硫酸钾氧化-紫外分光光度法测定,MC采用烘干法测定.
称取0.5g冻干土壤样品,使用E.Z.N.A.®土壤DNA提取试剂盒(Omega Bio-tek, Norcross,美国)进行土壤DNA提取,采用1%琼脂糖凝胶电泳检测DNA的提取质量,使用NanoDrop2000测定DNA 浓度和纯度.使用实时荧光定量PCR检测仪(ABI7500,美国)测定土壤中(AOA-、AOB-)、(、)、、功能基因的丰度.目标基因引物、序列和片段大小见表2,定量在20.0μL反应体系中进行,反应体系为:ChamQ SYBR Color qPCR Master Mix(2X)16.4μL、模板DNA 2μL、引物F(5mmol/L)0.8μL、引物R(5mmol/L) 0.8μL.PCR热循环条件为:初级阶段3min,然后在95℃/5s、55℃/30s和72℃/1min进行40个循环,扩增效率范围为85%~100%,2³99%.

表2 qPCR目的基因扩增引物序列
1.4 数据处理
每个阶段土壤剖面氮浓度或功能基因丰度为4层土壤的平均值,SІ和SⅡ分别为试验前60d和后60d土壤剖面氮浓度或功能基因丰度的平均值,F和D分别为0~30d + 60~90d和30~60d + 90~120d土壤剖面氮浓度或功能基因丰度的平均值.使用SPSS 24.0进行正态分布和显著差异性(<0.05)检验, Origin 2019b进行绘图,RDA分析和SEM通过R中“vegan”和“lavaan”包执行.
2 结果与分析
2.1 土壤氮浓度变化
浅层地下水中不同氮浓度及其水位升降引起的干湿交替均会造成土壤剖面中不同形态氮浓度变化.随土壤剖面干湿交替,SND和0ND处理各土层中氮浓度均呈相同变化趋势,NH4+-N在淹水阶段逐渐增加和落干阶段逐渐降低,NO3−-N和TDN却呈相反变化(图1).随土壤剖面持续淹水,SNF处理中各土层NH4+-N浓度呈现出前60d逐渐增加和后60d逐渐降低,NO3−-N和TDN浓度呈现整体性持续下降.3个处理中各形态氮浓度均表现为A层>B层>C层>D层.SND和0ND处理土壤剖面氮浓度在相同阶段均呈显著差异(图1),与SND处理相比,0ND处理中NH4+-N、NO3−-N和TDN浓度在SI阶段分别显著(<0.05)降低15%、13%和5%,SⅡ阶段NO3−-N和TDN浓度显著(<0.05)降低15%和10%.SNF处理中NO3−-N和TDN浓度在F阶段显著(<0.05)降低9%和增加11%,而在D阶段NH4+-N浓度显著(<0.001)增加81%(<0.001),NO3−-N与TDN浓度则显著(<0.001)降低55%和50%.相比SND处理,SNF处理的土壤剖面NO3−-N和TDN浓度在整个试验过程中分别降低37%和29%,0ND处理分别降低14%和7%.
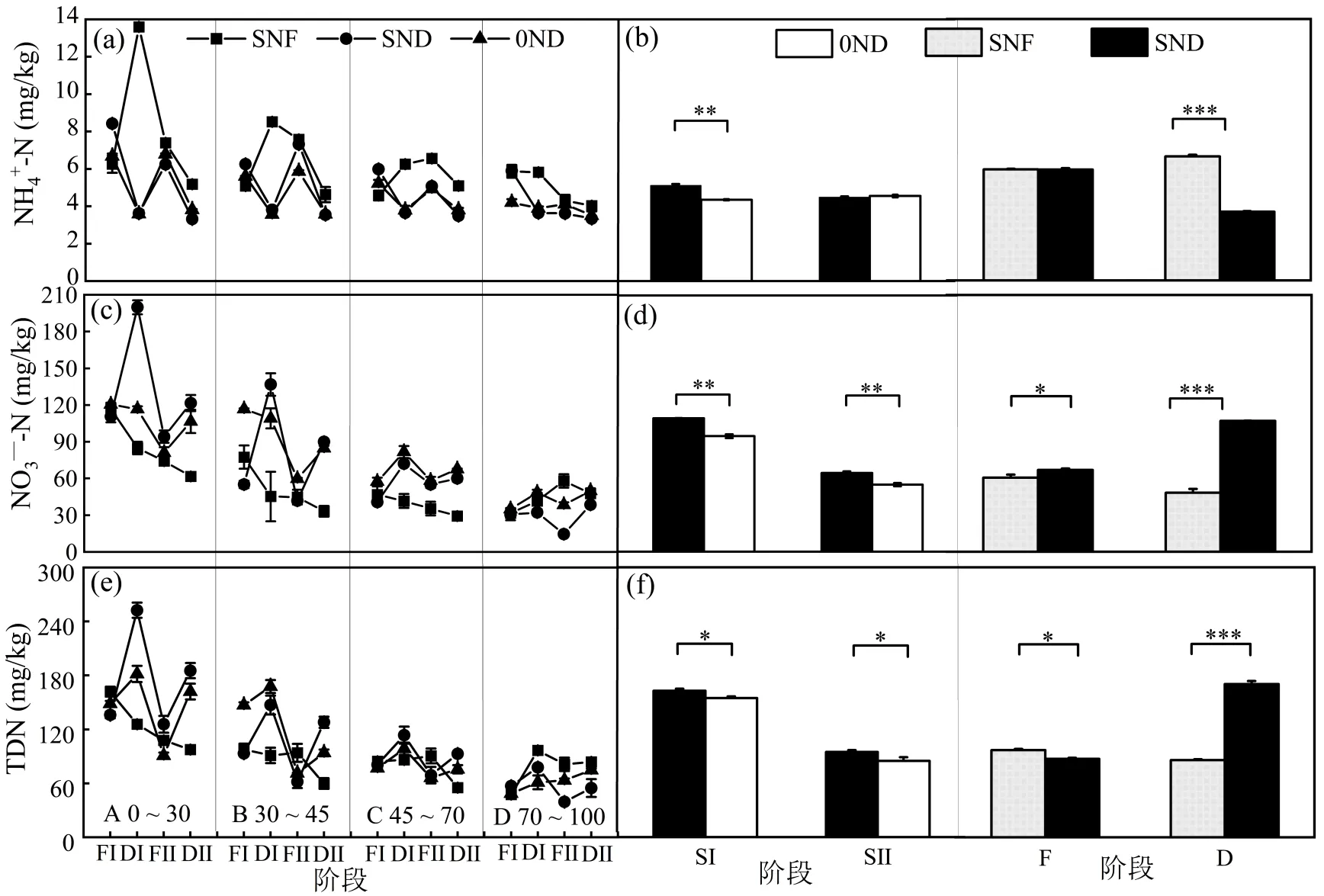
图1 土壤剖面氮浓度变化
(a)、(c)、(e)为不同处理相同土层的氮浓度变化,(b)、(d)、(f)为不同处理相同阶段的氮浓度变化;*表示处理间差异显著(*<0.05,**<0.01,***<0.001)
2.2 土壤氮转化功能基因丰度变化
浅层地下水中不同氮浓度及其水位升降引起的土壤干湿交替均会造成土壤剖面中氮转化功能基因丰度变化.随土壤剖面持续淹水,SNF处理的各土层中、、和丰度呈整体下降(图2);而随干湿交替,SND和0ND处理各土层中丰度表现为在淹水阶段下降而落干阶段增加,丰度呈整体下降;SND处理B、C、D层中、丰度在淹水阶段下降而落干阶段增加,0ND的B、D层中丰度也呈相同变化,其它土层中、丰度呈整体下降.3个处理的土层中功能基因丰度均表现为A层>B层>C层>D层.与SND处理相比(图2),0ND处理中、、丰度在SI阶段分别降低21%、39%和12% (<0.05),丰度增加69%(<0.05);SⅡ阶段和丰度降低19%和28%(<0.05),和丰度增加248%和21%(<0.001).水位升降引起的土壤干湿交替同样改变了氮转化功能基因丰度,与SND处理相比,SNF处理土壤剖面中和丰度在F阶段增加49%和53%(<0.05),和丰度降低7%和6%;而、和丰度在D阶段分别降低52%、38%和56%(<0.01),丰度增加41%(<0.05).与整个试验过程中SND处理功能基因丰度相比,SNF和0ND处理土壤剖面厌氧氨氧化功能基因丰度分别增加68%和7%,反硝化功能基因丰度则分别降低20%和1%,硝化功能基因丰度则在SNF处理中降低34%,而0ND处理则增加了23%.

图2 土壤剖面氮转化功能基因丰度变化
(a)、(c)、(e)、(g)为不同处理相同土层的功能基因丰度变化,(b)、(d)、(f)、(h)为不同处理相同阶段的功能基因丰度变化,*表示处理间差异显著(*<0.05,**<0.01,***<0.001)
3 讨论
3.1 浅层地下水中氮浓度和土壤干湿交替对土壤剖面氮浓度的影响
随土壤深度增加,土壤中有机物质会逐渐减少,微生物活性降低[19-20],造成各土层中氮浓度随剖面深度增加而降低.已有研究表明[6-7,9],水位滞留时间、地下水中氮浓度均与土壤氮浓度和流失量存在显著相关性,这在本研究结果中也被证明.在淹水阶段,各处理土层中NH4+-N逐渐升高,NO3−-N逐渐降低,这归因于:1)淹水造成土壤剖面形成厌氧环境,抑制了硝化微生物对NH4+-N的消耗,促进了反硝化微生物活性和加快对NO3--N的消耗[21];2)厌氧环境下异化硝酸盐还原为铵(DNRA)过程变得极为活跃,促进了土壤中NO3−-N转化为NH4+-N[9],同时,厌氧环境也促进了土壤有机氮矿化[22],土壤NH4+-N累积增加而消耗降低导致淹水阶段各土层中NH4+-N累积量增加,土层中NO3−-N却相反.然而,落干阶段,由于土壤逐渐由厌氧环境转变为好氧环境,提高了土壤硝化微生物活性,促进NH4+-N转化为NO3−-N[23],同时,反硝化及DNRA过程受到抑制[21],导致淹水阶段土壤剖面中累积的NH4+-N被消耗而NO3−-N逐渐累积.此外,通过SEM分析也发现(图3),MC分别与NH4+-N、NO3−-N和TDN存在直接显著(<0.05)正效应,这也证明土壤剖面持续淹水或干湿交替均显著影响氮形态浓度.这些原因造成SNF处理土壤剖面中NH4+-N浓度在整个试验过程中均高于SND,而NO3−-N浓度则相反.SNF处理持续淹水60d后,各土层NH4+-N浓度逐渐下降,可能是由于持续淹水抑制土壤有机氮矿化[24]和刺激了厌氧氨氧化微生物活性,促进NH4+-N转化为N2[25].此外,与SNF处理相比,SND处理的土壤剖面氮浓度在淹水与落干阶段波动幅度更大,这表明干湿交替加速了土壤剖面氮转化[26-27],主要因为干湿交替加速土壤剖面于好氧-兼氧-厌氧环境中不断循环,刺激了好氧或厌氧微生物活性[28],致使土壤剖面氮素不断转化和相互增加反应底物氮浓度.与0ND处理相比,SND处理中土壤剖面各氮形态浓度显著较高,一方面原因是地下水中NH4+-N很容易被土壤吸附[29],而NO3−-N虽然不易被土壤吸附,但外源氮大量输入,激发厌氧微生物利用外源氮来维持自身的代谢活动[30],很大程度上削减了SND处理的土壤剖面氮流失;另一方面是水-土中氮浓度存在较大的浓度差,低氮浓度的地下水与高氮浓度的土壤剖面相互作用,加速了氮从土壤剖面向地下水中释放,从而0ND处理的土壤剖面氮浓度显著降低.总体来说,无论持续淹水或干湿交替,土壤剖面NO3−-N和TDN浓度均呈下降趋势,这表明地下水位波动能够加速土壤剖面溶解性氮流失.
3.2 水位波动对土壤剖面氮转化功能基因丰度的影响
水位波动造成土壤剖面土壤氧化还原环境、氮浓度和含水率等发生变化[10-11,31],土壤底物碳氮浓度[32-33]、氧供应[34]、土壤理化性质[35]等重要因子主要通过影响氮转化功能基因丰度变化,进而影响氮的转化过程.通过RDA分析发现(图3),土壤NH4+- N、NO3−-N、TDN和MC是土壤氮转化功能基因丰度变化的主要驱动因子,SNF、SND和0ND处理的前两轴分别解释了95.5%、98.3%和99.8%的氮功能基因丰度变化.各土层中氮转化功能基因丰度随剖面深度增加而降低,这归因于土壤剖面中碳氮浓度和氧扩散能力随土壤深度增加而逐渐降低[36].通常,淹水可增加土壤孔隙中持水量和降低土壤剖面中溶解氧浓度,当溶解氧浓度低于2mg/L时[8],有利于形成反硝化发生的厌氧环境,与基因丰度理论应该增加.但研究发现,土壤剖面氮浓度整体呈现出的下降趋势与和基因丰度变化也一致,水位升降造成的土壤剖面NO3−-N浓度变化才是导致SND与0ND处理的土层中和基因丰度变化的主要原因,SEM分析结果也表明(图3),SND与0ND处理中NO3−-N也分别与和呈现出直接的正效应(<0.05),这说明NO3−-N作为反硝化过程的反应底物,其浓度高低也影响反硝化作用和氮转化功能基因丰度[37-38].相比0ND处理,SND处理的水中较高的NO3−-N浓度为土壤反硝化提供了外源氮,降低土壤剖面中NO3−-N流失,加之土壤孔隙水中NO3−-N浓度也是控制反硝化的关键因素[39],以至于SND处理中与基因丰度在SI和SⅡ阶段均显著高于0ND处理.SNF处理土壤剖面中与基因丰度呈持续下降趋势,在F和D阶段与SND处理均呈现出显著差异,且MC对、均有直接显著正效应(<0.01),这说明SNF处理中MC显著影响和丰度;而与NO3−-N浓度表现出微弱的负相关,这与Dandie等[40]研究一致,但并不能否认土壤剖面中NO3−-N浓度对其没有影响,长期淹水可能导致反硝化微生物所需的碳供应不足,抑制了反硝化酶活性.此外,F阶段SNF处理中和丰度比SND处理显著增加49%和53%,而D阶段则分别显著降低38%和56%.这是由于SNF处理处于持续淹水环境,更有利于促进反硝化微生物生长,但D阶段SND处理中累积的NH4+-N转化为NO3−-N,底物浓度增加刺激了反硝化微生物活性,提高了和丰度,这也表明水位升降引起的土壤氮浓度变化对和丰度起主导作用.然而,土壤干湿交替却显著影响各处理中丰度,SEM分析发现,3个处理中MC对均有直接的显著正效应(<0.05),并通过调控TDN和NO3−-N间接影响(<0.05),这表明MC是丰度变化的主要驱动因子.这主要由于AOA和AOB为好氧微生物,干湿交替造成的土壤氧化还原环境和土壤水分变化更有利于刺激硝化酶活性;另一方面,AOA和AOB对环境适应偏好并不同,如AOA更能适应低氧、酸性、低NH4+-N浓度环境[41],两者对环境的偏好可能掩盖了底物氮浓度的重要性.在本研究中,各处理丰度整体均呈现出持续下降趋势,这有两方面原因,一是土壤剖面NH4+-N较培养前增加,这增加了厌氧氨氧化电子供体,但NO3−-N浓度降低导致反硝化底物浓度缺乏,限制了NO2−-N的形成,从而造成厌氧氨氧化电子受体供应不足,限制了厌氧氨氧化酶活性[42];二是SND与0ND处理落干阶段形成好氧环境,并不利于厌氧氨氧化过程发生,通过SEM分析也发现MC均与呈现显著正效应(<0.05),这在D阶段SND处理中丰度显著低于SNF处理也得以体现.综上,浅层地下水升降及其水中氮浓度分别引起土壤剖面干湿交替和氮浓度变化,两者共同驱动土壤剖面氮转化功能基因丰度的变化,且浅层地下水中氮浓度影响强度更大,因为与SND处理相比,0ND处理土壤剖面氮转化功能基因丰度变化率远高于SNF处理.

图3 土壤氮转化功能基因丰度与环境因子的冗余度分析(RDA)和结构方程(SEM)
SEM中黑色和灰色箭头分别表示正效应和负效应,实线和虚线表示路径系数的显著和不显著,线宽度表示显著性程度(*<0.05,**<0.01,***<0.001)
4 结论
4.1 浅层地下水中的氮浓度及其水位波动显著影响土壤剖面中氮浓度,持续淹水和低氮浓度地下水波动将显著降低土壤剖面溶解性氮浓度.与初始阶段土壤剖面中溶解性氮浓度相比,常规氮浓度地下水波动下土壤剖面中NO3−-N和TDN分别下降28%和21%,无氮浓度地下水波动下NO3−-N和TDN下降率增加至38%和30%,持续淹水条件下土壤剖面NO3−-N和TDN下降率高达55%和44%.
4.2 地下水位波动及水中氮浓度引起土壤剖面干湿交替和氮浓度变化,共同驱动着土壤剖面氮转化功能基因丰度变化,且浅层地下水中氮浓度影响强度远高于水位波动.持续淹水和无氮浓度地下水波动条件下土壤剖面厌氧氨氧化功能基因丰度与常规氮浓度地下水波动相比,分别增加7%和68%,反硝化功能基因丰度则分别降低1%和20%,硝化功能基因丰度分别增加23%和降低34%.
[1] Kuypers M M M, Marchant H K, Kartal B. The microbial nitrogen-cycling network [J]. Nature Reviews Microbiology, 2018,16: 263–276.
[2] Yang X L, Lu Y L, Yan D, et al. Optimising nitrogen fertilisation: a key to improving nitrogen-use efficiency and minimising nitrate leaching losses in an intensive wheat/maize rotation (2008~2014) [J]. Field Crop Research, 2017,206:1–10.
[3] 姜姗姗,庞炳坤,张敬沙,等.减氮及不同肥料配施对稻田CH4和N2O排放的影响 [J]. 中国环境科学, 2017,37(5):1741–1750.
Jiang S S, Pang B K, Zahng J S, et al. Effects of reduced nitrogen and combined application of different fertilizers on CH4and N2O emissions in paddy fields [J]. China Environmental Science, 2017,37(5):1741– 1750.
[4] 刘 鑫,左 锐,孟 利,等.地下水位上升过程中硝态氮(硝酸盐)污染变化规律研究 [J]. 中国环境科学, 2021,41(1):232–238.
Liu X, Zuo R, Meng L, et al. Study on the variation law of nitrate pollution during the rise of groundwater level [J]. China Environmental Science, 2021,41(1):232–238.
[5] Arp D J, Stein L Y. Metabolism of inorganic N compounds by ammonia-oxidizing bacteria [J]. Critical Reviews in Biochemistry and Molecular Biology, 2003,38(6):471–495.
[6] Chen A Q, Zhang D, Wang H Y, et al. Shallow groundwater fluctuation: An ignored soil N loss pathway from cropland [J]. Science of the Total Environment, 2022,828:154554.
[7] Cui R Y, Zhang D, Liu G C, et al. Shift of lakeshore cropland to buffer zones greatly reduced nitrogen loss from the soil profile caused by the interaction of lake water and shallow groundwater [J]. Science of the Total Environment, 2022,803:150093.
[8] Liu X Y, Hu S H, Sun R, et al. Dissolved oxygen disturbs nitrate transformation by modifying microbial community, co-occurrence networks, and functional genes during aerobic-anoxic transition [J]. Science of the Total Environment, 2021,790:148245.
[9] Liu Y Y, Liu C X, Nelson W C, et al. Effect of water chemistry and hydrodynamics on nitrogen transformation activity and microbial community functional potential in Hyporheic Zone sediment columns [J]. Environmental Science & Technology, 2017,51(9):4877–4886.
[10] Zhang D, Cui R Y, Fu B, et al. Shallow groundwater table fluctuations affect bacterial communities and nitrogen functional genes along the soil profile in a vegetable field [J]. Applied Soil Ecology, 2020,146: 103368.
[11] 崔荣阳,雷宝坤,张 丹,等.浅层地下水升降对菜地土壤剖面硝化/反硝化微生物丰度的影响[J]. 环境科学学报, 2019,39(9):3099–3106.
Cui R Y, Lei B K, Zhang D, et al. Effects of shallow groundwater fluctuations on the abundances of nitrification and denitrification microbes in the soil profile of vegetable field [J]. Acta Scientiae Circumstantiae, 2019,39(9):3099–3106.
[12] 孙翼飞,沈菊培,张翠景,等.模拟水位下降与刈割对高寒湿地土壤氨氧化与反硝化微生物的影响[J]. 农业环境科学学报, 2017,36(11): 2356–2364.
Sun Y F, Shen P J, Zhang C J, et al. Effects of water table lowering and mowing on soil ammonia oxidizers and denitrifiers in alpine wetlands [J]. Journal of Agro-Environment Science, 2017,36(11):2356–2364.
[13] Gartner J D, Renshaw C E, Dade W B, et al. Time and depth scales of fine sediment delivery into gravel stream beds: Constraints from fallout radionuclides on fine sediment residence time and delivery [J]. Geomorphology, 2012,151–152:39–49.
[14] Böhlke K, Antweller R C, Harvey J W, et al. Multi-scale measurements and modeling of denitrification in streams with varying flow and nitrate concentration in the upper Mississippi River basin, USA [J]. Biogeochemistry, 2009,93(1/2):117–141.
[15] Landon M K, Green C T, Belitz K, et al. Relations of hydrogeologic factors, groundwater reduction-oxidation conditions, and temporal and spatial distributions of nitrate, Central-Eastside San Joaquin Valley, California, USA [J]. Hydrogeology Journal, 2011,19(6):1203–1224.
[16] Zhang D, Fan M P, Liu H B, et al. Effects of shallow groundwater table fluctuations on nitrogen in the groundwater and soil profile in the nearshore vegetable fields of Erhai Lake, southwest China [J]. Journal of Soils Sediments, 2020,20:42–51.
[17] Rivett V, Buss S R, Morgan P, et al. Nitrate attenuation in groundwater: a review of biogeochemical controlling processes [J]. Water Research, 2008,42:4215–4232.
[18] 李桂芳,杨 恒,叶远行,等.高原湖泊周边浅层地下水:氮素时空分布及驱动因素[J]. 环境科学, 2022,43(6):3027–3036.
Li G F, Yang H, Ye Y H, et al. Shallow groundwater around plateau lakes: spatiotemporal distribution of nitrogen and its driving factors [J]. Environmental Science, 2022,43(6):3027–3036.
[19] Wang S, Zhuang Q, Wang Q, et al. Mapping stocks of soil organic carbon and soil total nitrogen in Liaoning Province of China [J]. Geoderma, 2017,305:250–263.
[20] Liu Z, Shao M, Wang Y. Spatial patterns of soil total nitrogen and soil total phosphorus across the entire Loess Plateau region of China [J]. Geoderma, 2013,197–198:67–78.
[21] Szukics U, Abell G C J, Hödl V, et al. Nitrifiers and denitrifiers respond rapidly to changed moisture and increasing temperature in a pristine forest soil [J]. FEMS Microbiology Ecology, 2010,72(3):395– 406.
[22] Jia J, Bai J H, Gao H F, et al. In situ soil net nitrogen mineralization in coastal salt marshes (Suaeda salsa) with different flooding periods in a Chinese estuary [J]. Ecological Indicators, 2017,73:559–565.
[23] Li X, Li J, Xi B D, et al. Effects of groundwater level variations on the nitrate content of groundwater: a case study in Luoyang area, China [J]. Environmental Earth Sciences, 2015,74(5):3969–3983.
[24] Song G, Zhao X, Wang S Q, et al. Dissolved organic nitrogen leaching from rice-wheat rotated agroecosystem in southern China [J]. Pedosphere, 2015,25(1):93–102.
[25] Medinets S, Skiba U, Rennenberg H, et al. A review of soil NO transformation: associated processes and possible physiological significance on organisms [J]. Soil Biology Biochemistry, 2015,80:92– 117.
[26] Krüger M, Potthast K, Michalzik B, et al. Drought and rewetting events enhance nitrate leaching and seepage-mediated translocation of microbes from beech forest soils [J]. Soil Biology and Biochemistry, 2021,154:108153.
[27] Gao D C, Bai E, Li M H, Zhao C H, et al. Responses of soil nitrogen and phosphorus cycling to drying and rewetting cycles: a meta- analysis [J]. Soil Biology Biochemistry, 2020,148:107896.
[28] Sun D S, Li K J, Bi Q F, et al. Effects of organic amendment on soil aggregation and microbial community composition during drying- rewetting alternation [J]. Science of the Total Environment, 2017,574: 735–743.
[29] Huang P, Zhang J B, Zhu A N, et al. Nitrate accumulation and leaching potential reduced by coupled water and nitrogen management in the Huang-Huai-Hai Plain [J]. Science of the Total Environment, 2018, 610–611:1020–1028.
[30] Walton C R, Zak D, Audet J, et al. Wetland buffer zones for nitrogen and phosphorus retention: impacts of soil type, hydrology and vegetation [J]. Science of the Total Environment, 2020,727:138709.
[31] Peralta A L, Ludmer S, Matthews J W, et al. Bacterial community response to changes in soil redox potential along a moisture gradient in restored wetlands [J]. Ecological Engineering, 2014,73:246–253.
[32] Palta M M, Ehrenfeld J G, Groffman P M. “Hotspots” and “Hot Moments” of denitrification in urban brownfield wetlands [J]. Ecosystems, 2014,17(7):1121–1137.
[33] Hayakawa A, Nakata M, Jiang R, et al. Spatial variation of denitrification potential of grassland, windbreak forest, and riparian forest soils in an agricultural catchment in eastern Hokkaido, Japan [J]. Ecological Engineering, 2012,47:92–100.
[34] Zhang J, Cai Z, Cheng Y, et al. Denitrification and total nitrogen gas production from forest soils of Eastern China [J]. Soil Biology Biochemistry, 2009,41(12):2551–2557.
[35] Deng M, Kimura S D, Lee J, et al. Denitrification on Andosols in an intensive dairy farming region of central Japan [J]. Agriculture Ecosystems Environment, 2011,144(1):330–337.
[36] Zhu G, Wang S, Li Y, et al. Microbial pathways for nitrogen loss in an upland soil [J]. Environmental Microbiology, 2018,20(5):1723–1738.
[37] Song K, Lee S H, Mitsch W J, et al. Different responses of denitrification rates and denitrifying bacterial communities to hydrologic pulsing in created wetlands [J]. Soil Biology and Biochemistry, 2010,42(10):1721–1727.
[38] van Kessel M A, Speth D R, Albertsen M, et al. Complete nitrification by a single microorganism [J]. Nature 2015,528(7583):555–559.
[39] Hou J, Cao X, Song C, et al. Predominance of ammonia-oxidizing archaea and nirK-gene-bearing denitrifiers among ammonia- oxidizing and denitrifying populations in sediments of a large urban eutrophic lake (Lake Donghu) [J]. Canadian Journal of Microbiology, 2013,59(7):456–464.
[40] Dandie C E, Burton D L, Zebarth B J, et al. Changes in denitrifier community abundance over time in an agricultural fied and their relationship with denitrification activity [J]. Applied and Environmental Microbiology, 2008,74(19):5997–6005.
[41] Shen J P, Zhang L M, Di H J, et al. A review of ammonia-oxidizing bacteria and archaea in Chinese [J]. Frontiers in Microbiology, 2012, 3:296.
[42] Qian G, Wang J, Kan J, et al. Diversity and distribution of anammox bacteria in water column and sediments of the Eastern Indian Ocean [J]. International Biodeterioration & Biodegradation, 2018,133:52–62.
Effects of water table fluctuations and nitrogen concentration variations on the abundances of nitrogen-transforming functional genes in soil profiles.
CUI Rong-yang1,2, LIU Gang-cai1, HU Wan-li3, FU Bin3, CHEN An-qiang3*
(1.Key Laboratory of Mountain Surface Processes and Ecological Regulation, Chinese Academy of Sciences, Institute of Mountain Hazards and Environment, Chinese Academy of Sciences and Ministry of Water Conservancy, Chengdu 610041, China;2.University of Chinese Academy of Science, Beijing 100049, China;3.Agricultural Environment and Resources Institute, Yunnan Academy of Agricultural Science, Kunming 650201, China)., 2022,42(11):5378~5386
To explore the effects of nitrogen concentration in shallow groundwater and its water table fluctuations on the abundance of soil nitrogen-transforming functional genes, taking the undisturbed soil profile from cropland around Erhai as the object, changes in nitrogen concentrations and abundance of nitrogen-transforming functional genes in soil profiles under shallow groundwater table fluctuations (SND) and continuous flooding (SNF) with conventional nitrogen concentration, and shallow groundwater table fluctuations (0ND) without nitrogen were studied. The relationship between soil environmental factors and abundance of functional genes was discussed. The results indicated that, compared with the nitrogen concentrations in soil profile before the microcosmic experiment, the total dissolved nitrogen (TDN) concentrations in SNF, SND and 0ND decreased by 44%, 21% and 30%, and NO3−-N concentrations decreased by 55%, 28% and 38%, respectively. Meanwhile, compared with the abundance of nitrogen-transforming functional genes in soil profile in SND, the denitrification function gene abundances in 0ND and SNF decreased by 20% and 1%, while the anammox function gene abundances increased by 68% and 7%, and the nitrification function gene abundances decreased by 34% and increased by 23%, respectively. Changes in functional gene abundances were mainly driven by soil moisture content (MC), NH4+-N, NO3−-N and TDN. In conclusion, continuous flooding in soil profiles would significantly reduce dissolved nitrogen concentrations, and changes in alternation of drying-flooding and nitrogen concentrations in soil profile caused by the nitrogen concentrations in shallow groundwater and its water table fluctuations were the main drivers for changes in the abundance of nitrogen-transforming functional genes.
nitrogen-transforming functional gene;soil profile;alternation of drying-flooding;shallow groundwater table fluctuation
X172;X523
A
1000-6923(2022)11-5378-09
崔荣阳(1993-),男,云南昆明人,中国科学院、水利部成都山地灾害与环境研究所博士研究生,主要从事土壤氮素迁移转化及其环境效应研究.发表论文13篇.
2022-04-15
国家自然科学基金资助项目(41977319,42067052);云南省科技人才与平台计划项目(202205AM070002);云南省财政厅专项(530000221100000648476)
* 责任作者, 研究员, chaq163@163.com

