Two new species from Sulawesi and Borneo facilitate phylogeny and taxonomic revision of Engelhardia (Juglandaceae)
Hong-Hu Mng , Can-Yu Zhang , Shook Ling Low , Lang Li , Jian-Yong Shn ,Nurainas ,Yu Zhang ,Pi-Han Huang ,Shi-Shun Zhou ,Yun-Hong Tan ,g,Ji Li ,*
a Plant Phylogenetics and Conservation Group,Center for Integrative Conservation,Xishuangbanna Tropical Botanical Garden,Chinese Academy of Sciences,Kunming 650223, Yunnan, China
b Yunnan Normal University, Kunming 650500, Yunnan, China
c Bonn University Botanic Gardens, Meckenheimer Allee 171, D-53115 Bonn, Germany
d The Center for Gardening and Horticulture, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences Mengla 666303, Yunnan, China
e Department of Biology, Faculty of Math. & Nat. Sci., Andalas University, Padang 25163, West Sumatra, Indonesia
f Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Nay Pyi Taw 05282, Myanmar
g Center of Conservation Biology, Core Botanical Gardens, Chinese Academy of Sciences, Mengla 666303, Yunnan, China
h University of Chinese Academy of Science,100049, Beijing, China
Keywords:Engelhardia Taxonomic notes Morphological clustering Phylogeny Distribution
A B S T R A C T Engelhardia,a genus of Juglandaceae(the walnut family),is endemic to tropical and subtropical Asia.The rich Cenozoic fossil records and distinctive morphological characters of the living plants have been used to explore the evolutionary history and geographic distribution of Juglandaceae.However,the taxonomy of this genus has been suffered from a lack of in-depth investigation and good specimens across its distribution ranges. Species delimitation of Engelhardia was defined with seven species in 2020, but detailed information on the circumscription of the species still remains poorly understood.In this study,two new species are described from Sulawesi and Borneo, Engelhardia anminiana and E. borneensis. We also revised and reconstructed the phylogeny within Engelhardia using morphological,molecular(plastid and ribosomal),and distribution data.We sampled 787 individuals in 80 populations,and all the samples were genotyped using plastid regions, trnS-trnG, rps16, trnL-trnF, psbA-trnH, and rpl32-trnL; one ribosomal region, nuclear ribosomal internal transcribed spacer (nrITS). The all datasets were used to reconstruct the phylogenetic relationships. Then, the molecular analyses were combined for 738 sheets of specimens with 15 morphological characteristics to further explore the morphological clusters of Engelhardia. Cluster analysis using morphological data confirmed the delimitation of nine Engelhardia species. Also, phylogenetic analysis based on molecular data (i.e., plastid and ribosomal) supported the monophyly of Engelhardia and generated phylogenetic trees that included E. fenzelii, E. roxburghiana,
1. Introduction
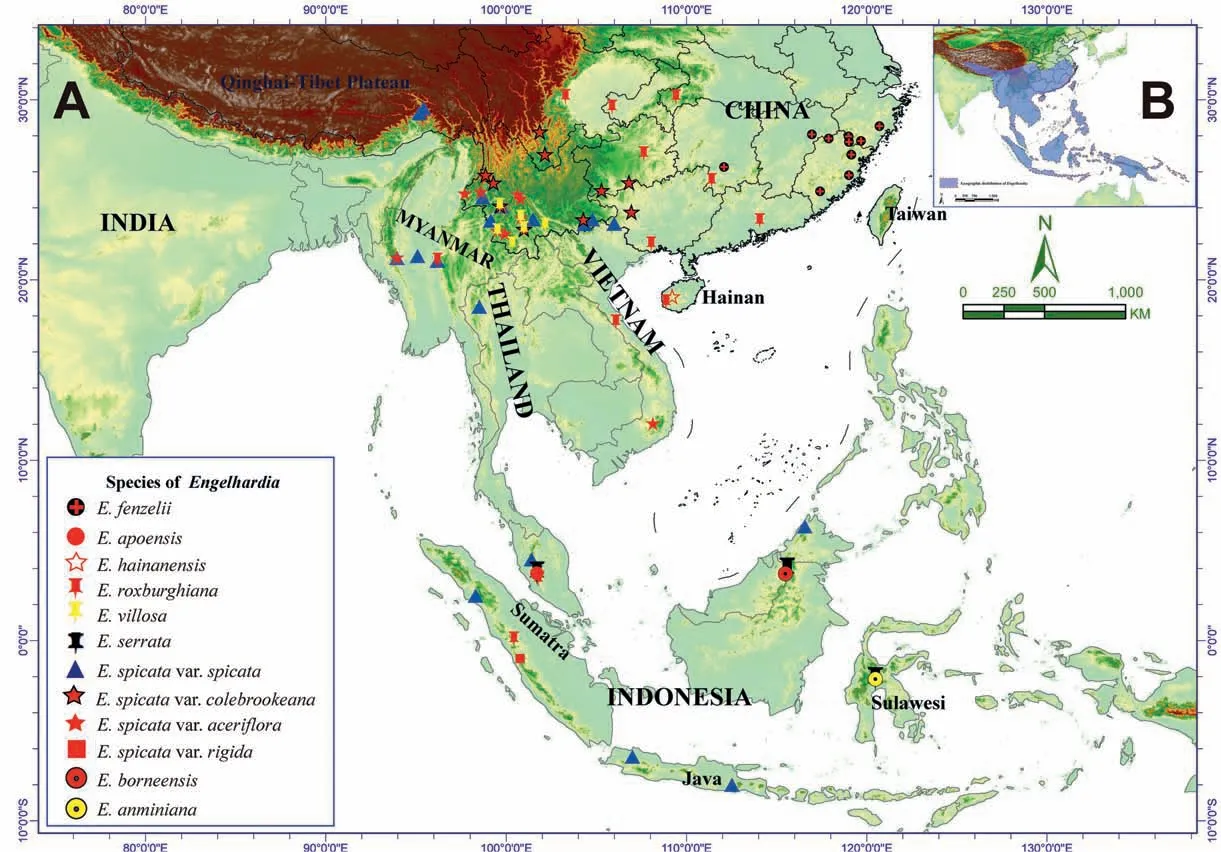
Fig. 1. The genus, Engelhardia, across its geographic distribution in tropical and subtropical Asia. A, locations where Engelhardia species were collected in this study. B, entire geographic range of Engelhardia distribution, as shown by blue shaded areas (adopted from Meng et al., 2015; Manchester,1987).
Engelhardia is a primitive genus of Juglandaceae (the walnut family) that is endemically distributed in tropical and subtropical Asia (Lu, 1982; Fig. 1B), specifically, the southern Himalayas, i.e.,parts of Nepal,southern Xizang in China,the south Yangtze River in China, the Indochina Peninsula, the Malay Peninsula, Borneo,Sulawesi,the Indonesian Archipelagoes,the Philippines,and Papua New Guinea(Jacobs,1960;Manning,1966;Stone,1993,1977;2010;Lu et al.,1999; Zhang et al., 2020). Although the genus was established by Blumevon Carl(1826)and adopted by Jacobs(1960)in the Flora Malesiana,it is still an open question as to how many species belong to Engelhardia (Lu et al.,1999), and how these species are related phylogenetically. Engelhardia species are either deciduous or evergreen, and are frequently monoecious. The trees of Engelhardia are characterized by having even-pinnate leaves,with 2-14 leaflets, both staminate and pistillate flowers on a catkins inflorescence, all of which are located terminally on new branches, or laterally on old wood. Engelhardia flowers (both staminate and pistillate) typically have between 1 and 4 sepals. Additionally, the male anemophilous flowers have a 3-lobed bract with 2 bracteoles,which are rarely absent, 3 to 15 stamens, and anterior-posterior carpels. The fruits of Engelhardia species are produced from the female flowers, which have a 3-lobed wing consisting of an intact oblong-ovate middle lobe and two lateral lobes that are apically obovate and have pinnate venation. The middle lobe is thicker at the base and gradually tapers to the apex; the nutlet is located at the base,which is round or hispid,and is sub-divided by a septum into two compartments (Jacobs, 1960; Manning, 1966; Lu et al.,1999; Meng et al., 2015; Zhang et al., 2020). Historically, Engelhardia possesses a rich Cenozoic fossil record; in particular the fruits fossils can be well-preserved in the sediment. This encouraged many paleobotanists and modern biologists to utilize both the paleo-distribution data and distinctive morphological characters to untangle the evolution and radiation of the Juglandaceae(Manchester, 1987, 1994; Manos and Stone, 2001; Manos et al.,2007; Stone, 2010; Meng et al., 2015, 2022; Song et al., 2020;Hazra et al., 2021; Zhang et al., 2022a).
Taxonomic descriptions of Engelhardia have been undertaken for several decades since it was established(Blume and von Carl,1826;Jacobs,1960;Manning,1966;Lu et al.,1999;Meng et al.,2015;Zhang et al., 2020). Most of these descriptions have used morphological traits (i.e., the bud, leaflets, and inflorescences) to propose subdivisions within Engelhardia(Jacobs,1960;Manning,1966;Lu et al.,1999), relying on specimens in herbaria, descriptions included in previous studies,flora,and local plant lists.However,the taxonomy of Engelhardia remains poorly understood. Fresh plant material is often inaccessible because Engelhardia are distributed over a large range,traversing various countries in Asia,large oceanic separation,and a vast latitudinal distribution range (Zhang et al., 2020).Furthermore, small-scale investigations commonly use multiple synonyms for a single Engelhardia species in different regions or countries(Manning,1966;Lu et al.,1999;Zhang et al.,2020).These limitations are exacerbated by the use of a single method (e.g.,morphological data)to resolve taxonomic issues.
Several studies have attempted to elucidate the taxonomy and phylogeny of Engelhardia by using integrative approaches. For instance, Engelhardia possesses a rich Cenozoic fossil record; in particular the fruits fossils can be well-preserved in the sediment.This has encouraged researchers to utilize both paleo-distribution data and distinctive morphological characters to untangle the evolution and radiation of the Juglandaceae (Manchester, 1987,1994; Manos and Stone, 2001; Manos et al., 2007; Stone, 2010;Meng et al.,2015,2022;Song et al.,2020;Hazra et al.,2021;Zhang et al., 2022a). In 2020, we integrated the morphology, molecular phylogeny, and a large-scale field investigation to provide insights into the species delimitation of Engelhardia. We confirmed the currently accepted species, i.e., E. roxburghiana, E. fenzelii,E. apoensis, E. serrata, E. hainanensis, E. villosa, E. spicata and the varieties (including E. spicata var. spicata, E. spicata var. rigida,E. spicata var. aceriflora, and E. spicata var. colebrookeana) (Zhang et al.,2020).
It is well known that misidentification and widespread errors in tropical plant collections are very common, and more than 50% of tropical specimens, on average, are likely to be incorrectly identified with the world's collections having more than doubled since 1970(Goodwin et al.,2015).Also,the use of multiple synonyms for a single Engelhardia species in different regions or countries is common for small-scale investigations. Thus, the taxonomy of Engelhardia is further complicated for such a large distribution by the different synonymous names in the different countries and regions (Manning, 1966; Lu et al., 1999; Zhang et al., 2020). For instance, based on limited information, especially for the rarest species (Manning,1966), and incomplete or damaged Engelhardia specimens (i.e., fragments of fruits which are typical for Engelhardia) from herbariums, E. apoensis was suspected to collect in the field and included in the species delimitation (Zhang et al., 2020).Generally, Engelhardia inhabits open areas along the road or mountain tracks,which is convenient for plant collecting.However,there is one exception where the trees grow in the wet valleys of the dense forests in Borneo. After a thorough comparison, we confirmed that it is an unnamed species. Consequently, given the above-mentioned issues, it is necessary to implement a comprehensive, critical revision of Engelhardia.
During the fieldwork in Sulawesi and Borneo, two new species have been identified. The unnamed Engelhardia species from Borneo is unique in that it grows in the wet valleys of dense forests.This tree represents an emergent species and is always higher than the surrounding species on the slope. The discovery of these two new species of Engelhardia has led us to revise the taxonomic foundation of our previous integrative study with updated morphological descriptions and geographic distributions. In this study,we(1)describe two new species and present the taxonomic treatments (including the key to species identification); (2)reconstruct the phylogeny of Engelhardia using the integrative evidence from the chloroplast DNA (cpDNA), nuclear ribosomal internal transcribed spacer (nrITS), and morphological traits.
2. Materials and methods
2.1. Materials
For the taxonomic revision of Engelhardia, we sampled 787 individuals from 80 populations (Fig. 1A; see Table S1 for the geographic coordinates) during the biodiversity surveys were conducted during different seasons spanning in the geographic distribution of Engelhardia in tropical and subtropical Asia from 2016 to 2019.For all specimens,the morphology was observed and measured based on living plants in the field;and dry specimens in herbaria(Table S2).The morphological characteristics based on the taxonomic criteria in Flora of China (FOC) were observed and measured for Engelhardia(26 in total),and 15 characters were used for principal component analysis (PCA; Tables S3 and S4). Species identity was verified by comparison to herbaria specimens and literature review; species names which we could not verify were omitted. Voucher specimens were deposited at the herbarium of Xishuangbanna Tropical Botanical Garden (HITBC).
2.2. Lab protocols, cluster and phylogenetic analyses
To test the placement of Engelhardia specimens in multivariate space, we used discriminant analysis and principal components analysis(PCA).A total of 26 morphological characters were used for discriminant analysis (Table S3). PCA was conducted using 15 morphological characters and following the procedure of Wold et al. (1987) (Table S4). Morphological characters of specimen flowers and seeds were measured during field surveys and checked in herbaria using the taxonomic criteria in Flora of China (FOC)(Tables S2-S4).All the calculations were performed using the IBM SPSS Statistics v.22.0 software(SPSS,Armonk, NY: IBM Corp).
Engelhardia phylogenetic trees were reconstructed using both cpDNA haplotypes and nuclear ribosomal DNA (nrDNA) ribotypes following protocols established by Zhang et al. (2020) and Meng et al. (2022). Total genomic DNA of Engelhardia was extracted using the Plant Genomic DNA Kit (Tiangen Biotech, China). Five cpDNA regions (trnS-trnG, rps16, trnL-trnF, psbA-trnH, and rpl32-trnL) and one ribosomal region (nrITS) were amplified by polymerase chain reaction (PCR). All the targeted sequences were aligned, concatenated, and edited manually using Geneious v.6.1.2(https://www.geneious.com/). The combined cpDNA haplotypes(H) and nuclear ribosomal DNA (nrDNA) ribotypes (R) were analyzed using DNASP v.6 (Rozas et al., 2017). The relationships between the haplotypes and ribotypes for the lineages were analyzed using a median-joining network in NETWORK v.2.0(Bandelt et al., 1999) and Splits Tree v.4.14.8 (Huson and Bryant,2006). Both the cpDNA haplotypes and nrITS ribotypes data sets were subjected to Bayesian analyses using MrBayes v.3.1.2(Huelsenbeck and Ronquist, 2001), and the maximum likelihood(ML)analyses were performed in IQ-TREE v.2.1.1(Minh et al.,2020).
3. Results
3.1. Taxonomic treatments
3.1.1. Engelhardia anminiana H.H. Meng sp.nov. (安民黄杞, ¯an mín hu′ang qǐ)
TYPE: INDONESIA. Sulawesi. 02°08′31′′S,120°28′23′′E, altitude(alt.)1314 m,October 1,2018,collected by Hong-Hu Meng,Can-Yu Zhang,and Shook Ling Low(holotype:S-3,HITBC;isotypes:S-1,S-2, S-4, HITBC, PE, PEY).
Distribution: Sulawesi, the south border line between S. Sulawesi and C. Sulawesi, near the mountain tracks, open areas.
Note: Engelhardia anminiana has the most unique leaf morphology in Juglandaceae,possessing scaly and large oval leaves,and present of scales on the leaf surface(Figs.2E,F and 3A),with a leaf length >20 cm, and width >10 cm. Individual plants were discovered during the flowering phase (October).
Phenology: Flowering in October.
Etymology: The specific epithet “anminiana” was named in honor of Prof.An-Min Lu from Institute of Botany,Chinese Academy of Sciences, Beijing. He has devoted his life to the phylogeny and evolution of angiosperms as well as plant taxonomy and geography.Further,he is an expert on the family Juglandaceae,and the leading author of this family in Flora of China(Kuang and Lu,1979;Lu et al.,1982,1999).
3.1.2. Engelhardia borneensis H.H. Meng sp. nov. (婆罗洲黄杞, p′o lu′o zh¯ou hu′ang qǐ)
TYPE: MALAYSIA. 03°42′08′′N,115°29′41′′E, alt.1147 m, Oct.18,2019, collected by Hong-Hu Meng, Lang Li, and Shook Ling Low(holotype:BM-009 HITBC;isotypes:BM-001,BM-002,BM-003,BM-004,BM-005,BM-006,BM-007,BM-008,BM-010,HITBC,PE,PEY).
Distribution:Bario to Miri,Sarawak,along the mountain tracks from Bario to Miri, habitat in dense forest.
Note: Engelhardia borneensis is the biggest tree in this genus(Fig. 4), growing in humid habitats. It is distributed in the valleys but is an emergent tree species that grows higher than the other nearby trees on the slope.The tree trunk of this species is huge,as shown in Fig.4,in which the author(Meng H.-H.)stands next to the trunk with plate-like roots.Its fruit is reddish,with a spike length of ca.7.2 cm (Figs. 4 and 5), and sometimes is longer than 10 cm.

Fig. 2. Morphological characteristics of Engelhardia anminiana. A, fruit branch and scaly leaf axial surface; B, branch and abaxial surface of scaly leaf; C, sprout; D, the female inflorescences; E, detailed feature of the scaly leaf axial surface; F, detailed feature of the abaxial surface of scaly leaf; G, fruit. Scale bar = 5 cm.
Phenology: Fruiting in October.
Etymology: The specific epithet “borneensis” was given in reference to the distribution locality,Borneo,where the species was discovered.Borneo is one of the global biodiversity hotspots where scientists have discovered many new plant species,and the epithet was nominated as “borneensis”.
3.2. Key to the species of Engelhardia and the taxonomic description
The trees are deciduous (e.g., Engelhardia spicata and the varieties) or evergreen, and monoecious or rarely dioecious (e.g.,E. spicata). The branchlets are with solid pith. Terminal buds are oblong,naked,green,or brown.Leaves are even-pinnate,rarely oddpinnate (few in E. roxburghiana and E. fenzelii); leaflets 2 to 14,margin of leaves are entire or serrate, axial and abaxial surface are glabrous,rarely scaly(e.g.,E.anminiana).Inflorescences are lateral or terminal on the old or new branch,respectively. Androgynous panicles of male and female spikes on the same tree or separate on different trees. Male spikes are solitary or clustered and pendulous,while female spikes are erect in flower and pendulous or recurved in tender fruit.Flowers are anemophilous.Male flowers have a 3-lobed bract;2 bracteoles are rarely absent.Sepals are 1-4 are rarely absent.There are 3-15 stamens adhered to the glabrous or pubescent anthers. Female flowers are light green or reddish-brown, subtended by an enlarged 3-lobed bract and 2 united bracteoles reduced to a low rim or forming a conspicuous, anterior prophyll and adnate to the base of the ovary.Sepals 4 pieces are adnate to the ovary and free at the apex.Styles are absent or elongate,and stigmas are carinal or commissural and 2-lobed,with 2-4 plumose branches or short and 4-lobed.Fruits spikes elongate,grow over time,and pendulous wing fruits are 3-lobed with 2-4-chambered nutlets at the base. The germination is epigeal. The above-mentioned full descriptions are adapted from Lu et al.(1999).
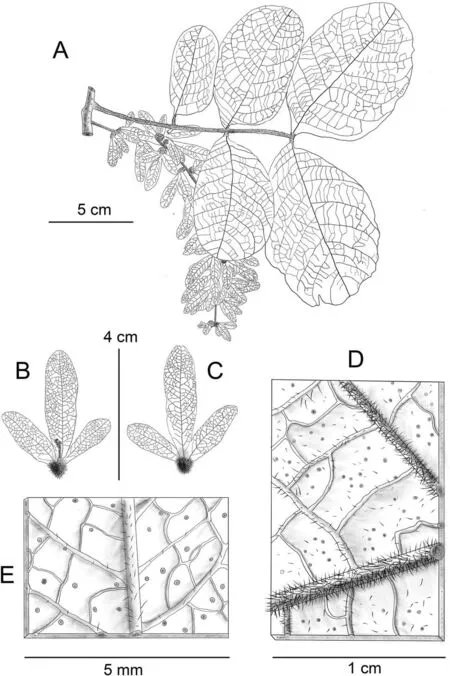
Fig.3. Line illustration of Engelhardia anminiana.A,fruit branch and scaly leaf axial surface;B,axial surface of fruit;C,abaxial surface of fruit;D,abaxial surface and the hispid hairs of leaflet; E, abaxial surface and the hispid hairs of fruit. Drawn by Jian-Yong Shen from Xishuangbanna Tropical Botany Garden.
3.3. Key to the species of Engelhardia (revised after Zhang et al.,2020)
1. Inflorescences are terminal; pistillate flowers and fruits are glabrous; prominently stalked; bracts at the fruit base and terminal bud glabrous and comb-like; leaves evergreen; leaflets entire,glabrous,and conspicuously stalked.

Fig. 4. Morphological characteristics of Engelhardia borneensis. A, fruit branch; B, bud; C, axial leaf surface and abaxial surface with glandular dots; D, leaf surface and branch; E,axial leaf surface and branch; F, fruit surface; G, fruit axial surface; H, the colour of fruit (reddish); I, trees of E. borneensis from the valley is always higher than the surrounding species on the slope, the yellow arrow denoted the big trees of E.borneensis;J,the biggest trees of Engelhardia with buttress roots,and the author(H.-H.Meng).Scale bar=5 cm.
2. Twigs are dark-brown or black;leaflets are usually in 3-5 pairs and most are shortly acuminate at the apex, secondary leaflet veins are in 7 (5-13) pairs………………………E. roxburghiana
2. Twigs are grayish-white; leaflets only 1-2 pairs and most leaflets acuminate at the apex; secondary leaflet veins in 4 (3-6)pairs…………………………………………………………E. fenzelii
1. Inflorescences are lateral; pistillate flowers and base of fruit with hispid hairs, typically subsessile; fruit are covered with bracts; terminal buds are hirsute; leaves evergreen or deciduous;leaflets serrate or entire,glabrous or hirsute,and stalked or sessile.
3. Leaflets entire and present with scales/ruga,axial surface lightly hirsute abaxially;elliptic at the apex,oblique at the base;similar leaflet size on the same branch……………………E. anminiana
3. Leaflets entire or serrate in spire/young leaf,glabrous or hirsute,acuminate or elliptic at the apex,rounded or oblique at the base;lower leaflets reduced in size or gradually becoming smaller.
4. Leaflets usually entire or serrate in the sapling (leaves of seedlings are serrate), somewhat variable in size and shape of the leaves and fruit,glabrous to densely hirsute on the fruits and/or leaves,acuminate or elliptic at the apex of the leaves,the lower leaflets usually reduced in size…………………………E. spicata
4. Leaflets are always serrate,glabrous or hirsute,and acuminate at the apex; or leaflets are gradually becoming smaller or lower leaflets strongly reduced in size.
5. Leaflets are sessile, glandular, glabrous to slightly pubescent along the midvein abaxially;leaflets are in clusters at the apex of the branches;lower leaflets strongly reduced in size;branchlets glabrous………………………………………………E. hainanensis
5. Leaflets sessile or subsessile; glabrous or hirsute; leaflets gradually becoming smaller; branchlets hirsute.
6. Leaflets sessile,hard serrate,glabrous,branchlets lightly hirsute;secondary leaflet veins in 7 (6-10) pairs…………………………………………………………………………………E. serrata
6. Young leaflets light yellow, sessile or subsessile, scales usually bright golden yellow in mature leaves but sometimes dark green, densely hirsute, branchlets densely hirsute; secondary leaflet veins in 6 (5-10) pairs…………………………E. villosa
7. Leaflets with brown glandular dots on both sides; axial surface lightly hirsute; ab-axial surface with dense brown-hirsute;back; fruit reddish with the dense reddish hirsute, secondary leaflet veins in 13 (10-16) pairs, humid habitat…………………………………………………………………E. borneensis
7. Leaflets strongly stalked, tapering from the base to the apex;reddish and scaly on the midrib,low leaflets about the same size as the others; petiole 3.5-4 cm, secondary leaflet veins in 8(7-10) pairs, open areas………………………………E. apoensis
3.3.1. Engelhardia roxburghiana Wallich, Pl. Asiat. Rar. 2: 85.1831. (黄杞, hu′ang qǐǐ)
According to the FOC, the species Alfaropsis roxburghiana,
Engelhardia chrysolepis,E.formosana,E.roxburghiana f.brevialata,E. spicata var. formosana and E. unijuga are considered as the synonyms. We accept the treatment of the taxonomy of Engelhardia in the FOC.
Diagnosis:This species differs from Engelhardia fenzelii in bracts at the base of the fruit;leaf and fruit are glabrous;bud and sprout are brown; leaflets are relatively numerous,ca. 2-7 pairs.
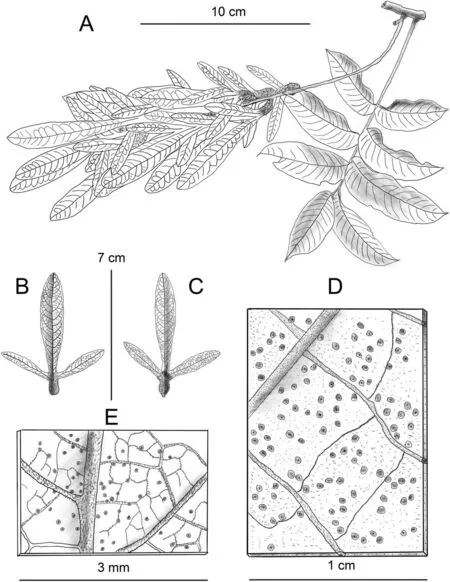
Fig.5. Line illustration of Engelhardia borneensis.A,fruit branch and scaly leaf surface; B,axial surface of fruit;C,abaxial surface of fruit;D,abaxial surface and the hispid hairs of leaflet; E, abaxial surface and the hispid hairs of fruit. Drawn by Jian-Yong Shen from Xishuangbanna Tropical Botany Garden.
Description:Trees are tall,up to 30 m tall;buds are brown.Evenpinnate leaves and rarely odd-pinnate, vary in scale from 1 to 25 cm;petiole 1-8 cm,glabrous;rachis glabrous;entire leaflets are 2-14,petiolule 2-15 mm,blades elliptic-lanceolate to long elliptic,leaf area about 14 × 5 cm; ab-axially glabrous, base oblique, apex acuminate or shortly acuminate. Globose and glabrous nutlets about 3-5 mm;glabrous wings,middle wings 1.5-5 cm and lateral wings 0.7-2.7 cm. Flowering phase is in May-June, fruiting in August-September. 2n = 32.
Distribution: This species is widely distributed from the south Yangtze River to the Indo-China Peninsula, the Malaya Peninsula,Borneo, the Indonesian Archipelago, and Papua New Guinea.
Additional specimens examined: See Table S5.
3.3.2. Engelhardia fenzelii Merrill. Lingnan Sci. Journ. 7: 300.1929. (少叶黄杞, shǎo y‵e hu′ang qǐ)
Diagnosis:This species differs from Engelhardia roxburghiana by having bracts are at the fruit base; leaves and fruit are glabrous;buds and sprouts are bright green; leaflets relatively few, ca.1-3 pairs.
Description: Trees are tall at 10-15 m, with green buds. Leaves even-pinnate, rarely odd-pinnate, 8-16 cm; petiole 1.5-4 cm,glabrous; rachis glabrous; entire leaflets 2-6, petiole is 2-15 mm,blades are elliptic-lanceolate to long elliptic. Leaf area is about 14 × 5 cm; abaxially glabrous, base oblique, apex acuminate or shortly acuminate. Globose nutlets are about 3-5 mm, glabrous;wings glabrous, middle wings are 6-8 cm, and lateral wings are 1.5-2.2 cm. Flowering phase in May to July, fruiting in July-September. 2n = 32.
Distribution: This species is mainly distributed in eastern China, i.e., Guangdong, Fujian, Jiangxi, and Zhejiang. There is a geographic substitution and/or overlap between Engelhardia roxburghiana and E. fenzelii.
Additional specimens examined: See Table S5.
3.3.3. Engelhardia anminiana H.H. Meng sp. nov. (安民黄杞, ¯an mín hu′ang qǐǐ; Figs. 2 and 3)
Diagnosis:This species differs from other species of Engelhardia by having scaly leaves, which is a unique leaf characteristic in the genus; buds and sprouts are bright green; fruit are encased with bracts with hispid hairs; leaves and fruits are glabrous; buds and sprouts are bright green; leaflets are bigger but relatively fewer,1-4 pairs.
Description: Trees are tall at 10-20 m. Leaves have scales along the veins (unique leaf morphology in Engelhardia). Bright green buds and sprouts (Fig. 2C). Even-pinnate leaves are 8-20 cm;petiole 2-5 cm, brown hairy beneath or sessile or both; rachis hairy; entire leaflets 2-8, petiolule 1-1.5 mm, blades elliptic and broad.Leaf area about 11×24 cm;hispid along the abaxial rachis;leaflets with oblique base, apex shortly acuminate, scales present along the veins. Nutlets are globose, 3-6 mm, hairy bracteoles;wings hairy, middle wings 4-6 cm, lateral wings 2-5 cm. Flowering phase is in October.
Distribution:This species is distributed along the road and edges of the primary forest on rocky and steep slopes, near the border between South Sulawesi and Central Sulawesi.
Additional specimens examined: See Table S5.
3.3.4. Engelhardia spicata Leschenault ex Blume, Bijdr.10: 528.1825. (云南黄杞, yún n′an hu′ang qǐ)
In this species, Engelhardia aceriflora and Pterilema aceriflorum are considered as the synonyms of Engelhardia spicata var. aceriflora. Engelhardia colebrookeana, E. esquirolii, E. pterococca var.colebrookeana,E.spicata var.integra are considered as synonyms of E. spicata var. colebrookeana.
Diagnosis:This species differs from other species of Engelhardia by having various leaf and fruit characteristics. For example,E. spicata has entire, sessile, or petiolulate leaves; blade that are abaxially glabrous or pubescence and elliptic,elliptic-lanceolate,or elliptic-ovate; buds and sprouts are brown; fruit are encased with hispid-hairs on the bracts. The young leaves of E. spicata are pubescent and serrate, however, adult leaves vary. Specifically, the adult leaflets of E. spicata var. spicata are glabrous; the mature leaflets of E. spicata var. colebrookeana are white and gray pubescent or tomentose;the mature leaflets of E.spicata var.aceriflora are thick but glabrous; the mature leaflets of E. spicata var. rigida are similar to those of E. spicata var. spicata but with small fruit(1.5-3 cm).
Description: Trees are tall at 20-30 m. Even-pinnate leaves(rarely odd-pinnate)are 15-35 cm;glabrous and pubescent petiole is about 2.5-11.5 cm; rachis are glabrous or pubescent; entire,sessile, or petiolulate leaflets are 4-14, blades elliptic, ellipticlanceolate, or elliptic-ovate. Leaf area is about 7-15 × 2-7 cm,abaxially glabrous or pubescent,base broadly cuneate,apex shortly acuminate. Globose or ovoid nutlets are about 3-6 mm, hispid;wings hispid at the base,middle wings 2.5-3.5 cm,lateral wings ca.1.5-2 cm. Flowering phase is from November to April, fruiting in January-August. 2n = 32.
1a. Leaflets strongly petiolulate and abaxially glabrous; long fruiting spike(22-)30-45(-60)cm......................4a.var.spicata(云南黄杞, yún n′an hu′ang qǐǐ)
1b. Leaflets are short, strongly petiolulate, abaxially glabrous;fruiting spike short and the fruit is very small, about 1.5-3 cm long and 1-2 cm wide......................4b.var.rigida(小果黄杞,xiǎo guǒ hu′ang qǐǐ)
1c. Leaflets are sessile or petiolulate, abaxially pubescent;fruiting spike short,13-30 (-40) cm.
2a. Leaflets are thick, sessile or shortly petiolulate, abaxially slightly pubescent or obtuse, some are hairless from young to mature,apex acuminate or obtuse......................4c.var.aceriflora(爪哇黄杞, zhǎo w¯a hu′ang qǐǐ)
2b.Leaflets are abaxially tomentose from young to mature,and petiolulate or rarely sessile,apex obtuse or acute......................4d.var. colebrookeana (毛叶黄杞, m′ao y‵e hu′ang qǐ)
Distribution: This species and the varieties are always considered as species complex, that are widely distributed from southwest China to the Indo-China Peninsula, the Malaya Peninsula,Borneo, the Indonesian Archipelago, and Papua New Guinea.
Additional specimens examined: See Table S5.
3.3.5. Engelhardia hainanensis Chen, Acta Phytotax. Sin.19: 251.1981. (海南黄杞, hǎi n′an hu′ang qǐǐ)
Diagnosis: This species differs from Engelhardia serrata and E. villosa by having lanceolate and abaxially glabrous leaflets with glandular dots; buds and sprouts are brown; fruit are encased by bracts with hispid hairs.
Description:Trees up to 30 m tall.Even-pinnate leaves are about 15-23 cm; pubescent or glabrescent petiole is 4.8-7 cm, rachis pubescent; leaflets 6-10, petiolule 2-3 mm, blades oblong-ovate or oblong-elliptic. Some leaflets are clustered and leaf area is about 5-10.5×2.5-4 cm with glandular dots,abaxially pubescent along midvein and with scattered hairs in the vein axils, base oblique,subobtuse or subcordate,margin serrate,apex acuminate.Nutlets obovoid,8-10×5-7 mm,hispid;hispid wings at the base,middle wings 5-6 cm,and lateral wings 2.5-2.8 cm.Fruiting phase is in December to January.
Distribution:This species is mainly distributed in Hainan Island,i.e., Bawangling, Dongfang, and Jianfengling; and it could also be distributed in the neighboring mainland, Guangxi (personal communication with colleagues).
Additional specimens examined: See Table S5.
3.3.6. Engelhardia serrata Miquel,Fl.Ind.Bat.Suppl.1:346.1862.(齿叶黄杞, chǐ y‵e hu′ang qǐ)
Diagnosis:This species differs from Engelhardia villosa by having thick short lanceolate leaflets with serrate leaf margin;in addition,these leaflets are abaxially glabrous but lack glandular dots; buds and sprouts are brown; and fruit encased by bracts with hispid hairs.
Description: Trees up to 15-20 m. Leaflets elongate, subsessile,6-18, always serrate at least in part, with crowded smoothmargined yellowish or brownish scales; apex acute to acuminate;stamens 3-7; staminate catkins 1-7 cm long, fruiting catkins 8-15 cm long; fruit 1.5-3 cm long.
Distribution:Manning(1966)mentioned the presence of several varieties of this species that are distributed across Malaysia,Indonesia and the Philippines. We only collected species from Sumatra and Sarawak.Therefore,we were unable to examine all the varieties and conclude whether this species should be considered as a species or species complex.
Additional specimens examined: See Table S5.
3.3.7. Engelhardia villosa Kurz, Forest Flora British Burma.2:491.1877. (柔毛黄杞, r′ou m′ao hu′ang qǐǐ)
We suggested that Engelhardia serrata var. cambodica is considered as a synonym.
Diagnosis:This species differs from Engelhardia serrata by having the light and thin leaflets with tomentose;lanceolate leaflet margin is irregularly serrate or entire; the lower leaflets is highly reduced in size; buds and sprouts are yellow and bright green; fruit is encased with hispid hairs bracts.
Description: Trees up to 12 m tall. Even-pinnate leaves (rarely odd-pinnate) are 15-25 cm; tomentose petiole is 1-2 cm; rachis tomentose; leaflets 6-14, sessile to shortly petiolulate, blades elliptic or elliptic-lanceolate.Leaf area is about(2-)6-13×(1.5-)2.5-4.5 cm, abaxially tomentose, base broadly cuneate to obtuse,margin irregularly serrate or entire,lower leaflets is highly reduced in size; apex acute or shortly acuminate. Golden yellow scales intermixed with hairs abaxially on the leaflets.Nutlets globose,ca.3 mm, hispid; wings hispid at the base, middle wings 2-2.5 cm,lateral wings ca.1.3 cm. Flowering in February, fruiting in April.
Distribution: This species is distributed sporadically from southwest China to the Indochina Peninsula, and the Malay Peninsula.
Additional specimens examined: See Table S5.
3.3.8. Engelhardia borneensis H.H.Meng sp.nov.(婆罗洲黄杞,p′o lu′o zh¯ou hu′ang qǐ)
Diagnosis: This species differs from Engelhardia spicata var. spicata and E.apoensis in having brown glandular dots present on both sides of the leaflets with brown tomentose; leaflets with broadly cuneate to obtuse base;buds and sprouts are brown;reddish fruit is encased with bracts and hispid hairs.
Description: Trees to 40-50 m tall. Even-pinnate leaves (rarely odd-pinnate) 15-25 cm and brown glandular dots are on both sides; axial surface lightly hirsute, abaxially densely brown-black hirsute; tomentose petiole about 5-8 cm; rachis tomentose; leaflets are 4-12,sessile to shortly petiolulate,blades elliptic or ellipticlanceolate. Leaf area is about 3 × 8 cm, abaxially tomentose, base broadly cuneate to obtuse; apex acute or shortly acuminate, secondary leaflet veins in 13 (10-16) pairs; reddish fruit with dense reddish hirsute, globose nutlets ca. 5 mm, with reddish hispid;wings hispid at the base and middle wings 7-9 cm,lateral wings ca.4 cm. Flowering phase is unsure, fruiting in October.
Distribution: This species represents the biggest trees of Engelhardia that were recorded in our field investigation.The species is,so far,the only species that prefers a humid habitat and not in open areas. It was discovered in Bario and Miri, Sarawak, Malaysia.
Additional specimens examined: See Table S5.
3.3.9. Engelhardia apoensis Elmer ex Nagel,Bot.Jahrb.Engler.50:477.1914. (阿波黄杞, ¯a b¯o hu′ang qǐ)
Diagnosis: This species differs from Engelhardia borneensis and E. spicata var. spicata by having leaflets like E. spicata, but with brown tomentose; buds and sprouts are brown; fruit are encased with reddish pubescent or hairy bracts.
Description:Trees 20-30 m tall,dioecious.Brown buds;leaflets are strongly stalked, uniform, 6-10; even-pinnate leaves (rarely odd-pinnate) are about 15-25 cm; staminate flowering not been reported.Catkins of the cluster are elongate and slender,8-11 cm;fruit with elongated body up to 5-7 cm, reddish pubescence.
Distribution:This species was recorded in Mindanao,Philippines and we collected individuals from Fraser's Hill,Malaysia.The type is Elmer 11744 from Mindanao, Philippines, and the specific epithet“apoensis”was named after the distribution region,Apo Mount,the summit of the Philippines.
Additional specimens examined: See Table S5.
3.4. Morphological clustering
The morphological traits of 738 specimens were explored and examined as morphological clusters using a PCA (Fig. 6a) and discriminant analysis(Fig.6b).The first three principal components accounted for 62.71%of the variation across all the characters,with the variation in the first principal component explaining 39.15%of the variation(Fig.6a).The morphological traits of the first PCA axis(value score >0.5) were the terminal/lateral inflorescences, inflorescences of old/new branches, branchlet hairs, fruit hairs,inflorescence position, terminal bud hairs, leaflet hairs, number of leaflets, petiole length, and leaflet arrangement. The second principal component of the total morphological information had traits inclusive of the leaflet apex, leaflet hairs, and leaflet thickness,which explained 13.04% of the variation. The third principal component was associated with the leaflet arrangement, twig color, and leaflet margins explained 10.52% of the variance. The results of the PCA suggested that there were nine distinct groups for Engelhardia,and all nine species were well-identified except for the E. spicata complex. The morphology of E. roxburghiana is similar with that of E. fenzelii but different from the other species, i.e.,
E.apoensis,E.anminiana,E.borneensis,E.serrata,E.hainanensis,and E. villosa. Based on these morphological traits, all the species exhibited clear species boundaries (Fig. 5a).
The group centroid from the discriminant analysis had similar results to the PCA, i.e., the groups were separated for each species except the E. spicata complex (four varieties of E. spicata; Fig. 6b).The results of the discriminant analysis for the respective characters showed a good discriminant function, with a total of 88.1% of the original grouped cases and 85.9%of the cross-validated grouped cases correctly classified.Overall,the morphological analyses from the PCA and discriminant analysis showed very similar morphological clustering (Fig. 6). Therefore, the morphological clusters support the delimitation of the E. spicata and the other sister species.
3.5. Phylogeny from cpDNA and nrITS
From all the sampled individuals in the populations (Table S1),five aligned cpDNA (trnS-trnG + rps16 + trnL-trnF + psbAtrnH+rpl32-trnL)spacers were sequenced,resulting in a dataset of 4647 bp and 48 haplotypes were determined. Also, the nrITS resulted consisted in a dataset of 755 bp, and 39 ribotypes were determined(Table S1).The haplotype and ribotype sequences were deposited in NCBI with the following accession numbers:OM296106-OM296114, OM304894-OM304938, and MN307497-MN307736.
In general, the reconstructed phylogenetic tree that was based on the cpDNA had a well-resolved phylogenetic backbone from the multi-locus DNA and provided clear major clades for Engelhardia.The topologies of the phylogenetic trees reconstructed with Bayesian and ML methods were nearly identical. Although the cpDNA and nrITS phylogenetic trees of Engelhardia were slightly conflicting, the relationships among all the species of Engelhardia could be clearly identified except for the varieties of E. spicata var.spicata and E. spicata var. rigida that were more complicated than the other taxa (Fig. 7). The obtained phylogram supports the species delimitation of Engelhardia according to Zhang et al. (2020).
The phylogeny of the cpDNA haplotypes and nrDNA ribotypes showed E. hainanensis as a distinct lineage that is distributed in Hainan Island, supporting E. hainanensis as a veritable endemic species. Although E. hainanensis had a close affinity with E.borneensis in the cpDNA trees,the long distance between Hainan Island and Borneo suggests that E. borneensis is a good species.E.fenzelii is only distributed in Southeast China and was identified as a sister lineage to E. roxburghiana and E. apoensis, where the unique morphology of the species indicated that E. roxburghiana,
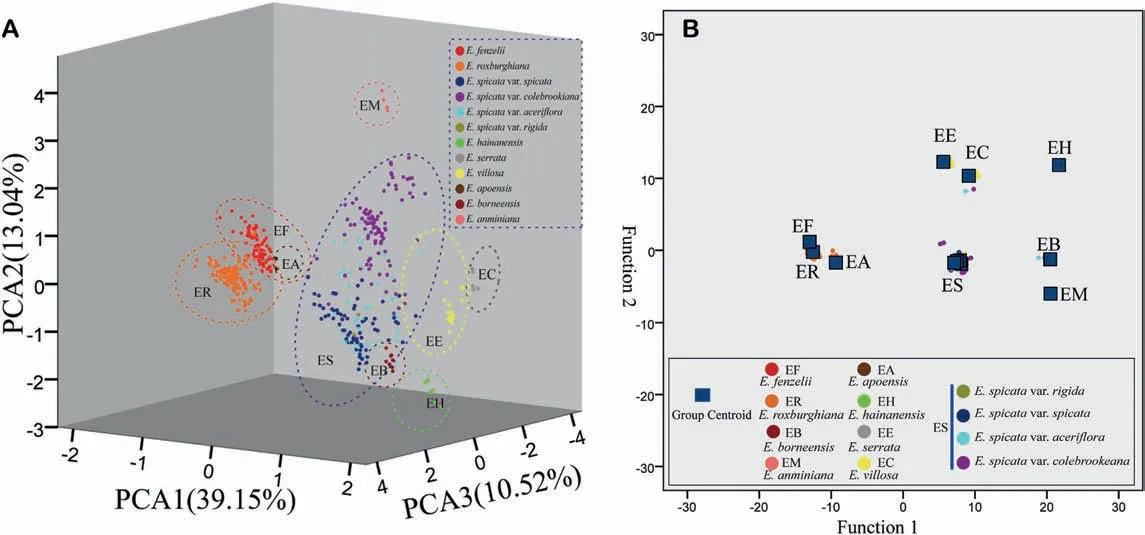
Fig. 6. A, Scatterplots based on principal component analysis (PCA) scores for each individual (shown as a dot) evaluated, and the coloured and dotted circles enclose individuals belonging to the same component;B,canonical discriminant analysis,the blue squares represent each group centroid of classification,and the dots represent off-centre individuals.The list of taxa and colour scheme are the same in A and B.
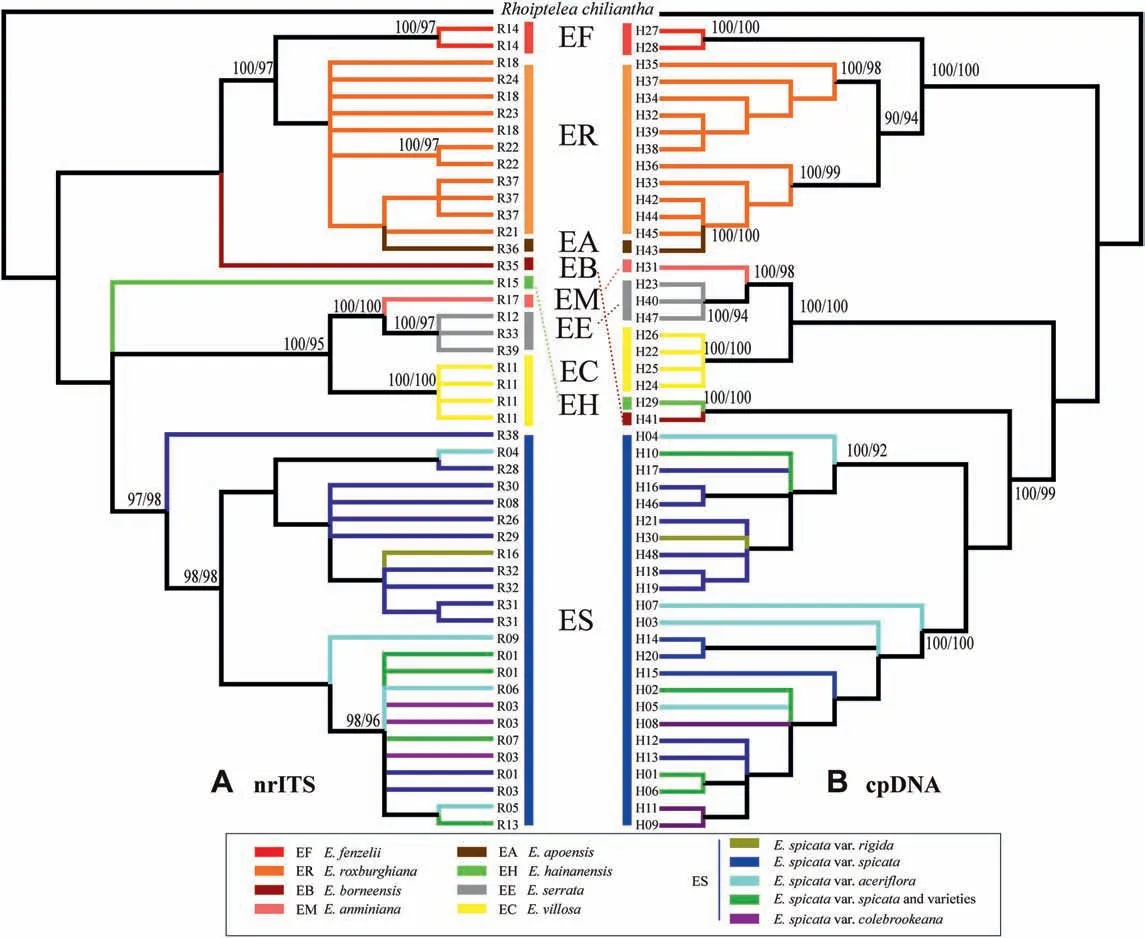
Fig. 7. Phylogeny of Engelhardia from Bayesian consensus tree based on nrITS ribotypes (A, left) and the combined cpDNA haplotypes (B, right); posterior probability support of Bayesian (before) and bootstrap support values from ML analyses (after) are given above the major branches (>90% values).
E. fenzelii and E. apoensis are three species. E. anminiana and E. serrata had a close affinity, and the two species were closer to E. villosa. This result was supported from the phylogeny from cpDNA and nrITS data (Fig. 7). Specifically, E. roxburghiana,E. borneensis, and E. fenzelii were sister lineages in the clades.Additionally, the clear morphological traits among the three species, the remote distance from E. apoensis, and the two established-well species indicated the subclades of the three species, E. apoensis, E. fenzelii and E. roxburghiana. The phylogenetic position of the new species,E.borneensis,differed depending on the data set used for phylogenetic analysis.For instance,when we used nrITS data, E. borneensis was identified as a close relative of E.roxburghiana,E.apoensis and E.fenzelii(Fig.7A).However,when cpDNA data was used, E. borneensis was more closely related to with E. hainanensis (Fig. 7B). Further, our analysis indicated that E.anminiana was a closer relative to E.serrata,which occurs in the same region in Sulawesi(Fig.7).In addition,E.serrata and E.villosa were confirmed to be the distinct lineages. Both cpDNA and nrITS analyses indicated that E.spicata var.rigida and the other varieties(i.e.,E.spicata var.spicata,E.spicata var.aceriflora,and E.spicata var.colebrookeana) are intermixed, forming a species complex (Fig. 7).
4. Discussion
4.1. Taxonomic notes for Engelhardia
The taxonomic description of Engelhardia has been pursued for several decades (Manning, 1966). However, the phylogeny of Engelhardia was less well described and mainly focused on the Juglandaceae (Manos and Stone, 2001; Song et al., 2020; Zhang et al., 2022a), possibly due to the inaccessibly of specimen materials for fine investigation. Moreover, the lack of representative specimens from throughout its distribution ranges limited the taxonomic description of Engelhardia, and the species numbers of Engelhardia remained unresolved(Lu et al.,1999).Therefore,it was necessary to revise the description of Engelhardia by integrating their morphology, molecular phylogeny, and geographic distribution. This integration led to the first study on detailed species delimitation on Engelhardia (Zhang et al., 2020). Despite this, there were still some inevitable weaknesses in the revision of Engelhardia.In this study,we describe two new species of Engelhardia from Indonesia, E. anminiana and E. borneensis. We also integrated field investigations,literature reviews,and molecular data to accurately delimit all known species of Engelhardia. Here,we provide several useful taxonomic notes on fallible species of Engelhardia.
Firstly,in terms of the fruit traits,Engelhardia roxburghiana and E.fenzelii are the only two species that possess glabrous fruits at the fruit base without hispid hairs; whereas the rest of the species in Engelhardia are characterized by having bracts with hairs at the fruit base.The taxonomy of E.roxburghiana and E.fenzelii has been controversial (Manos and Stone, 2001; Manos et al., 2007; Stone,2010). Iljinskaya (1993) proposed that E. roxburghiana should be described as a new monotypic genus with the only one species,Alfaropsis roxburghiana. However, Alfaropsis has since been generally treated as a synonym of Engelhardia(Lu et al.,1999;Manos and Stone, 2001; Stone, 2010; Meng et al., 2015, 2022; Hermsen and Gandolfo, 2016). Morphological and molecular analyses in our study confirm that A.roxburghiana is a synonym of E.roxburghiana;thus, we reject resurrecting Alfaropsis as a monophyletic genus in the Juglandaceae. Previous studies have also combined E.roxburghiana and E.fenzelii as a synonym for the dry specimens in herbaria which generally do not show some distinct characters,such as the color of the twigs and buds. However, relying on the number of leaflets has limited the identification of both species.Although the two species are similar in the dry specimens,they can be distinguished by several traits,such as the glabrous flowers and fruits and terminal inflorescences. Specifically, E. fenzelii possesses the greyish white twigs with 1-2 pairs of leaflets that have 4(3-6)pairs of secondary veins; while E. roxburghiana has dark brown or black twigs with 3-5 pairs of leaflets and 7 (5-13) pairs of secondary leaflet veins (Kuang and Lu 1979). Additionally, the distribution range of the two species has a substitution and/or some overlapping, i.e., E. roxburghiana is distributed widely across tropical and subtropical Asia, while E. fenzelii is restricted to the eastern China.
Secondly, leaf traits in Engelhardia, but caution should be exercised when using these traits for species identification. For example, the leaflets of E. anminiana have scale-like structures along the veins and brown-hispid hairs, which are unique in Juglandaceae. The leaves of E. villosa are thin and yellowish,whereas the leaves of E.bornensis has brown glandular dots on both sides.Even within the leaves of E.spicata complex,leaf traits differ among varieties. For instance, E. spicata var. aceriflora has thick leaves, whereas E. spicata var. colebrookeana has white and yellow pubescence on the surface of leaves,E.anminiana has brown-hispid hairs. Leaflets margin is usually smooth, except for adult E. serrata has essentially serrate from the base to apex of leaflets.Importantly,not all the species with serrate leaflets are E.serrata,e.g.,the young trees of E. spicata complex and E. villosa, which also have serrate leaflets. Furthermore, E. hainanensis has leaflets that are slightly serrated with glandular dots. According to Manning (1966),E. serrata has some varieties. Nevertheless, we collected the sclerophyll with serrate leaflets and considered it to be E.serrata.Thus,caution must be taken when working with E.serrata,or any variety with serrate leaflets.We recommend using the presence of serrate leaflets in conjunction with other characters for species identification.
Thirdly, based on the ecological habitat, most Engelhardia species are in sunny and open areas. In contrast, our field investigations revealed that E. borneensis inhabits humid and wet habitats in the valleys of dense forests.This newly described species also has the biggest trees in Engelhardia (Fig. 4).
4.2. Phylogeny and morphological cluster for Engelhardia
Phylogenetic analyses based on nrITS ribotypes and combined cpDNA haplotypes of all Engelhardia species showed clear species boundaries (Fig. 7). Furthermore, the topology of Engelhardia phylogenetic trees generated from increased numbers of samples and species in this study supported our previous topology (Zhang et al., 2020). Our nrITS phylogeny indicated that there are eight clear species lineages, i.e., E. fenzelii, E. roxburghiana, E. borneensis,E. hainanensis, E. anminiana, E. serrata, E. villosa, and the E. spicata complex.Two new species described in this study,E.borneensis and E.anminiana,are embedded within the clades comprising E.serrata,E.villosa and E.hainanensis.Although E.apoensis falls into the clade of E. roxburghiana with closer affinity (Fig. 7A), the unique morphological character (as listed in the taxonomic section) between both species that distinctly differentiates them implies that E. apoensis should not be placed as a synonym of E. roxburghiana.Further, E. apoensis is mainly distributed in Borneo and the Philippines, while E. roxburghiana is largely distributed in tropical and subtropical Asia. The topology of phylogram based on combined cpDNA was very similar to the topological structure based on nrITS phylogeny. However, the placement of E. borneensis differed between phylograms based on cpDNA and nrITS data. In the phylogenetic tree of the combined cpDNA,E.borneensis is the sister taxa to E.hainanensis(Fig.7B),However,the special characteristics,e.g., the fruit with reddish pubescence and the leaflets of E. borneensis, have indicated a different morphology from E. hainanensis. The morphological characters possessed by E.fenzelii,E.roxburghiana and E.apoensis also indicate that they are three different species too. In addition, the distribution locality of E.borneensis does not overlap with the geographical localities of the other four species(Fig.1);thus far,it has only been discovered at its type localities in Sarawak, Malaysia. E. hainanensis possesses a narrow distribution range of in Hainan Island as well as E. borneensis in Malaysia. Thus, E. borneensis is considered as good species, even though there is a taxonomic conflict presents between the nrITS and cpDNA phylograms (Fig. 7).
Morphological cluster analysis using PCA and discriminant analysis indicated the distinct species delimitation of Engelhardia(Fig. 6),i.e., E.roxburghiana,E. fenzelii,E. borneensis, E. hainanensis,E.anminiana,E.serrata,E.villosa,E.apoensis and the E.spicata.In the discriminant analysis, E. spicata var. rigida clusters together with the E. spicata (also seen in the phylogenetic trees; Fig. 7). Nevertheless, the distinct morphological traits of the small leaflets and fruit support the affiliation of the E. spicata var. rigida from the E. spicata complex, and other sister species. Additionally, the morphological cluster generated in the PCA showed E. apoensis embedded in the morphological cluster of E. fenzelii (Fig. 6A), but the distribution ranges in eastern China (i.e., E. fenzelii) vs. Borneo and the Philippines (i.e., E. apoensis), and the different morphological traits implied that E. fenzelii and E. apoensis are different species.
4.3. Distribution of Engelhardia
Engelhardia is an endemic genus to tropical and subtropical Asia(Lu,1982;Lu et al.,1999;Stone,2010;Meng et al.,2015;Zhang et al.,2020). However, not all the species of Engelhardia are distributed across Southeast Asia. In this study, we updated the distribution information of Engelhardia species through our field observations.The detailed information on the distributions is summarized as follows:E. roxburghiana and E. spicata var. spicata are ubiquitously distributed at a large scale from subtropical China to Indonesia;E. fenzelii is narrowly distributed in eastern China; E. borneensis is rare and was discovered in Bario and Miri, Borneo; E. anminiana was discovered in one locality in central Sulawesi;E.hainanensis is mainly distributed in Hainan Island, China; E. serrata is mainly distributed from Malaya, to Sumatra, Java, Borneo, and the Philippines(according to Manning,1966);E.villosa has a scattered distribution from China to Malaya;E.apoensis is mainly distributed in Malaysia, Borneo, and the Philippines; E. spicata var. rigida is mainly distributed in Java, Sumatra, Borneo and New Guinea (according to Manning,1966), and we collected it from Sumatra and Sarawak; E. spicata var. colebrookeana and E. spicata var. aceriflora have a scattered distribution from Southwest China to the Indochina Peninsula.
Our large-scale field investigation has re-evaluated which Engelhardia species are rare. Manning (1966) suggested E. apoensis was the only rare species in the genus,as it had only been collected 12 times. We found that E. apoensis is distributed in peninsular Malaysia and Borneo, where several individuals have been discovered in each locality. Furthermore, our field work indicates that additional Engelhardia species are rare: E. hainanensis, E. anminiana and E.borneensis.Importantly,these species have thus far only been observed at their type locality(see the taxonomic section).
Engelhardia habitats are mainly distributed in mountainous regions, and less commonly in the lowlands and plains (Fig.1). This pattern of distribution may be a consequence of human activity(e.g.,infrastructure,farming,cash crops,and deforestation)in Asia in terms of (Meng et al., 2019a). Thus, the human footprint has restricted the expansion of Engelhardia in such anthropogenic zones.Moreover,it is difficult to find the trees of Engelhardia in the deep mountains and forests. The major distribution regions of Engelhardia are in global biodiversity hotspots. However, these regions have also been identified as global priorities for the restoration of biodiversity due to climate change and other criteria(Strassburg et al., 2020). Global warming and anthropogenic activity are contributing to an imminent biodiversity crisis, and studies on biodiversity conservation (Meng et al., 2019b, 2021),which have identified biodiversity loss and climate change as the two crises in the Anthropocene (Corlett, 2020). Thus, the distribution of Engelhardia should be recognized from widespread species to narrowly distributed species, and then the fine-scale ecological and evolutionary processes and/or patterns can be determined in the context of the Anthropocene and against the background of climate change and biodiversity loss.
5. Conclusion
Overall, our study provides a comprehensive revision of the Engelhardia species from the south Yangtze River in China to Indonesia using a large-scale investigation framework.Our findings show that there is an urgent need for inventories,herbarium work,nomenclature, identification, and comparative studies of plant diversity in Southeast Asia, the global biodiversity hotspots. As mentioned,therefore,there is a need to attract young scientists to specialize in plant taxonomy in Southeast Asia (Yang et al., 2020).Over the past twenty years,the Chinese Plant Names Index (CPNI)has added many vascular plans of China(Du et al.,2020).However,surveys onto other plants are still needed, especially in the biodiversity hotpots from China to Southeast Asia.
Species concept and delimitation, also, are fundamental to taxonomic and evolutionary studies (Su et al., 2020). The identification of species is the basis of evolutionary biology and ecology.With in-depth surveys, new delimitations and/or species of the specific taxa are being identified.Thus,the comprehensive revision of specific taxa is becoming a crucial and urgent issue. Southeast Asia has been designated as global biodiversity hotspots and conservation priority for over two decades (Myers et al., 2000).Recognition of the negative effects of ecosystem degradation and conversion on biodiversity has elicited ambitious efforts at national, regional, and global levels (Strassburg et al., 2020). Biodiversity conservation based on recognizing species has had major obstacles to in-depth surveys, without which some plants will disappear before being discovered (Meng et al.,2019a).
After in-depth surveys, this taxonomic revision of Engelhardia from the phylogeny of nrITS and cpDNA showed consistency with the morphological cluster analyses and will further benefit plant taxonomy in Southeast Asia and global biodiversity hotspots.Here, two new and rare species of Engelhardia, E. anminiana and E. borneensis, were discovered in Sulawesi and Borneo. And the discovery of two new species of Engelhardia suggests that new species remain to be discovered in untraversed areas across scattered islands in the Pacific Ocean.Additionally,this study may improve our understanding the plant diversity of subtropical China to tropical Asia, e.g., tropical-subtropical transitions(Ashton and Zhu, 2020), global biodiversity patterns, latitudinal diversity gradient, and maintaining biodiversity in Asia (Raven and Wackernagel, 2020; Zhang et al., 2022b). The systematic position of these two new species was reconstructed, and the phylogenetic relationships within Engelhardia were revised based on expanded morphological and molecular data.Although studies on two genera from Central America (i.e., Alfaroa and Oreomunnea) are necessary to reconstruct the phylogeny of Engelhardioideae in future, the current study provides the most comprehensive revision of Engelhardia species across its most geographic distribution from the southern Yangtze River in China to Indonesia.
Author contributions
HHM designed this research.HHM,CYZ,SLL,LL,N,SSZ and YHT collected the materials.HHM and CYZ analyzed data.JYS drew the line illustrations.HHM,CYZ,JL,SLL and YZ,PHH wrote the paper;all authors contributed to revision of the manuscript.
Declaration of competing interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Acknowledgments
We would like to thank Prof.An-Min Lu,Prof.Zhi-Duan Chen,Dr.Su Liu and Dr. Jian-Wen Zhang for their valuable suggestions of taxonomic treatments in the manuscript. Also, Prof. Ismail, Mr.Dasriel Effendi, Mr. Putong, Dr. Richard Chung-Cheng Kong and Prof. Harry are grateful for the assistance in the field work and herbarium specimens checking in Southeast Asia.The fieldwork in Sarawak (Borneo, Malaysia) was conducted under the research permit no. (90)JHS/NCCD/600-7/2/107/Jld.2 and park permit no.WL45/2019. This work was supported by the National Natural Science Foundation of China (No. 42171063); Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences (No.Y4ZK111B01); Youth Innovation Promotion Association, CAS (No.2018432); and the CAS “Light of West China” Program. Finally, the three reviewers are grateful for the valuable suggestions and comments that help to improve the quality of this study.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2022.08.003.
- 植物多样性的其它文章
- Genetic analysis of walnut cultivars from southwest China:Implications for germplasm improvement
- Development of genomic resources for Wenchengia alternifolia(Lamiaceae) based on genome skimming data
- Fertile Woodwardia from the middle Eocene of South China and its implications for palaeogeography and palaeoclimate
- Does the critically endangered Rhododendron amesiae deserve top priority for conservation?
- An ethnobotanical study of medicinal plants in Güce district,north-eastern Turkey
- Borana rangeland of southern Ethiopia: Estimating biomass production and carrying capacity using field and remote sensing data

