植物对PFAS的富集机制研究进展
何 强,颜 正,智 悦,钱深华,王小铭,陈 一,刘彩虹,程 呈,胡学斌
植物对PFAS的富集机制研究进展
何 强,颜 正,智 悦*,钱深华,王小铭,陈 一,刘彩虹,程 呈,胡学斌
(重庆大学,三峡库区生态环境教育部重点实验室,重庆 400045)
本文通过文献梳理,分析了全(多)氟烷基化合物(PFAS)对植物的暴露途径;统计了已发表文献中36种植物对19种PFAS的转运、富集特征;系统地阐释了PFAS从环境介质到植物组织内的迁移、积累机制;讨论了PFAS分子结构(如全氟碳链长度、头部官能团)、植物生理特性、环境因素对该富集过程的影响,并提出了未来有关植物富集PFAS可关注的重点和方向,以期能深入认识PFAS在环境介质-植物根际-植物组织内的赋存与迁移转化特征,更好地管控评估PFAS污染场地并制定植物修复方案,为开展生态与健康风险评价提供参考.
全(多)氟类化合物;PFAS;植物;转运;富集
全(多)氟烷基化合物(PFAS)是一类高度氟化的有机物,具有优良的表面活性、疏水疏油性能、化学惰性和耐高温能力[1-2],已被广泛应用于工业生产和日常生活中[3-6].近10a来,PFAS在全球范围内各种环境介质中被广泛检出,包括大气、水、土壤、沉积物、植物、动物(如南极企鹅,海鸥等)和极地冰盖,发达国家PFAS污染比发展中国家更为严重[7-13].目前人体血液和尿液中已经多种PFAS[14-15],其中全氟辛烷磺酸(PFOS)和全氟辛烷羧酸(PFOA)在人体内半衰期平均值分别为3.4a和2.7a[15].毒理学研究表明PFAS会对人体产生多种毒性作用,包括肾脏、免疫系统、神经、发育和内分泌干扰毒性[16-19].
由于PFAS特殊的理化性质,其在进入环境中后可远距离输送[20-22].PFAS可以通过大气干湿沉降、地表径流和固废(如工业固废、生活垃圾、污泥)处置等多种途径进入土壤造成污染[23-25],因此土壤是PFAS重要的“汇”.赋存于土壤中的PFAS可从土壤颗粒脱附、迁移、转运、富集于植物组织,并通过陆生食物链传递和生物放大,危害生态系统和人类健康[26].通常污染场地中的PFAS污染面积广、浓度痕量、环境持久(难以被光解、水解、生物降解)[27-29],故PFAS污染场地修复技术已成为亟待解决的挑战.植物修复是一种低成本、低扰动、低二次污染的原位场地修复技术,已被证明是可用于痕量、微量PFAS污染治理的处理技术之一[30-31].然而目前对于植物吸收PFAS的研究集中于农作物等可食用植物,研究的重点围绕在食物链富集和放大以及生态环境风险.研究的污染物对象集中在全氟烷基羧酸和磺酸(PFCA和PFSA)两类,尤其是PFOA和PFOS,而对于其他复杂分子结构PFAS的植物富集行为还鲜有报道.植物特性、环境因素对植物转运、富集PFAS的影响尚不明确.本文对已发表的文献数据进行了梳理,统计了植物对包括PFCA、PFSA、全氟聚醚(PFEA)在内的多种PFAS的生物富集行为;阐释了PFAS在植物体内的积累富集机制;讨论了PFAS分子结构、植物生理特性、环境因素对生物富集过程的影响,并提出了未来有关植物富集PFAS研究可以关注的重点和方向.以期为全面认识PFAS在土-植物体系中的赋存状况与迁移转化机制、更好地管控评估PFAS污染场地制定植物修复方案以及为生态与健康风险评价提供参考.
1 PFAS对植物的暴露途径
PFAS对植物的暴露途径包括由植物根系暴露在PFAS污染地表径流或污染土壤,或者植物叶片暴露于大气中携带PFAS的颗粒和水滴[23,32-34].土壤中PFAS的来源包括大气沉降、地表径流和生活垃圾或污水厂活性污泥的堆放、垃圾场渗滤液(特别是食品包装材料、消防防火服等垃圾)等多种污染途径[35-37].具体来说,由于PFAS难以被传统污水处理工艺降解和去除[22,36,38-39],通过污水管网进入到污水厂的生产废水及医疗废水中的大量PFAS无法被降解和去除,其大部分会沉积、吸附在活性污泥相.部分污泥会作为土壤改良剂被回用于农田[39-41],土壤改良剂投入农田后其中的PFAS首先解吸、脱附,再重新吸附于表层土壤颗粒,并随灌溉水向土壤深处渗流.垃圾渗滤液也是PFAS进入土壤和水环境的一种重要载体,其可以通过直接(渗滤液泄露或穿透防渗层)或间接(渗滤液经污水处理后排放)的方式被排入环境[42],进而暴露于植物根系.
空气中以蒸汽相和颗粒相呈现的PFAS及其前体物可以吸附到植物叶片和树皮等部位并被植物吸收,即从周围空气中直接吸收气相化学物质或通过质量交换的方式吸收[43-44].也可通过大气沉降以雨雪水的方式被带回陆地表面,进入土壤水体污染植物根系[45-46].有报道称垃圾填埋场、氟化物加工厂中的挥发性和半挥发性PFAS前体物可能会逸入到大气中.在此过程中,离子型PFAS前体如氟调聚物醇(FTOH)、全氟辛烷磺酰胺(FOSA)和全氟辛烷磺酰胺乙醇(FOSE),先通过空中运输,后被植物叶片吸收,再在植物体内被进一步代谢为PFCA和PFSA[47]. Tian等[24]分析了天津垃圾填埋场附近的植物中PFAS的浓度水平和分布情况,证实植物叶片能有效吸收空气中多种PFAS,包括近来受到广泛关注的PFAS前体物多氟烷基磷酸二酯(diPAPS),植物叶片被发现能有效地反映PFAS环境污染情况,因此可作为一种被动采样方法,用于监测植物点源附近的空气质量.
2 植物对PFAS的生物富集
2.1 植物对PFAS的生物富集浓度
目前研究最广泛的PFAS种类是PFCA和PFSA 2个系列,分别被研究了108次和84次,前期研究集中于PFOA(C7,-COOH)和PFOS(C8,-SO3H)两种物质.36种实验植物包括草本植物及木本植物.表1列出了植物对PFAS的富集浓度水平.针对已报道的36种植物经由暴露培养后对19种PFAS均有生物富集(表1).
总结前期研究结果发现,对于同种植物,PFAS的全氟碳链链长和头部官能团均会影响其在植物体内的转运和富集.如木贼()对全氟己烷羧酸(PFHxA,C5,-COOH)的富集浓度高达2.4×104ng/g,但对全氟己烷磺酸(PFHxS,C6,-SO3H)的富集浓度只有2.8×102ng/g;黑柳()对全氟戊烷羧酸(PFPeA,C4,-COOH)的富集浓度为3.2×104ng/g,而对PFOA(C7,-COOH)的富集浓度仅为3.7×103ng/g.除了PFCA和PFSA外,Zhang等[48]研究了包含全氟-3-甲氧基丙酸(PFMoPrA)、全氟-4-甲氧基丁酸(PFMoBA)、全氟(2-甲基-3-氧代己酸)铵(GenX)、4,8-二氧-3氢-全氟壬酸铵(ADONA)、氯代多氟烷基醚磺酸盐(F-53B)在内的PFEA,相比于PFCA和PFSA,长毛苔草()作为研究对象对5种PFEA的富集浓度范围仅为8.0~1.3×102ng/g,而同为莎草科的纸莎草()对PFCA和PFSA则显示出更高的富集能力,对PFOA和PFOS的富集浓度分别达到3.4×103ng/g和1.2×104ng/g,然而,目前对PFEA的研究数据还相对较少,且暴露时间、环境因素(温度、光照、湿度等)、培养介质中的初始浓度等均对富集效果有显著影响.相比于典型PFAS,新型PFAS醚基类替代品是否具有相似的生物富集行为亟需更多数据支持.
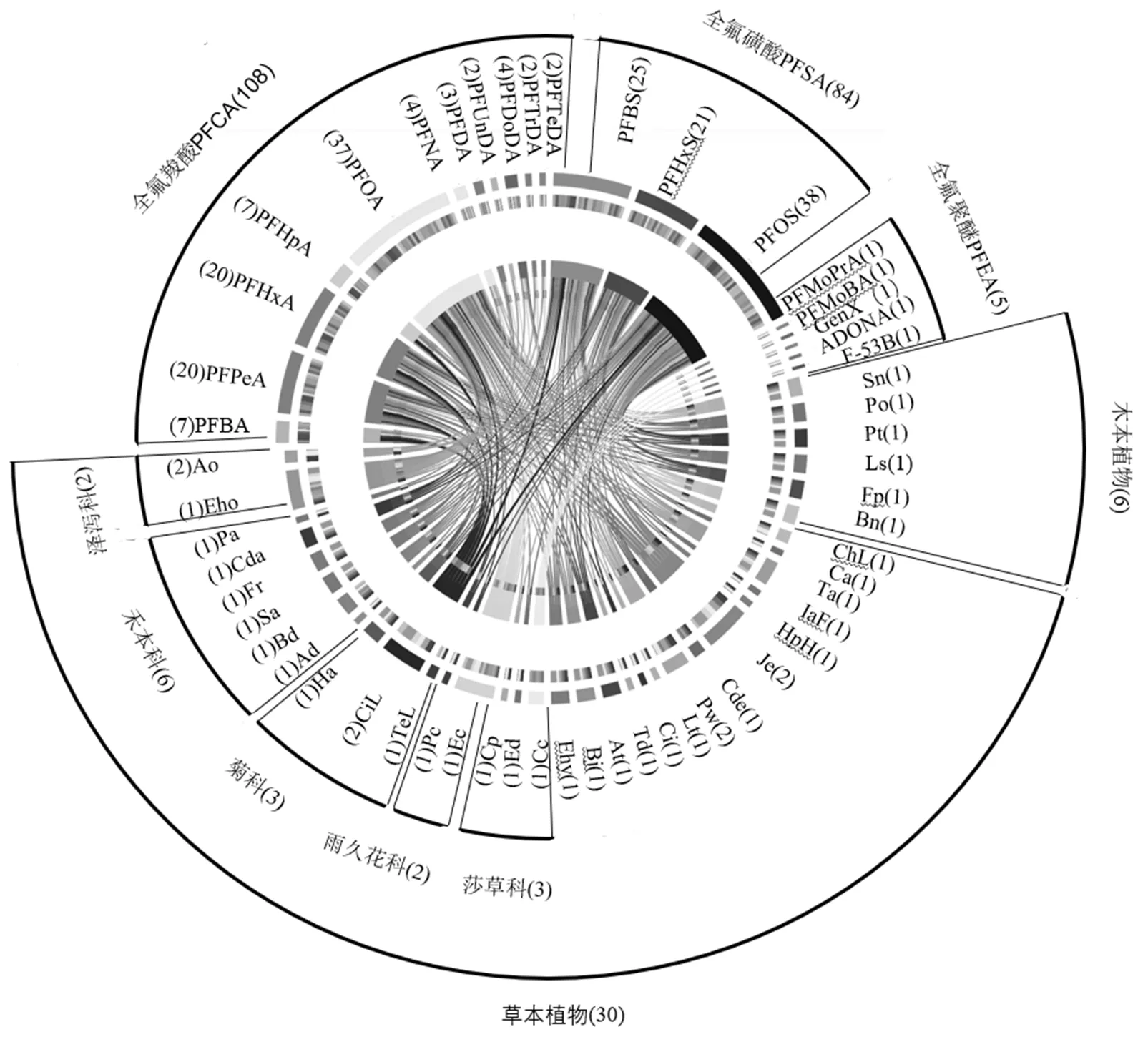
图1 植物对PFAS的生物富集数据汇总
已报道数据截至2021年4月,色带的厚度表示所对应的PFAS或植物的研究类型多少,植物或者PFAS前、后的数字表示其被研究的频率
比较植物对PFAS生物富集水平可筛选出具有植物修复潜力的PFAS高耐性物种.已发表文献中,植物对PFAS生物富集浓度范围为1.4~8.8×105ng/g, 不同植物科属对PFAS的富集能力差异较大(表1).其中灯芯草(灯芯草科)显示出最高的富集能力(8.8×105ng/g,干重),其次是大安水蓑衣(爵床科)4.6×104ng/g (干重),皆远超其他植物种类.相较而言,富集能力最低的物种为万寿菊(菊科)和线叶水马齿(水马齿科),其对PFAS的富集能力仅为1.40~95.00ng/g(干重)和6.56~ 14.16ng/g(干重).由表1可知,针对禾本科类植物研究报道最多,且其具有较高的PFAS生物富集能力,6种禾本科类植物(芦苇、狗牙根、紫阳茅、水茅、双雄雀麦和芦竹)对7种PFAS(全氟丁烷羧酸PFBA、PFPeA、PFHxA、PFOA、全氟葵烷羧酸PFDA、全氟丁烷磺酸PFBS、PFHxS和PFOS)的富集浓度在2.2×102~3.4×104ng/g之间.包含玉米、水稻、燕麦在内的禾本科植物已被证明对重金属[49]、有机物(如多环芳烃[50])及石油污染土壤[51]有高生物量耐性,其可能是因为禾本科植物的根系具有优良的根系形态,可以通过根系固定、根系吸收和传输、植物挥发、植物降解及根系微生物降解过程去除PFAS.
相比于草本植物,目前对木本植物的研究较少(图1),木本植物由于其多年生,根茎粗壮,木质部发达的特点,也具有PFAS污染场地修复的潜力. Gobelius和Huff调研了瑞典斯德哥尔摩机场附近消防训练场[含PFAS的水性灭火泡沫(AFFF)污染场地]植物群落的不同植物物种即白桦()、挪威云杉()、鸟樱桃()、山灰()、地接骨木()、长山毛榉()和野草莓()对PFAS的富集行为.其发现白桦叶和云杉针叶中PFAS的浓度分别高达97ng/g和94ng/g(湿重),单棵树可以分别富集高达11mg和1.8mg的总PFAS[52].该研究表明木本植物每年可以从重度PFAS污染场地去除 1.4g总PFAS/hm2,是一种可行的场地修复手段[34,52].

表1 植物对PFAS的富集浓度水平
注:所列浓度为整株植物对该种PFAS富集的浓度值(ng/g); ND为浓度数据缺失.
2.2 根系富集系数(RCF)
水培或土培过程中,植物根系中PFAS的浓度与培养基质中PFAS的浓度比为根系富集系数(RCF,公式1),该参数表示植物根系对PFAS的富集潜力,是描述PFAS在植物体内累积趋势的重要指标.

由图2a所示,36种植物对19种PFAS(包括PFCA、PFSA、PFEA系列)的RCF值介于0.04~1030,跨越6个数量级;PFAS链长和头部官能团均会影响RCF值,对于PFCA系列,从PFBA(C3,-COOH)到PFHxA(C5,-COOH),RCF的数值变化不显著(1.295~ 1.69),而从PFHxA(C5,-COOH)到全氟十二烷羧酸(PFDoDA,C12,-COOH),RCF的数值随全氟碳链链长显著增加(1.69~701.33);PFSA系列(C4,C6,C8)也显示出类似趋势,RCF也随着全氟碳链长度的增加显著升高(2.42~81.84),该趋势说明随着碳链长度的增加,根系对PFAS的富集能力呈现递增的现象.前期研究显示长链PFAS(如PFOS、PFOA)显示出较高的RCF,易在根部富集,且长链PFAS的富集程度与根部蛋白质、脂质含量存在显著正相关关系[63-65].
2.3 转运系数(TF)
PFAS从根部向上的转移潜力通常用转运系数(TF)来表示,其等于植物地上部分PFAS含量与地下部分PFAS含量的比值.

对于11种PFCA、3种PFSA和5种PFEA,其TF的范围为0.01~18.54.随着链长的增加,PFCA和PFSA系列的TF值均显示出下降趋势(图2b).TF随链长降低可能是由于长链PFSA疏水性更强,易于吸附在植物根系,并受卡氏带的限制,从而向上转移的潜力较弱,而溶解度更高、疏水性较弱、碳链更短的PFAS可以穿越卡氏带屏障,通过蒸腾流被转运茎部[54,58].对于新型氧杂型PFEA,TF的范围为0.1~ 14.2,分子质量最高的6:2Cl-PFESA(F-53B的主要成分)明显低于其他4种PFEA.PFEA相比于相似分子质量的PFCA、PFSA,其TF值略高,说明植物对其向上转运的能力更强.由于目前PFEA的数据样本较少,上述现象还亟需更多的实验证实.
比较图2a和图2b,TF(0.01~18.54)的值要远小于RCF(0.04~1030)的值,其可能是因为根维管组织的吸附能力远大于茎维管组织的吸附能力或根表面与卡氏带之间的植物组织的吸附能力[66],这也是植物吸收污染物的重要机制之一.
3 PFAS在“土-水-根-茎-叶”多介质体系的传质过程与机制分析
3.1 PFAS在“土-水-根”体系的传质过程
前期研究表明,土壤是PFAS在生态系统不同环境介质中交换的重要周转站,土壤-水是PFAS进入植物体内,并且进一步进入食物链的重要途径[67-68]. PFAS首先从其污染源直接或间接释放进入土壤环境,由于PFAS难以被降解,吸附和淋溶是影响PFAS在土壤-水体系中迁移和生物有效性的两个重要过程[69-70].PFAS与土壤颗粒之间的吸附-淋溶机制包括疏水作用、静电作用、氢键、离子交换和范德华力等,以上几种作用力可能同时存在[70].分子结构上, PFAS在土壤颗粒-孔隙水体系中的吸附和淋溶行为与C-F链长、特征官能团有关;前期针对PFAS在沉积物中的吸附行为研究发现:碳氟链上每增加一个CF2基团,logOC(有机碳吸附常数)值即增加0.3~ 0.6;碳链长度相同时,磺酸类比羧酸类在沉积物上吸附高约0.2个对数单位[71].土壤理化性质方面,土壤对PFAS的吸附、淋溶和吸附滞后性与土壤有机质含量、pH值、阴/阳离子交换容量、共存阳离子、离子强度、比表面积、矿物质含量等有关[70,72-73],而且主流研究认为土壤中有机质含量是最主要的因素[71,74],此结论不仅仅针对典型传统PFAS,最新的研究表明,新型醚类替代物F-53B的最大吸附量也与土壤有机质含量呈正相关[75].
3.2 植物根系对PFAS的吸收以及PFAS的跨膜机制
PFAS吸附在植物根系表面之后,先被运输到根表皮细胞,再被径向运输到皮层.附着在根表皮的PFAS可通过共质体运输和质外体运输两种途径被根吸收[55,76-77].共质体运输是指植物细胞间通过胞间连丝进行PFAS转运,而质外体运输是指植物细胞间通过原生质体以外的自由空间进行PFAS转运.前期研究指出,PFAS可通过质外体和共质体途径被转运.在质外体运输中,PFAS会被内皮层中垂直周壁分布的卡氏带(Casparian strip)阻断,由于卡氏带由疏水的木栓质和木质素组成,故对长链、大分子PFAS在植物体内的转运有阻碍作用,而小分子PFAS可通过卡氏带被径向转运[78].
植物根系对PFAS的吸收包括伴随蒸腾流,通过扩散作用进入植物体内的被动吸收和通过植物细胞膜对污染物选择性运输的主动吸收(图3).主动和被动过程对PFAS吸收的作用取决于植物生理、PFAS的亲脂性、分子结构以及培养方式[79-80].有研究者推测PFAS(特别是小分子短链PFAS)可以通过被动扩散的方式被植物细胞吸收.有研究者基于PFAS在磷脂双分子层膜中的平衡分配发现,PFAS与磷脂膜的融合程度与氟化碳原子的个数(n)成正相关,且全氟磺酸(-SO3H)的融合程度强于全氟羧酸(-COOH)[53].
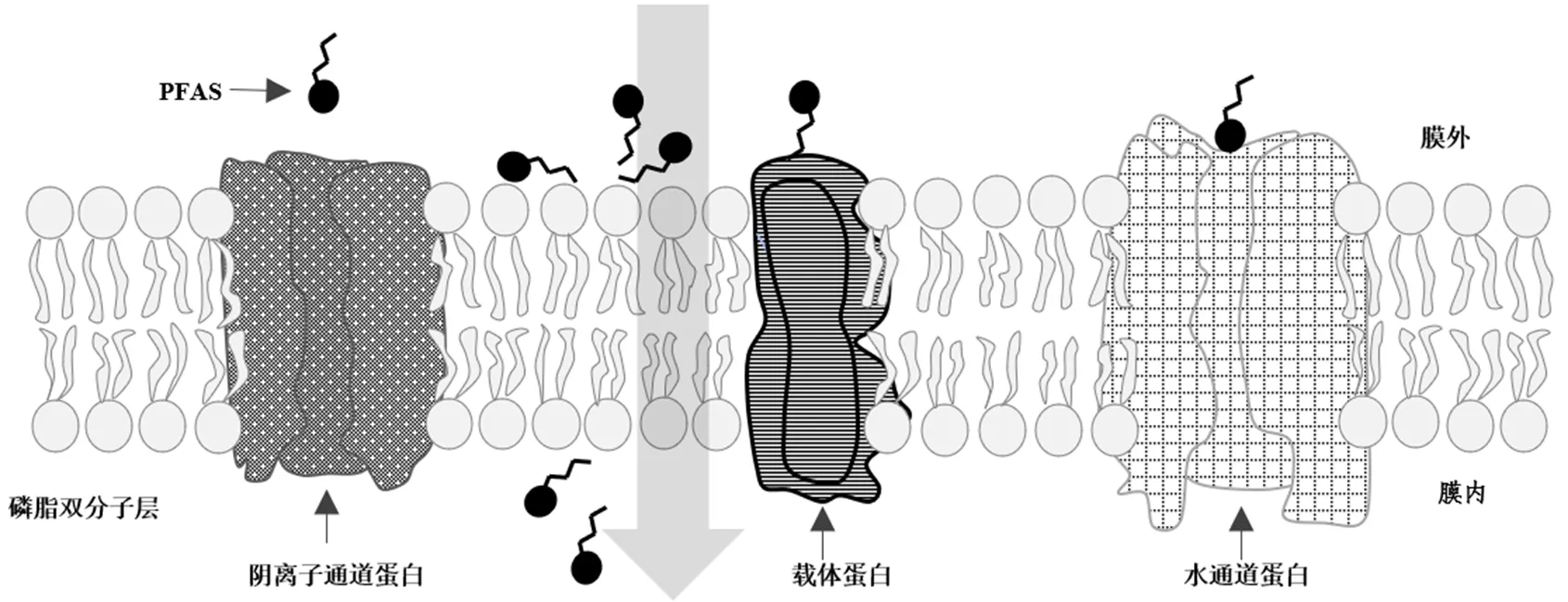
图3 PFAS在植物细胞中的潜在主动运输通道
相比之下,PFAS的主动运输可能需要依赖膜上的载体蛋白、阴离子通道和水通道蛋白[33,53,55,81].当前关于PFAS跨膜机制的研究,主要是通过添加代谢抑制剂(停止能量依赖过程)、水和阴离子通道蛋白(水转运蛋白)抑制剂来推测其吸收机制.为揭示植物吸收PFAS的路径,Wen等[77]通过使用2,4-二硝基苯酚(2,4-DNP)、NaN3和Na3VO4作为代谢抑制剂,发现PFOA被玉米()根部吸收的过程是需要消耗能量的主动运输,而PFOS则是在载体帮助下的被动扩散过程;Wen等[76]发现小麦()对PFBA、PFHxA、PFOA和PFOS的吸收也是需要消耗能量的主动过程;有研究者分别通过添加甘油和硝酸银竞争、抑制水通道蛋白,Wen等[77]发现水通道蛋白被抑制后,小麦对PFOA的吸收无影响,而PFOS的吸收过程被压制,甘油组和硝酸银组PFOA/PFOS植物吸收分别降低25%和31%[77];而Wang等[53]发现加入甘油和硝酸银后,东方泽泻()对PFOA和PFOS的吸收均受到了不同程度的抑制(PFOA降低25.3%~30.9%、PFOS降低22.3%~33.9%),表明水通道蛋白是东方泽泻根部吸收PFOA和PFOS的重要通道.另外,通过加入9-蒽甲酸(9-AC)、4,4¢-二异硫氰酰-2,2¢-基二磺酸二钠盐(DID)和5-硝基-2-(3-苯丙胺)苯甲酸(NPPB)作为阴离子通道阻断剂,Wen等[77]发现玉米对PFOA的富集仅受9-AC影响,而对PFOS的富集却主要受DID和NPPB影响,且PFOS和PFOA共存时,植物对他们的吸收不具有竞争关系,添加PFOS前后PFOA的生物富集浓度无显著性变化,说明植物根部对不同PFAS物质具有不同的转运路径.相比之下,Wang等[53]却发现PFOS和PFOA在东方泽泻根部的吸收过程同样依赖阴离子通道的参与,但PFOA和PFOS却显示出竞争关系,添加PFOS前后PFOA生物富集浓度变化显著(<0.01),在PFOS和PFOA同时存在时,PFOS和PFOA的富集浓度分别降低14.7%和31%.另一方面,Zhang等[82]研究了小麦对PFBA、PFHxA、PFOA和PFOS的生物富集,发现甘油、硝酸银、9-AC、DID和NPPB对小麦吸收上述PFAS均无影响,推测其转移过程是借由小麦根部特异性载体蛋白的主动运输.上述结果的不一致可部分归因为植物种间的巨大差异.综上,目前学术界对PFAS的跨膜机制尚未统一,植物对PFAS生物转运富集机制尚不明确,特别是植物对分子结构不同PFAS的选择性生物富集亟待明确.
3.3 PFAS从根系向地上部分的转运机制
有机化合物被植物根系吸收后,穿过表皮、皮层、内皮层和维管束鞘到达维管束,然后通过木质部从根部向地上部分传输并积累[55,61,83-84].在此过程中,位于木质部中的卡氏带会限制PFAS从根向中央维管系统的迁移,卡氏带通过封闭细胞和细胞膜(质外体)之间的非活性空间,防止化合物通过内皮层的被动运输[85].Wang等[55]利用DESI-MS(吸附解吸电喷雾电离质谱成像)和TEM-EDS(透射电子显微镜结合X射线能谱)观测了PFOA和PFOS在8种湿地植物中向上转运运输的路径,其中再力花、芦苇仅通过共质体运输向上转移,而美人蕉、风车草则是通过共质体运输和质外体运输两种过程同时向上转移,推测其可能是与植物特异性向上转移模式和植物不同的茎截面生理结构差异性有关.
PFAS从植物根系向地上部分传输的驱动力为植物蒸腾作用产生的水势梯度.蒸腾作用的强度可通过测定植物蒸腾速率()和气孔导度(Gs)来量化,其受植物生理结构(如气孔的数量和大小)和环境因素(如光照、温度、湿度、风速)影响[81].蒸腾流富集因子(TSCF)是木质部汁液中污染物浓度与根区水培液或土壤溶液中污染物浓度的比值,被广泛用于描述化学物质在植物中的传输能力.TSCF可以根据植物组织中PFAS的质量和累积蒸腾水分的体积估算:

根据化合物的TSCF数值大小可将化合物分为3类:植物对化合物的吸收速度高于对水分的吸收速度,TSCF值>l;与水分吸收具有相同的速度的化学物质,TSCF=1;吸收速度低于水分的化合物,TSCF值 PFAS在植物中的吸收、富集和转运特征与PFAS分子结构(如logow值、全氟碳链长度、官能团类型、分子尺寸)[66,86,89–92]、植物根和茎的组成(如蛋白质含量、脂质含量、蛋白质结构)[77,81,93]、植物根和茎的生理特征(如水分蒸腾量、气孔导度、生物量、根生理形态)[66,89,94-95]、环境因素(培养基质性质、土壤有机质含量、盐度、湿度和温度)[87,96]、暴露浓度[86]、时间(如污染物浓度、污泥施用频率)[83,90,97]有关. PFAS在植物中的吸收、富集和转运特征与PFAS分子结构有关.Felizeter等[66]发现PFCA系列(C6-C11)的RCF和logow呈现正相关关系,但是在PFDoDA(C12)之后,RCF不会进一步增加,其推测原因为碳链过长的PFAS物质亲脂性过高,分子尺寸过大,其在植物根部表皮的吸附达到饱和.Wang等[55]基于可视化技术DESI-MS和TEM-EDS观察到PFOS和PFOA在植物表皮细胞、皮层和维管束中均有分布,但其在维管束中的浓度远低于维管束周围相邻皮层中浓度,表明长链物质如PFOA和PFOS主要是吸附于植物根系.综上所述,根对PFAS的吸收很可能受到其在植物根类脂质固体上吸附作用的支配,而从根到叶的迁移受到PFAS疏水性(ow)、分子量的限制;随碳链长度的增加,根转运系数RCF呈现递减的现象.由此,短链PFAS(如PFBA、PFHxA)更易向上运输,并更多的富集在茎叶、果实中;而长链PFAS(如PFOS、PFOA)则显示出较高的根富集因子(RCF)和较低的根-茎叶迁移因子(TF),更易在根部富集[63-64]. PFAS头部官能团也会影响其生物富集行为.对比同种植物(灯芯草,菊苣)对相同链长(C3、C5、C8)的PFCA和PFSA的RCF值发现,其富集PFCA和PFSA的RCF值分别在0.12~13和0.23~50.97之间;所综述植物的根系对PFOS的RCF整体介于2.3~1030,其数值显著高于PFOA(RCF 介于1.4~72),因此可以推断官能团对根系的生物富集行为有显著性影响[60,62,83,98].其可能是由于磺酸基比带羧基的PFCA更容易吸附到土壤中,即与同链长的PFCA相比,PFSA具有更低的根际土壤迁移能力和生物有效性,故其更不容易被富集到植物中. 植物体内的蛋白质、脂质及其物理特征等都会影响其吸收PFAS.植物细胞内的蛋白质是影响植物吸收PFAS的重要因素.不同的蛋白质可能在载体(或通道)相关和蒸腾驱动的吸收PFAS的过程中发挥不同的作用,且蛋白质的类型和功能在植物品种中也具有一定的特异性.之前的文献发现,PFAS与某些动物组织中的特定蛋白具有较高的亲和力[81],且检测出PFAS在富含蛋白质的组织中分布更多[93]. Wen等[93]和Yu等[81]指出植物体内蛋白质越高,植物体内富集的PFAS可能越高,即PFAS含量与植物地上部份蛋白质和植物根系蛋白质含量的比值呈正相关,即蛋白质含量越高的植物,显示出更高的RCF和TF值.但如前文所述,目前学术界关于植物蛋白对PFAS富集过程的影响尚不明确. 植物组织的脂质含量也会影响PFAS的富集水平.植物细胞中存在的脂质有助于形成细胞膜并调节细胞极性,从而影响PFAS在植物细胞的进出.Wen等[77]报道植物根系脂质对根系中PFAS的积累具有抑制作用,并且对PFSA的抑制作用强于PFCA. PFAS在根系的富集量与脂质的含量呈负相关[93].脂质可能与PFAS竞争根上的蛋白质结合位点,故对PFAS吸收的抑制能力取决于特定化合物与蛋白质的结合强度.然而Wang等[55]的研究显示脂质含量与PFAS的RCF值呈正相关,这与Wen等[77]的研究结论相悖.由于目前的研究数量有限,未来亟需后续的研究补充数据,进一步揭示脂质对PFAS富集过程的作用机制. 除此之外,植物的生理形态特性包括根系形态[99]、根系比表面积[100]、气孔导度[81]等也会影响植物富集PFAS.由于土壤相中PFAS的流动性低于水相,由此植物根系的长度和比表面积对传质过程至关重要.气孔导度则主要影响蒸腾作用,由于植物富集PFAS的驱动力为蒸腾拉力,所以气孔导度(气孔的数量和大小)会影响蒸腾作用的强度,进一步影响PFAS在植物内的转运效率. 环境介质中的pH值、盐度、土壤有机碳含量、温度均会影响植物吸收PFAS.首先,Krippner等[92]研究了pH值(水培实验,培养液pH值分别为5、6、7)对玉米富集PFAS的影响,发现只有PFDA的富集速率随pH值变化有显著的区别(pH=5时2.51μg/(g·d),干重,pH=7时1.52μg/(g·d),干重),而其他PFAS对pH值的依赖性表现并不一致,如PFBA和PFHxA的富集速率在pH=7时较pH=5时有明显的提高,这与PFDA的表现恰好相反,而其他类型的PFAS(PFPeA,全氟庚烷羧酸PFHpA, PFOA,全氟壬烷羧酸PFNA, PFBS, PFHxS和PFOS)随pH值变化无显著差异.pH值变化对PFAS富集的潜在机制仍然未知,亟需后续研究进一步解释. 培养基质盐度的增加有利于水培系统植物对PFAS的吸收,而在土培系统中,随着盐度的增高, PFAS吸附在土壤颗粒上的比例反而增高[101].盐度效应主要影响PFAS的根吸收和转运,高盐度可能会通过盐析机制促使PFAS更多地进入根系的有机成分中,PFAS富集的增强可归因为植物在溶液电解质浓度升高的情况下增加水分吸收以维持适当的渗透压,随着盐度的增加,溶液的离子强度增加,这可能导致植物根系对PFAS的吸收更高[102],在Zhao等[101]的研究中发现,相比对照组,小麦在高盐度水培介质中对所研究的4种PFCA(PFBA、PFHpA、PFOA和PFDoDA)的TF值高出3.1~4.9倍,而在土壤介质中高盐度则是增强了土壤的吸附性,通过静电相互作用使得PFAS更多的附着在土壤表面,但目前并没有盐度对植物在土培条件下吸收PFAS的影响研究,还需要后续实验证实. 土壤有机碳含量(oc,%)也对植物吸收PFAS有重要的影响[89,103].PFAS在土壤有机质中的吸附会导致有机质微孔的重排、构象变化或改变,进而增加PFAS在土壤相的赋存和滞留,降低PFAS的生物可利用性[104-105].Higgins等[106]证实PFAS在土壤中的吸附与土壤有机碳含量成正相关,风化的有机质具有更强的吸附能力[107-108],影响土壤中PFAS的生物有效性,并抑制其向植物体内积累. 温度是影响植物生长的另一个环境因素,可以通过影响水和营养物质的吸收间接影响植物对污染物的吸收,也可以促进植物的生理活动,如蒸腾作用.一般情况下,当植物生长在适宜温度范围内时,生长温度的升高可以提高养分的扩散速率,降低水分的粘度,为光合作用提供更多的能量,从而增加对污染物的吸收.Zhao等[102]认为温度的升高也有利于植物对PFAS的吸收,随着温度升高,所研究的4种PFCA(PFBA、PFHpA、PFOA和PFDoDA)的TF值显著升高,且植物吸收长链PFCA相较短链PFCA对温度的变化更明显. 除此之外,植物组织中PFAS的富集浓度与培养介质的暴露浓度相关.Blaine等[89]发现在莴苣叶中所有PFAS的浓度均显示出与灌溉水中PFAS浓度(50~105ng/L)存在正相关关系.在此范围内,植物组织中检测到的 PFAS 对数浓度随灌溉水中PFAS 线性增加而未达到饱和.Zhang等[62]在高浓度(640~ 4300μg/L)下对灯芯草富集8种PFAS(PFBA、PFPeA、PFHxA、PFHpA、PFOA、PFBS、PFHxS、PFOS)进行了研究,发现在高浓度下植物对PFOA、PFHxS和PFOS之外的PFAS都没有生物富集效果,得出结论灯芯草对PFAS的吸收能力可能随溶液初始浓度增加而降低. 本文综述了目前已发表文献中36种植物对19种PFAS的暴露途径、生物富集水平以及在植物内的转运、积蓄、跨膜机制,并且系统地分析了PFAS分子结构、植物生理、环境因素和暴露方式对该生物富集行为的影响.本文解析了PFAS分子结构(全氟链长、特征头部官能团等)与多环境多介质的行为(RCF、TF、TSCF)的相关关系,这对于正确评估PFAS环境行为和潜在的生态风险效应具有指导意义.另外,部分植物显示出对PFAS胁迫的高耐受性,可用于植物场地修复技术,作为成本低廉、环境友好、低影响的手段去除污染场地中各类有机氟化污染物. 综上,目前植物富集PFAS的研究还较少,亟需后续的研究进行扩充论证:①研究的植物的种类集中于农作物,需要补充大量的数据,遴选出具有高生长速率和对PFAS高耐受性物种;②由于PFAS普遍分布于水环境中,并可能沿水生食物链生物放大,故对藻类和水生植物的研究十分必要;③目前的研究集中在PFSA和PFCA,对于新型替代品、前体物质鲜有涉及,而PFAS的前体物质可能作为PFAS生产过程中的副产品进入环境,也可能在生产过程中逃逸到大气中,故还需要进一步探索前体物质的迁移、植物转运富集过程,明确前体物的生态环境风险;④大部分对植物中PFAS的实验集中于室内水培,忽略了根际效应,即根际土、根际微生物对PFAS的作用,且实验室内的植物生长条件与野外实际修复环境差异较大,实验室条件下得出的结论还需要进一步田间实验验证. [1] Krafft M P, Riess J G. Selected physicochemical aspects of poly- and perfluoroalkylated substances relevant to performance, environment and sustainability-Part one [J]. Chemosphere, 2015,129:4-19. [2] O’hagan D. Understanding organofluorine chemistry. An introduction to the C–F bond [J]. Chemical Society Reviews, 2008,37(2):308-319. [3] Herzke D, Olsson E, Posner S. Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in consumer products in Norway - A pilot study [J]. Chemosphere, 2012,88(8):980-987. [4] Hekster F M, Laane R W P M, De Voogt P. Environmental and Toxicity Effects of Perfluoroalkylated Substances [J]. Rev Environ Contam Toxicol, 2003,179:99-121. [5] Buck R C, Franklin J, Berger U, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins [J]. Integrated Environmental Assessment and Management, 2011,7(4):513-541. [6] Phong Vo H N, Ngo H H, Guo W, et al. Poly-and perfluoroalkyl substances in water and wastewater: A comprehensive review from sources to remediation [J]. Journal of Water Process Engineering, 2020, 36:101391. [7] Sheng N, Cui R, Wang J, et al. Cytotoxicity of novel fluorinated alternatives to long-chain perfluoroalkyl substances to human liver cell line and their binding capacity to human liver fatty acid binding protein [J]. Archives of Toxicology, 2018,92(1):359-369. [8] Jusko T A, Oktapodas M, Palkovičová Murinová, L, et al. Demographic, Reproductive, and Dietary Determinants of Perfluorooctane Sulfonic (PFOS) and Perfluorooctanoic Acid (PFOA) Concentrations in Human Colostrum [J]. Environmental Science and Technology, 2016,50(13):7152-7162. [9] Liu Z, Lu Y, Shi Y, et al. Crop bioaccumulation and human exposure of perfluoroalkyl acids through multi-media transport from a mega fluorochemical industrial park, China [J]. Environment International, 2017,106:37-47. [10] Lal M S, Megharaj M, Naidu R, et al. Uptake of perfluorooctane sulfonate (PFOS) by common home-grown vegetable plants and potential risks to human health [J]. Environmental Technology and Innovation, 2020,19:100863. [11] Xiao F, Simcik M F, Halbach T R, et al. Perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in soils and groundwater of a U.S. metropolitan area: Migration and implications for human exposure [J]. Water Research, 2015,72:64-74. [12] Muir D, Bossi R, Carlsson P, et al. Levels and trends of poly- and perfluoroalkyl substances in the Arctic environment – An update [J]. Emerging Contaminants, 2019,5:240-271. [13] Houde M, De SilvA A O, Muir D C G, et al. Monitoring of perfluorinated compounds in aquatic biota: An updated review [J]. Environmental Science and Technology, 2011,45(19):7962-7973. [14] Cakmak S, Lukina A, Karthikeyan S, et al. The association between blood PFAS concentrations and clinical biochemical measures of organ function and metabolism in participants of the Canadian Health Measures Survey (CHMS) [J]. Science of the Total Environment, 2022,827:153900. [15] Peng L, Xu W, Zeng Q, et al. Exposure to perfluoroalkyl substances in waste recycling workers: Distributions in paired human serum and urine [J]. Environment International, 2022,158:106963. [16] Herrick R L, Buckholz J, Biro F M, et al. Polyfluoroalkyl substance exposure in the Mid-Ohio River Valley, 1991~2012 [J]. Environmental Pollution, 2017,228:50-60. [17] Steenland K, Kugathasan S, Barr D B. PFOA and ulcerative colitis [J]. Environmental Research, 2018,165:317-321. [18] Ballesteros V, Costa O, Iñiguez C, et al. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: A systematic review of epidemiologic studies [J]. Environment International, 2017,99:15-28. [19] Coakley J, Bridgen P, Mueller J, et al. Polybrominated diphenyl ethers and perfluorinated alkyl substances in blood serum of New Zealand adults, 2011~2013 [J]. Chemosphere, 2018,208:382-389. [20] Liu Z, Lu Y, Wang T, et al. Risk assessment and source identification of perfluoroalkyl acids in surface and ground water: Spatial distribution around a mega-fluorochemical industrial park, China [J]. Environment International, 2016,91:69-77. [21] Quinete N, Wu Q, Zhang T, et al. Specific profiles of perfluorinated compounds in surface and drinking waters and accumulation in mussels, fish, and dolphins from southeastern Brazil [J]. Chemosphere, 2009,77(6):863-869. [22] Rahman M F, Peldszus S, Anderson W B. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: A review [J]. Water Research, 2014,50:318-340. [23] Paterson S, Mackay D, Mcfarlane C. A model of organic chemical uptake by plants from soil and the atmosphere [J]. Environmental Science & Technology, 2002,28(13):2259-2266. [24] Tian Y, Yao Y, Chang S, et al. Occurrence and phase distribution of neutral and ionizable per- and polyfluoroalkyl substances (PFASs) in the atmosphere and plant leaves around landfills: A case study in Tianjin, China [J]. Environmental Science & Technology, 2018, 52(3):1301-1310. [25] 胡国成,郑 海,张丽娟,许振成,等.珠江三角洲土壤中全氟化合物污染特征研究[J]. 中国环境科学, 2013,33(S1):37-42. Hu G C, Zheng H, Zhang L J, et al Contamination characteristics of perfluorinated compounds in soil from Pearl River Delta, South China [J]. Chinese Environmental Science, 2013,33(S1):37-42. [26] 张宏娜,温 蓓,张淑贞.全氟和多氟烷基化合物异构体的分析方法、环境行为和生物效应研究进展[J]. 环境化学, 2019,38(1):42-50. Zhang H N, Wen B, Zhang S Z. Analytical methods, environmental behaviors and biological effects of per- and polyfluoroalkylisomers [J]. Environmental Chemistry, 2019,38(1):42-50. [27] 夏 慧.全氟化合物的暴露风险及其与人体功能蛋白相互作用的研究[D]. 上海:上海交通大学, 2018. Xia H. The investigations of exposure risk of perfluorinated compounds and their interactions with human functional proteins [D]. Shanghai: Shanghai Jiao Tong University, 2018. [28] Jiao X, Shi Q, Gan J. Uptake, accumulation and metabolism of PFASs in plants and health perspectives: A critical review [J]. Critical Reviews in Environmental Science and Technology, 2021,51(23): 2745-2776. [29] Groffen T, Rijnderd J, Van Doorn L, et al. Preliminary study on the distribution of metals and persistent organic pollutants (POPs), including perfluoroalkylated acids (PFAS), in the aquatic environment near Morogoro, Tanzania, and the potential health risks for humans [J]. Environmental Research, 2021,192:110299. [30] 王效举.植物修复技术在污染土壤修复中的应用[J]. 西华大学学报(自然科学版), 2019,38(1):65-70. Wang X J. Practical application of phytoremediation technology of contaminated soils [J]. Journal of Xihua Univerity(Natural Science Edition), 2019,38(1):65-70. [31] Sharma S, Singh B, Manchanda V K. Phytoremediation: role of terrestrial plants and aquatic macrophytes in the remediation of radionuclides and heavy metal contaminated soil and water [J]. Environmental Science and Pollution Research, 2015,22(2):946-962. [32] D. Collins C, Finnegan E. Modeling the plant uptake of organic chemicals, including the soil−air−plant pathway [J]. Environmental Science & Technology, 2010,44(3):998-1003. [33] Huang D, Xiao R, Du L, et al. Phytoremediation of poly- and perfluoroalkyl substances: A review on aquatic plants, influencing factors, and phytotoxicity [J]. Journal of Hazardous Materials, 2021, 418:126314. [34] Huff D K, Morris L A, Sutter L, et al. Accumulation of six PFAS compounds by woody and herbaceous plants: potential for phytoextraction [J]. International Journal of Phytoremediation, 2020,22(14): 1538-1550. [35] Brusseau M L, Anderson R H, Guo B. PFAS concentrations in soils: Background levels versus contaminated sites [J]. Science of the Total Environment, 2020,740:140017. [36] Xiao F, Simcik M F, GULLIVER J S. Mechanisms for removal of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) from drinking water by conventional and enhanced coagulation [J]. Water Research, 2013,47(1):49-56. [37] Nickerson A, Maizel A C, Kulkarni P R, et al. Enhanced extraction of AFFF-associated PFASs from source zone soils [J]. Environmental science & technology, 2020,54(8):4952-4962. [38] Eschauzier C, Beerendonk E, Scholte-Veenendaal P, et al. Impact of treatment processes on the removal of perfluoroalkyl acids from the drinking water production chain [J]. Environmental Science and Technology, 2012,46(3):1708-1715. [39] Tanaka S, Fujii S, Kunacheva C, et al. PFAA distribution in source water and their effective treatment technologies [J]. Reproductive Toxicology, 2012,33(4):588-589. [40] Bossi R, Strand J, Sortkjær O, et al. Perfluoroalkyl compounds in Danish wastewater treatment plants and aquatic environments [J]. Environment International, 2008,34(4):443-450. [41] Dauchy X, Boiteux V, Colin A, et al. Poly- and perfluoroalkyl substances in runoff water and wastewater sampled at a firefighter training area [J]. Archives of Environmental Contamination and Toxicology, 2019,76(2):206-215. [42] 魏潇潇.三峡库区垃圾渗滤液和江水全多氟化合物赋存特征[D]. 重庆:重庆大学, 2020. Wei X X. Occurrence characteristics of per- and polyfluoroalkyl substances in the Three gorges reservoir area leachates and Yangtze river water [D]. Chongqing: Chongqing university, 2019. [43] Armitage J, Cousins I T, Buck R C, et al. Modeling global-scale fate and transport of perfluorooctanoate emitted from direct sources [J]. Environmental Science and Technology, 2006,40(22):6969-6975. [44] Weber A K, Barber L B, Leblanc D R, et al. Geochemical and hydrologic factors controlling subsurface transport of poly- and perfluoroalkyl substances, Cape Cod, Massachusetts [J]. Environmental Science and Technology, 2017,51(8):4269-4279. [45] C hropeňová M, K arásková P, Kallenborn R, et al. Pine needles for the screening of perfluorinated alkylated substances (PFASs) along ski tracks [J]. Environmental Science and Technology, 2016,50(17): 9487-9496. [46] Scott B F, Spencer C, Martin J W, et al. Comparison of haloacetic acids in the environment of the Northern and Southern Hemispheres [J]. Environmental Science and Technology, 2005,39(22):8664-8670. [47] García-Valcárce A I, M olero E, Escorial M C, et al. Uptake of perfluorinated compounds by plants grown in nutrient solution [J]. Science of the Total Environment, 2014,472:20-26. [48] Zhang W, Cao H, Liang Y. Plant uptake and soil fractionation of five ether-PFAS in plant-soil systems [J]. Science of the Total Environment, 2021,771:144805. [49] 李德志,李经纶,秦艾丽.用禾本科植物富集修复土壤重金属污染的方法:中国, 103071669 [P]. 2013-05-01. Li D Z, Li J L, Qin A L. A method for remediation of heavy metal pollution in soil by enrichment of gramineous plants [P]. 2013-05-01. [50] 沈源源,腾 应,骆永明,等.几种豆科、禾本科植物对多环芳烃复合污染土壤的修复[J]. 土壤, 2011,43(2):253-257. Shen Y Y, Teng Y, Luo Y M et al. Remediation efficiency of several legumes and grasses in PAH-contaminated soils [J]. Soils, 2011, 43(2):253-257. [51] 许端平,董天骄,吕俊佳.典型禾本科植物对石油污染土壤的修复作用[J]. 环境工程学报, 2012,6(4):1398-1402. Xu D P, Dong T J, Lv J J. Remediation of petroleum contaminated soil by typical gramineous plants [J]. Chinese Journal of Environmental Engineering, 2012,6(4):1398-1402. [52] Gobelius L, Lewis J, Ahrens L. Plant uptake of per- and polyfluoroalkyl substances at a contaminated fire training facility to evaluate the phytoremediation potential of various plant species [J]. Environmental Science and Technology, 2017,51(21):12602-12610. [53] Wang T T, Ying G G, He L Y, et al. Uptake mechanism, subcellular distribution, and uptake process of perfluorooctanoic acid and perfluorooctane sulfonic acid by wetland plant[J]. Science of the Total Environment, 2020,733:139383. [54] Pi N, Ng J Z, Kelly B C. Uptake and elimination kinetics of perfluoroalkyl substances in submerged and free-floating aquatic macrophytes: Results of mesocosm experiments with Echinodorus horemanii and Eichhornia crassipes [J]. Water Research, 2017,117: 167-174. [55] Wang T T, Ying G G, Shi W J, et al. Uptake and translocation of perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) by wetland plants: Tissue-and cell-level distribution visualization with desorption electrospray ionization mass spectrometry (DESI-MS) and transmission electron microscopy equipped with energy-dispersive spectroscopy (TEM-EDS) [J]. Environmental Science and Technology, 2020,54(10):6009-6020. [56] Gredelj A, Nicoletto C, Polesello S, et al. Uptake and translocation of perfluoroalkyl acids (PFAAs) in hydroponically grown red chicory (.): Growth and developmental toxicity, comparison with growth in soil and bioavailability implications [J]. Science of the Total Environment, 2020,720:137333. [57] Mudumbi J B N, Daso A P, Okonkwo O J, et al. Propensity of Tagetes erecta L., a medicinal plant commonly used in diabetes management, to accumulate perfluoroalkyl substances [J]. Toxics, 2019,7(1):18. [58] Zhang D Q, Wang M, He Q, et al. Distribution of perfluoroalkyl substances (PFASs) in aquatic plant-based systems: From soil adsorption and plant uptake to effects on microbial community [J]. Environmental Pollution, 2020,257:113575. [59] Chen Y C, Lo S L, Lee Y C. Distribution and fate of perfluorinated compounds (PFCs) in a pilot constructed wetland [J]. Desalination and Water Treatment, 2012,37(1-3):178-184. [60] Wilkinson J L, Hooda P S, Swinden J, et al. Spatial (bio)accumulation of pharmaceuticals, illicit drugs, plasticisers, perfluorinated compounds and metabolites in river sediment, aquatic plants and benthic organisms [J]. Environmental Pollution, 2018,234:864-875. [61] Li X Q, Hua Z L, Wu J Y, et al. Removal of perfluoroalkyl acids (PFAAs) in constructed wetlands: Considerable contributions of submerged macrophytes and the microbial community [J]. Water Research, 2021,197:117080. [62] Zhang D, Zhang W, Liang Y. Distribution of eight perfluoroalkyl acids in plant-soil-water systems and their effect on the soil microbial community [J]. Science of the Total Environment, 2019,697:134146. [63] Wen B, Li L, Zhang H, et al. Field study on the uptake and translocation of perfluoroalkyl acids (PFAAs) by wheat (Triticum aestivum L.) grown in biosolids-amended soils [J]. Environmental Pollution, 2014,184:547-554. [64] Felizeter S, Mclachlan M S, De Voogt P. Root uptake and translocation of perfluorinated alkyl acids by three hydroponically grown crops [J]. Journal of Agricultural and Food Chemistry, 2014,62(15):3334–3342. [65] 伍兆诚,王少锐,江龙飞,等.全氟羧酸化合物在氟化工园区土壤-植物中的分布及组成特征[J]. 地球化学, 2021,50(5): 525-535. WU Z C, Wang S R, Jiang L F. Distribution and composition of perfluorocarboxylic acid substances in the soil-plant system around a fluorochemical manufacturing park [J]. GEOCHIMICA, 2021,50(5): 525-535. [66] Felizeter S, Jürling H, Kotthoff M. Influence of soil on the uptake of perfluoroalkyl acids by lettuce: A comparison between a hydroponic study and a field study [J]. Chemosphere, 2020,(260):127608. [67] Ghisi R, Vamerali T, Manzetti S. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review [J]. Environmental Research, 2019,169:326-341. [68] Lesmeister L, Lange F T, Breuer J, et al. Extending the knowledge about PFAS bioaccumulation factors for agricultural plants - A review [J]. Science of the Total Environment, 2020,766:142640. [69] Houtz E F, Higgins C P, Field J A, et al. Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil [J]. Environmental Science and Technology, 2013,47(15):8187-8195. [70] Mejia-Avendaño S, Zhi Y, Yan B, et al. Sorption of polyfluoroalkyl surfactants on surface soils: Effect of molecular structures, soil properties, and solution chemistry [J]. Environmental Science and Technology, 2020,54(3):1513-1521. [71] Higgins C P, LuthyR G. Sorption of perfluorinated surfactants on sediments [J]. Environmental Science and Technology, 2006,40(23): 7251-7256. [72] Zhi Y, Liu J. Column chromatography approach to determine mobility of fluorotelomer sulfonates and polyfluoroalkyl betaines [J]. Science of The Total Environment, 2019,683:480-488. [73] Zhi Y, Liu J. Sorption and desorption of anionic, cationic and zwitterionic polyfluoroalkyl substances by soil organic matter and pyrogenic carbonaceous materials [J]. Chemical Engineering Journal, 2018,346:682-691. [74] Li Y, Oliver D P, Kookana R S. A critical analysis of published data to discern the role of soil and sediment properties in determining sorption of per and polyfluoroalkyl substances (PFASs) [J]. Science of the Total Environment, 2018,628:110-120. [75] Wei; C, Song X, Wang Q, et al. Influence of coexisting Cr(VI) and sulfate anions and Cu(II) on the sorption of F-53B to soils [J]. Chemosphere, 2019,216:507-515. [76] Wen B, Li L, Zhang H, et al. Field study on the uptake and translocation of perfluoroalkyl acids (PFAAs) by wheat (L.) grown in biosolids-amended soils [J]. Environmental Pollution, 2014,184:547-554. [77] Wen B, Li L, Liu Y, et al. Mechanistic studies of perfluorooctane sulfonate, perfluorooctanoic acid uptake by maize (L. cv. TY2) [J]. Plant and Soil, 2013,370(1/2):345-354. [78] Niko G. Casparian strips [J]. Current Biology, 2013,23(23):R1025- R1026. [79] Collins C, Fryer M, Grosso A. Plant uptake of non-ionic organic chemicals [J]. Environmental Science and Technology, 2006,40(1):45-52. [80] Zhan X H, Ma H L X et al, Zhou L. Accumulation of phenanthrene by roots of intact wheat(L.) seedlings: Passive or active uptake? [J].BMC Plant Biol, 2010,10:52. [81] Yu P F, Xiang L, Li X H, et al. Cultivar-dependent accumulation and translocation of perfluorooctanesulfonate among lettuce (L.) cultivars grown on perfluorooctanesulfonate-contaminated soil [J]. Journal of Agricultural and Food Chemistry, 2018,66(50): 13096-13106. [82] Zhang L, Sun H, Wang Q et al. Uptake mechanisms of perfluoroalkyl acids with different carbon chain lengths (C2-C8) by wheat (L.) [J]. Science of the Total Environment, 2019,654:19-27. [83] Sima M W, Jaffé P R. A critical review of modeling poly- and perfluoroalkyl substances (PFAS) in the soil-water environment [J]. Science of the Total Environment., 2021,757:143793. [84] Zhang W, Zhang D, Zagorevski D v., et al. Exposure ofto seven perfluoroalkyl acids: Uptake, accumulation and phytotoxicity [J]. Chemosphere, 2019,233:300-308. [85] Liu Y, Hou X, Chen W, et al. Occurrences of perfluoroalkyl and polyfluoroalkyl substances in tree bark: Interspecies variability related to chain length [J]. Science of the Total Environment, 2019,689:1388- 1395. [86] 王团团,李贝贝,王 赛.泽泻()对全氟化合物的吸收和传输特征-浓度的影响[J]. 环境科学, 2019,40(12):5394-5400. WANG T T, Li B B, Wang S. Concentration-dependent accumulation and translocation of PFASs by wetland plant[J]. Environmental Science, 2019,40(12):5394-5400. [87] Blaine A C, Rich C D, Hundal L S, et al. Uptake of perfluoroalkyl acids into edible crops via land applied biosolids: Field and greenhouse studies [J]. Environmental Science and Technology, 2013, 47(24):14062-14069. [88] Limmer M A, Burken J G. Plant Translocation of organic compounds: Molecular and physicochemical predictors [J]. Environmental Science and Technology Letters, 2014,1(2):156-161. [89] Blaine A C, Rich C D, Sedlacko E M, et al. Perfluoroalkyl acid uptake in lettuce () and strawberry () irrigated with reclaimed water [J]. Environmental Science and Technology, 2014,48(24):14361-14368. [90] Felizeter S, Mclachlan M S, De Voogt P. Uptake of perfluorinated alkyl acids by hydroponically grown lettuce () [J]. Environmental Science and Technology, 2012,46(21):11735-11743. [91] Krippner J, Falk S, Brunn H, et al. Accumulation potentials of perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkyl sulfonic acids (PFSAs) in maize () [J]. Journal of Agricultural and Food Chemistry, 2015,63(14):3646-3653. [92] Krippner J, Brunn H, Falk S, et al. Effects of chain length and pH on the uptake and distribution of perfluoroalkyl substances in maize () [J]. Chemosphere, 2014,94:85-90. [93] Wen B, Wu Y, Zhang H, et al. The roles of protein and lipid in the accumulation and distribution of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in plants grown in biosolids-amended soils [J]. Environmental Pollution, 2016,216:682-688. [94] Lechner M, Knapp H. Carryover of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from soil to plant and distribution to the different plant compartments studied in cultures of carrots (), potatoes (), and cucumbers () [J]. Journal of Agricultural and Food Chemistry, 2011,59(20):11011-11018. [95] Müller C E, LeFevre G H, Timofte A E, et al. Competing mechanisms for perfluoroalkyl acid accumulation in plants revealed using anmodel system [J]. Environmental Toxicology and Chemistry, 2016,35(5):1138-1147. [96] Blaine A C, Rich C D, Sedlacko E M, et al. Perfluoroalkyl acid distribution in various plant compartments of edible crops grown in biosolids-amended soils [J]. Environmental Science and Technology, 2014,48(14):7858-7865. [97] Stahl T, Heyn J, Thiele H, et al. Carryover of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from soil to plants [J]. Archives of Environmental Contamination and Toxicology, 2009,57(2): 289-298. [98] Madikizela L M, Ncube S, Chimuka L. Uptake of pharmaceuticals by plants grown under hydroponic conditions and natural occurring plant species: A review [J]. Science of the Total Environment, 2018:477- 486. [99] 王团团.湿地植物对全氟辛酸和全氟辛烷磺酸的累积特征和吸收机制研究[D]. 北京:中国科学院大学, 2020. Wang T T. Accumulation characteristics and uptake mechanism of perfluorooctanoic acid and perfluorooctane sulfonic acid in wetland plants [D]. Beijing: University of Chinese Academy of Sciences, 2020. [100] Zhan X, Liang X, Xu G, et al. Influence of plant root morphology and tissue composition on phenanthrene uptake: Stepwise multiple linear regression analysis [J]. Environmental Pollution, 2013,179:294-300. [101] Jeon J, Kannan K, Lim B J, et al. Effects of salinity and organic matter on the partitioning of perfluoroalkyl acid (PFAs) to clay particles [J]. Journal of Environmental Monitoring, 2011,13(6):1803-1810. [102] Zhao H, Qu B, Guan Y, et al. Influence of salinity and temperature on uptake of perfluorinated carboxylic acids (PFCAs) by hydroponically grown wheat (L.) [J]. SpringerPlus, 2016,5(1):541-548. [103] Ghisi R, Vamerali T, Manzetti S. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review [J]. Environmental Research, 2019,169:326-341. [104] Liu M, Tian S, Chen P, et al. Predicting the bioavailability of sediment-associated polybrominated diphenyl ethers using a 45-d sequential Tenax extraction [J]. Chemosphere, 2011,85(3):424-431. [105] Xu Y, Gan J, Wang Z, et al. Effect of aging on desorption kinetics of sediment-associated pyrethroids [J]. Environmental Toxicology and Chemistry, 2008,27(6):1293-1301. [106] Higgins C P, Luthy R G. Sorption of perfluorinated surfactants on sediments [J]. Environmental Science and Technology, 2006,40(23): 7251-7256. [107] Zhang J, He M, Shi Y. Comparative sorption of benzo[α]phrene to different humic acids and humin in sediments [J]. Journal of Hazardous Materials, 2009,166(2/3):802-809. [108] Zhao L, Zhu L, Yang L, et al. Distribution and desorption of perfluorinated compounds in fractionated sediments [J]. Chemosphere, 2012,88(11):1390-1397. Research progress on bioaccumulation of per-and polyfluoroalkyl compounds by plants. HE Qiang, YAN Zheng, ZHI Yue*, QIAN Shen-hua, WANG Xiao-ming, CHEN Yi, LIU Cai-hong, CHENG Cheng, HU Xue-bin (Key Laboratory of the Three Gorges Reservoir Region’s Eco-Environment, Ministry of Education, Chongqing University, Chongqing 400045, China)., 2022,42(11):5395~5407 This review summarized the literature data, including bioaccumulation of 19 kinds of per- and polyfluoroalkyl compounds (PFAS) by 36 plants. Exposure, transport, and bioaccumulation feature data are summarized and interpreted; the mechanism of migration and accumulation of PFAS from environmental media to plant tissues was systematically elucidated; effects of PFAS molecular structure (such as perfluorocarbon chain length and head functional group), plant physiological characteristics, and environmental factors on the bioaccumulation process were discussed. In addition, future studies of phytoremediation and combined remediation of PFAS contaminated sites were prospected. The information can be used to manage and evaluate PFAS-contaminated sites, formulate phytoremediation plans, and assess ecological and health risk of PFAS contaminated sites. poly- and perfluoroalkyl substances;PFAS;plants;translocation;bioaccumulation X173 A 1000-6923(2022)11-5395-13 何 强(1965-),男,江苏江阴人,教授,博士,研究方向为小城镇污水处理、城市排水管网、城市水环境综合整治.发表论文200余篇. 2022-04-15 国家自然科学基金资助项目(U20A20326,52200225);中国博士后科学基金资助项目(265648) * 责任作者, 助理研究员, yuezhi6170@163.com4 植物吸收PFAS的影响因素
4.1 PFAS分子结构
4.2 植物的生理特征
4.3 环境因素和暴露方式
5 结论与展望

