Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
Mia Levite
Abstract T cells are essential for a healthy life,performing continuously: immune surveillance,recognition,protection,activation,suppression,assistance,eradication,secretion,adhesion,migration,homing,communications,and additional tasks.This paper describes five aspects of normal beneficial T cells in the healthy or diseased brain.First,normal beneficial T cells are essential for normal healthy brain functions: cognition,spatial learning,memory,adult neurogenesis,and neuroprotection.T cells decrease secondary neuronal degeneration,increase neuronal survival after central nervous system(CNS) injury,and limit CNS inflammation and damage upon injury and infection.Second,while pathogenic T cells contribute to CNS disorders,recent studies,mostly in animal models,show that specific subpopulations of normal beneficial T cells have protective and regenerative effects in several neuroinflammatory and neurodegenerative diseases.These include Multiple Sclerosis (MS),Alzheimer’s disease,Parkinson’s disease,Amyotrophic Lateral Sclerosis (ALS),stroke,CNS trauma,chronic pain,and others.Both T cell-secreted molecules and direct cell-cell contacts deliver T cell neuroprotective,neuroregenerative and immunomodulatory effects.Third,normal beneficial T cells are abnormal,impaired,and dysfunctional in aging and multiple neurological diseases.Different T cell impairments are evident in aging,brain tumors (mainly Glioblastoma),severe viral infections (including COVID-19),chronic stress,major depression,schizophrenia,Parkinson’s disease,Alzheimer’s disease,ALS,MS,stroke,and other neuro-pathologies.The main detrimental mechanisms that impair T cell function are activation-induced cell death,exhaustion,senescence,and impaired T cell stemness.Fourth,several physiological neurotransmitters and neuropeptides induce by themselves multiple direct,potent,beneficial,and therapeutically-relevant effects on normal human T cells,via their receptors in T cells.This scientific field is called “Nerve-Driven Immunity”.The main neurotransmitters and neuropeptides that induce directly activating and beneficial effects on naïve normal human T cells are: dopamine,glutamate,GnRH-II,neuropeptide Y,calcitonin gene-related peptide,and somatostatin.Fifth,“Personalized Adoptive Neuro-Immunotherapy”. This is a novel unique cellular immunotherapy,based on the “Nerve-Driven Immunity” findings,which was recently designed and patented for safe and repeated rejuvenation,activation,and improvement of impaired and dysfunctional T cells of any person in need,by ex vivo exposure of the person’s T cells to neurotransmitters and neuropeptides.Personalized adoptive neuro-immunotherapy includes an early ex vivo personalized diagnosis,and subsequent ex vivo → in vivo personalized adoptive therapy,tailored according to the diagnosis.The Personalized Adoptive Neuro-Immunotherapy has not yet been tested in humans,pending validation of safety and efficacy in clinical trials,especially in brain tumors,chronic infectious diseases,and aging,in which T cells are exhausted and/or senescent and dysfunctional.
Key Words: aging;dopamine;glutamate;nerve-driven immunity;neurological diseases;neuropeptides;neurotransmitters;Personalized Adoptive Neuro-Immunotherapy;T cells
From the Contents
1.Normal Beneficial T cells– Brief Introduction and Main T Cell Types 1165
2.Normal Beneficial T Cells Are Needed for Normal Brain Development and Function 1166
3.Normal T Cells,Primarily CD4+ T Cells and CD4+CD25+ Regulatory T Cells,Have Protective and Regenerative Roles in Various Central Nervous System Disorders1166
4.T Cells Often Undergo Pathological Changes due to Various Causes and Conditions 1166
5.T Cell Impairments and Dysfunction Are Common and Have Broad Catastrophic Implications 1168
6.T Cells Are Abnormal and Dysfunctional in Aging and Various Neurological Diseases and Injuries 1168
7.Specific Normal Beneficial T Cell Subpopulations Are Therapeutic in Some Neurological Diseases,According to Findings in Animal Models 1172
8.Nerve-Driven Immunity: Neurotransmitters and Neuropeptides Induce Direct,Powerful,and Beneficial Effects on T Cells,via Their Own Functional Receptors 1172
9.“Personalized Adoptive Neuro-Immunotherapy”– A Novel Adoptive T Cell Immunotherapy,Based on Ex Vivo Activation and Improvement of a Person’s T Cells by Neurotransmitters and Neuropeptides,and Their Subsequent Repeated Delivery into the Body 1175
10.Concluding Remarks 1176
1.Normal Beneficial T cells– Brief Introduction and Main T Cell Types
Healthy functional life cannot take place without normal functioning T cells.The ongoing and essential T cell tasks,carried out by various T cell types and subtypes,are numerous,including recognition,positive and negative selection,education,antigen-specific expansion,eradication,assistance,activation,suppression,secretion,adhesion,migration,homing,extravasation,communication,cooperation,and more.
For performing all these tasks at all times,different types and subtypes of T cells are needed and exist.The major types of T cells and how they divide the work among them are summarized below in a very laconic and general manner.
Cytotoxic T cells actively destroy cells infected by infectious organisms (viruses,bacteria or other) and eradicate cancer cells if they express tumor antigens that T cells recognize as foreign.The cytotoxic T cells contain granules that are exocytosed during specific interactions with target cells.In this process,the granule contents,including the lethal protein perforin and granzymes,a family of serine esterases,are secreted and delivered to the target cells,where they cause them to burst open in a process called apoptosis.In therapeutic setups of organ transplantation,it is the cytotoxic T cells that cause undesired and harmful graft rejection,when the transplanted organs are unmatched immunologically with the recipient body.In such setups,the cytotoxic T cells attack the foreign transplanted organ as they would attack an infected organ.Helper T cells activate cytotoxic T cells and macrophages,and stimulate antibody production by B cells.Regulatory T cells suppress the actions of B and T cells,to decrease the immune response when a highly active response is no longer warranted,and to prevent detrimental autoimmunity.Natural Killer T cells distinguish infected or cancerous cells from normal body cells,and attack cells that do not contain molecular markers that identify those cells as the body’s own.Memory T cells protect against previously encountered antigens,and may provide lifetime protection against some pathogens that previously invaded the body,and initiated an immune response against them.T cells have different effects on the CNS,depending on whether they are normal and beneficial,or autoimmune and detrimental.The different T cells “faces” in the brain (the term “faces” in this context means their condition,involvement,and contribution),are shown schematically inFigure 1.This paper focuses on normal beneficial T cells from several (yet surely not all) neurological perspectives.
Search strategy
Studies cited in this review,with regards to each of the multiple reviewed topics,were searched by PubMed,using sets of keywords specific for each topic.
2.Normal Beneficial T Cells Are Needed for Normal Brain Development and Function
The brain needs healthy beneficial T cells for normal brain function (Kipnis et al.,2004,2012;Filiano et al.,2017;Dantzer,2018;Evans et al.,2019;Hodo et al.,2020).
Healthy beneficial T cells are essential for cognition,spatial learning and memory,adult neurogenesis and neuroprotection,as well as for decreasing secondary neuronal degeneration,increasing neuronal survival after CNS injury,and limiting CNS inflammation and damage after injury and infection(Kipnis et al.,2004,2012;Filiano et al.,2017;Dantzer,2018;Evans et al.,2019;Hodo et al.,2020).
In addition,in animal models of a variety of neurological impairments and diseases,normal human T cells have been shown to be beneficial and therapeutic.
3.Normal T Cells,Primarily CD4+ T Cells and CD4+ CD25+ Regulatory T Cells,Have Protective and Regenerative Roles in Various Central Nervous System Disorders
While the pathogenic activities of T cells,primarily autoimmune and cytotoxic T cells,in CNS disorders are well-established,recent studies have shown that normal beneficial T cells have potent protective and regenerative roles in various neurological diseases,ranging from tissue protection to regeneration(Evans et al.,2019).These opposing functions of beneficialversusdetrimental T cells are carried out by either normal T cells or pathogenic T cells,respectively,and by different T cell subsets.
Evans et al.(2019) recently reviewed the beneficial influence of normal T cells in multiple neuroinflammatory and neurodegenerative diseases,including Multiple Sclerosis (MS),Alzheimer’s disease (AD),Parkinson’s disease (PD),Amyotrophic lateral sclerosis (ALS),stroke,and CNS trauma.Both T cellsecreted mediators and direct cell contact-dependent mechanisms deliver neuroprotective,neuroregenerative,and immunomodulatory signals in these diseases.Some of the experimental evidences for the positive contribution of normal beneficial T cells in these neurological diseases,are specified in Part 7 of this review.Each section of Part 7 summarizes the evidences for a different disease.Taken together,all these findings may pave the way to novel therapeutic pathways for neurological disorders,using beneficial T cells.
4.T Cells Often Undergo Pathological Changes due to Various Causes and Conditions
Multiple abnormal and pathological factors,processes,conditions and diseases can adversely affect the vitality,function and longevity of T cells,some of which are mentioned by name inFigure 2.
T cells are impaired in aging and various neurological diseases in the brain,some of which are listed inFigure 3.Herein is a brief summary of the most common pathological processes and mechanisms that impair T cell function under various abnormal conditions.
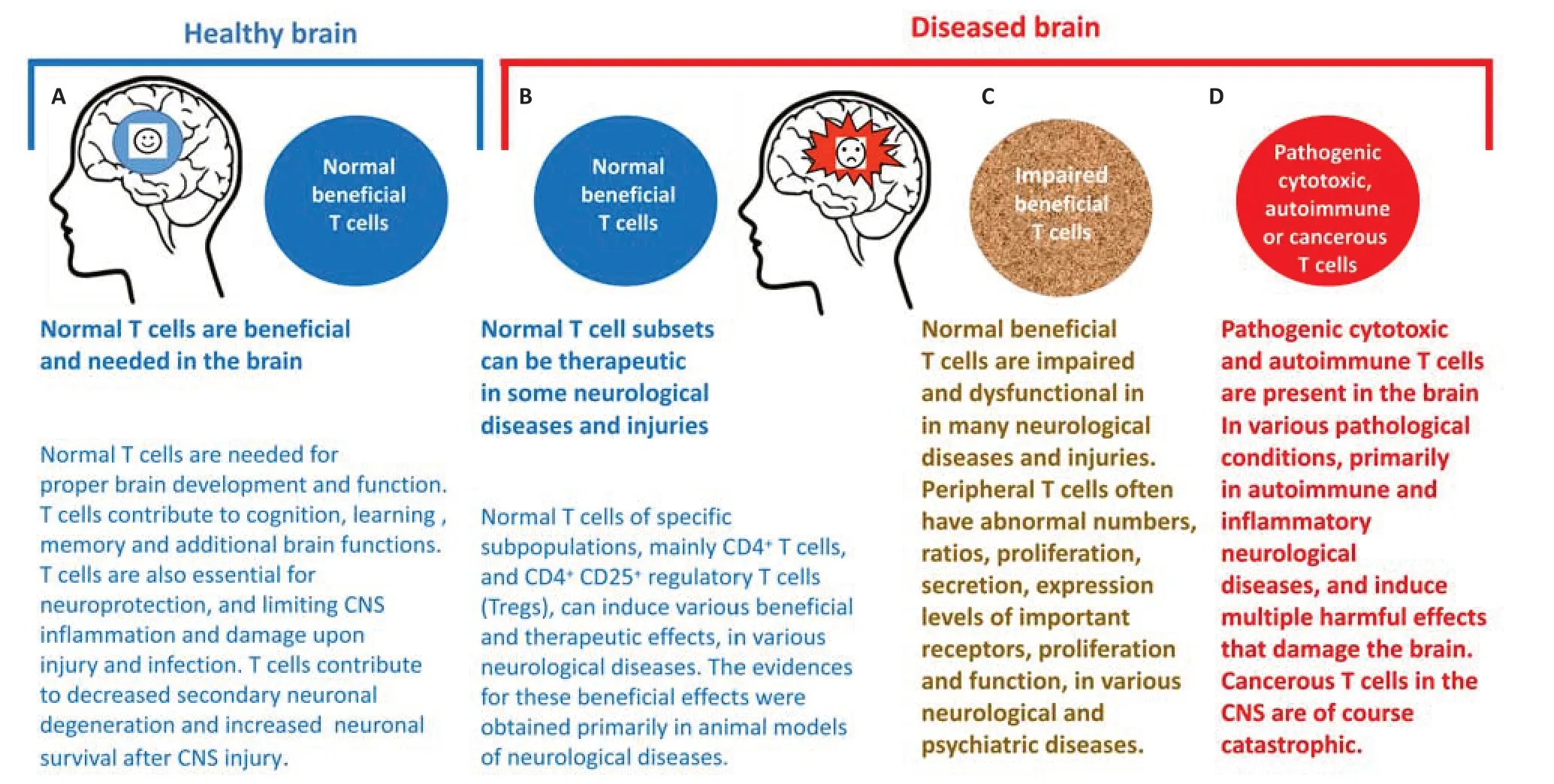
Figure 1|Different neuro faces of beneficial and detrimental T cells in the healthy and diseased brain.
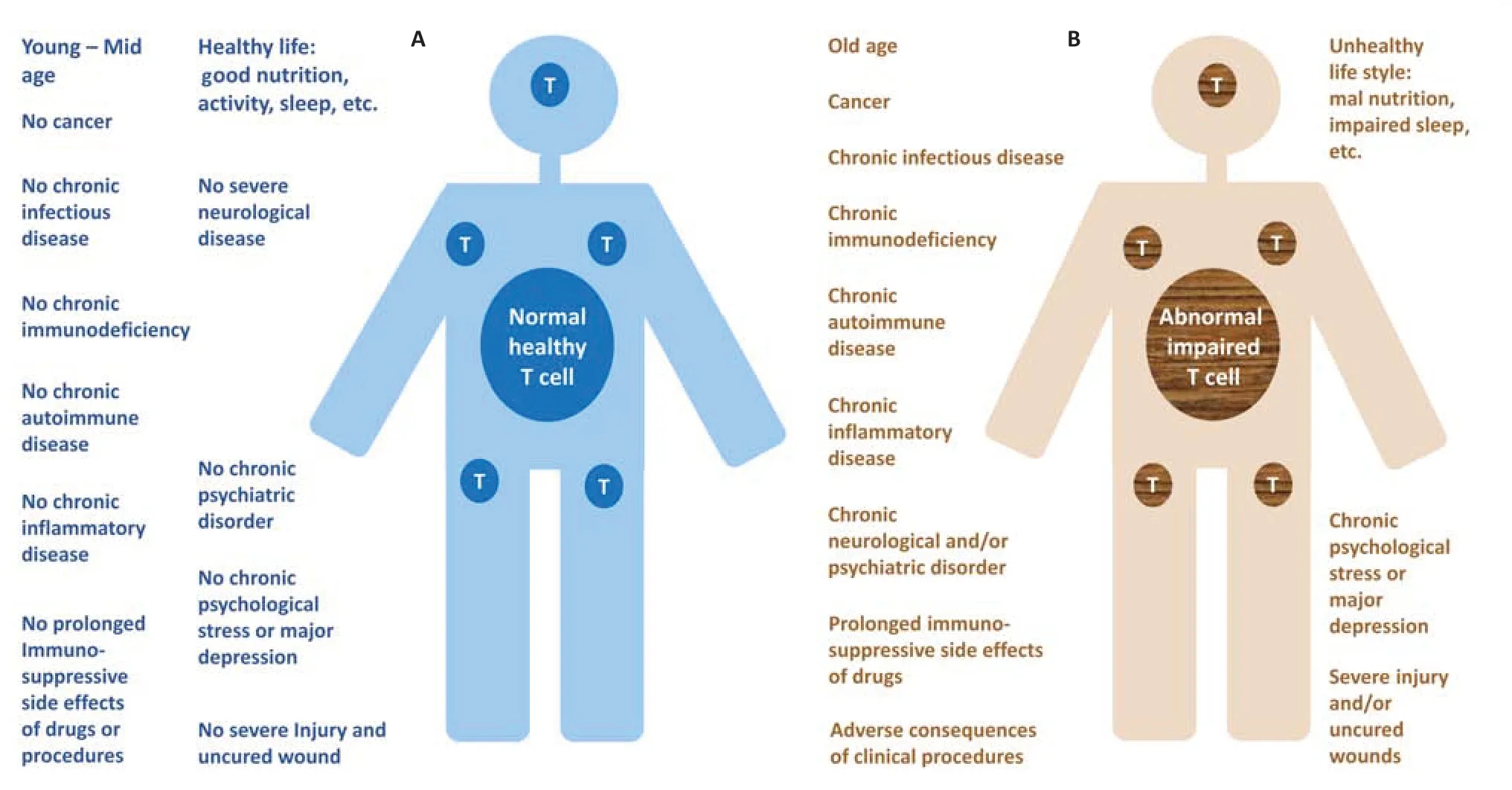
Figure 2|T cells of numerous human beings are abnormal and express a kaleidoscope of impairments in numbers,ratios,features,and functions.
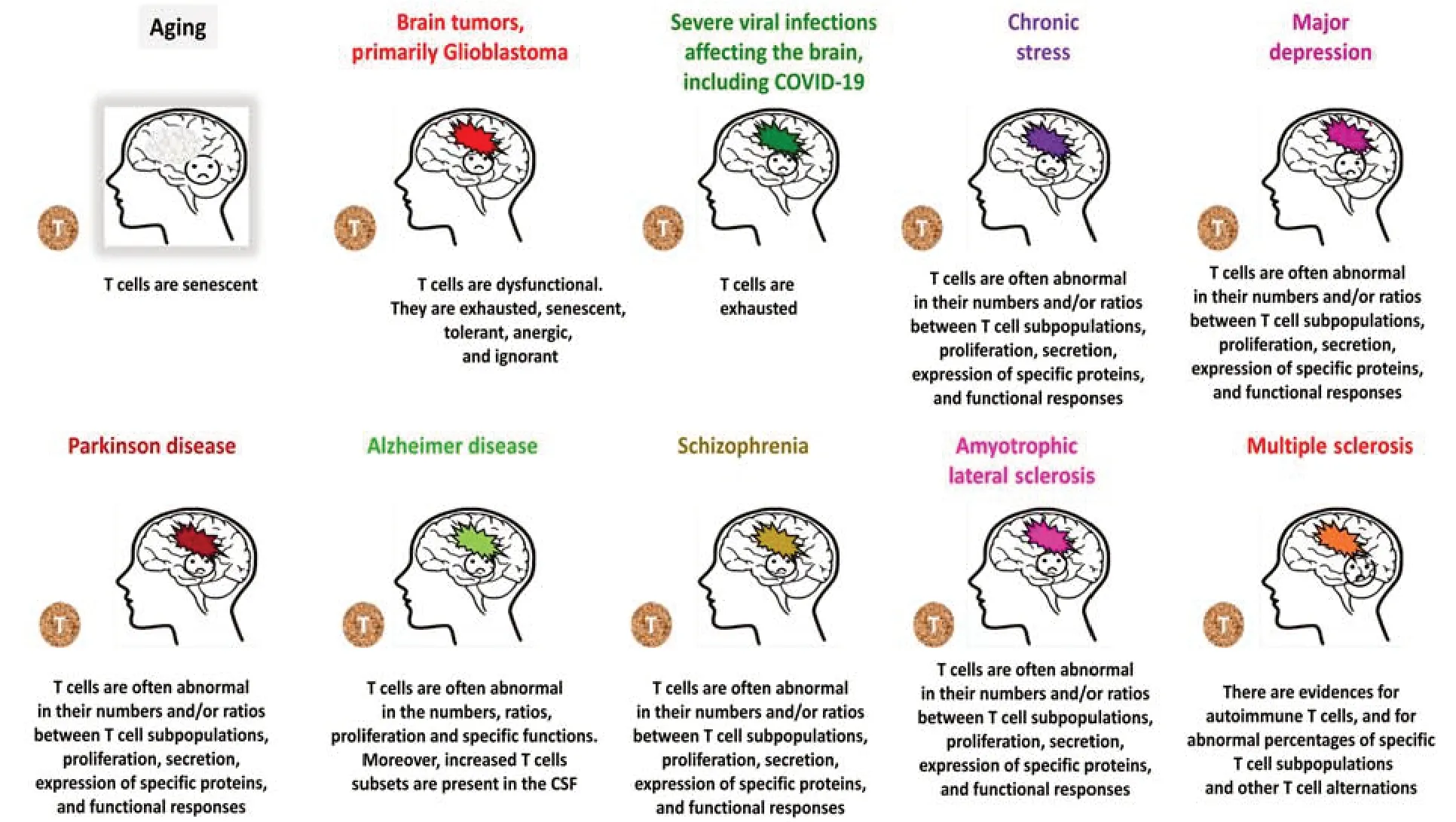
Figure 3|T cells are abnormal in aging,various neurological diseases,chronic stress,and major depression.
Activation-induced cell death of T cells
Activation-induced cell death (AICD) of T cells is a negative regulating process that activated T cells undergo,when their T cell receptor (TCR) is stimulated repeatedly (Arakaki et al.,2014).AICD helps to maintain peripheral immune tolerance.Alteration of the AICD process may lead to autoimmune disease.AICD is triggered by the switch from life to death through several signaling molecules,and takes place primarily by Fas/FasL-mediated apoptosis (Arakaki et al.,2014).Specifically,AICD is caused by the interaction of Fas receptors(Fas,CD95) and Fas ligands (FasL,CD95 ligand).
In practice,AICD of T cells has both beneficial and detrimental effects on the fate of T cells,depending on the context,and whether the body is healthy or diseased.
In a healthy body,AICD is an essential and beneficial process that helps to maintain proper immune tolerance and prevent autoimmunity,by eliminating harmful over-activated and autoimmune T cells.
Yet,the opposite is true in infectious diseases and cancer.In these diseases,AICD of beneficial T cells inherent to the body,or of therapeutic T cells delivered to the body by adoptive transfer,due to continuous T cell exposure to increasing doses of antigens of the respective infectious organisms or tumor cells,is a highly undesired process that eliminates effective antigenspecific T cells,and by doing so decreases the ability of the immune system to fight the related disease or cancer (Huan et al.,2022).
T cell exhaustion
T cell exhaustion refers to a deterioration,dysfunction,or physical elimination of T cell functions.T cell exhaustion occurs primarily in cancer and various chronic infections (Crespo et al.,2013;McKinney et al.,2015;McKinney and Smith,2016;Fisicaro et al.,2017;Hashimoto et al.,2018;Saeidi et al.,2018;Stelekati et al.,2018;Kurachi,2019;McLane et al.,2019;Vardhana et al.,2020;Gonzalez et al.,2021).
Exhausted T cells suffer from low proliferation capacity in response to antigenic stimulation,and from progressive loss of multiple effector functions,such as cytokine production and secretion,killing infected cells and cancer cells,and others.Exhausted T cells express multiple inhibitory receptors including PD-1,Tim-3,LAG-3,TIGIT,CTLA-4,CD160 and others,and suffer from various metabolic alterations.Impaired mitochondrial oxidative phosphorylation limits the self-renewal of T cells exposed to the persistent antigens (Vardhana et al.,2020).
T cell senescence termed also immunosenescence or biological aging
T cell senescence refers to a process of gradual deterioration of functional characteristics of T cells and irreversible growth arrest (Crespo et al.,2013;Childs et al.,2015;Regulski,2017;Xu and Larbi,2017;Pangrazzi and Weinberger,2020).
T cells that replicate multiple times due to repeated or continuous antigenic stimulation by infectious organisms or cancer cells,can lose their proliferation capacity and reach the stage of replicative senescence.The senescent cells stop dividing due to many different stress-inducing factors,amongst them both environmental and internal damaging events,abnormal cellular growth,oxidative stress,autophagy factors,and others.The inability of T cells to proliferate is partly due to the erosion of telomeres and the loss of telomerase activity (Childs et al.,2015;Xu and Larbi,2017).
Impaired T cell stemness
T cells,like various other cells in the body,must maintain the capacity to self-renew and be multipotent (Vodnala et al.,2019;Gonzalez et al.,2021).T cell stemness combines the ability of T cells to perpetuate their lineage,give rise to differentiated cells,and interact with their environment,in order to maintain a tightly-regulated balance between quiescence,proliferation,and regeneration.
T cell stemness and exhaustion coexist as two key contrasting phenomena during chronic antigenic stimulation,such as in chronic infection,mismatched organ transplantation cancer,and autoimmunity.
Vodnala et al.(2019) recently published a paper inScienceentitled “T cell stemness and dysfunction in tumors are triggered by a common mechanism”,which describes very important facets of T cell stemness.This study reveals that an overabundance of potassium in the tumor microenvironment triggers both suppression of T cell effector function,and preservation of stemness.The researchers show in this study that high levels of extracellular potassium constrain T cell effector programs by limiting nutrient uptake,thereby inducing autophagy and reduction of histone acetylation at effector and exhaustion loci,which in turn produce CD8+T cells with improvedin vivopersistence,multipotency,and tumor clearance.
In addition,recent studies on the exhaustive differentiation of T cells in chronic infection and cancer highlighted the stemness of “precursors of exhausted” T cells,prior to their terminal differentiation to exhausted cells.The transcriptional and epigenetic regulations of T cell stemness and exhaustion,and their clinical implications,were recently discussed (Gonzalez et al.,2021).
5.T Cell Impairments and Dysfunction Are Common and Have Broad Catastrophic Implications
Many people,in a kaleidoscope of abnormal conditions and diseases,have various severe and prolonged T cell impairments,which do not allow for effective and optimal eradication of infectious organisms and cancer cells,and impair additional essential T cell activities in the periphery and brain.
The multiple different abnormal conditions and diseases in which people’s T cells are impaired in various features,and weakly or severely dysfunctional,are listed in general terms inFigure 2,and discussed further in (Levite,2021).Some of these conditions are reviewed later,in Part 6 of this paper.
In addition,since the focus of this paper is beneficial T cells in the context of the nervous system,Figure 3specifies some neurological diseases and damages in which T cells were found to be abnormal,while the different sections of Part 6 specify some of the T cell impairments in each of these neuropathological conditions.
T cell abnormalities of various types can lead to multiple detrimental outcomes,listed briefly inFigure 4,and summarized below and in Levite (2021).
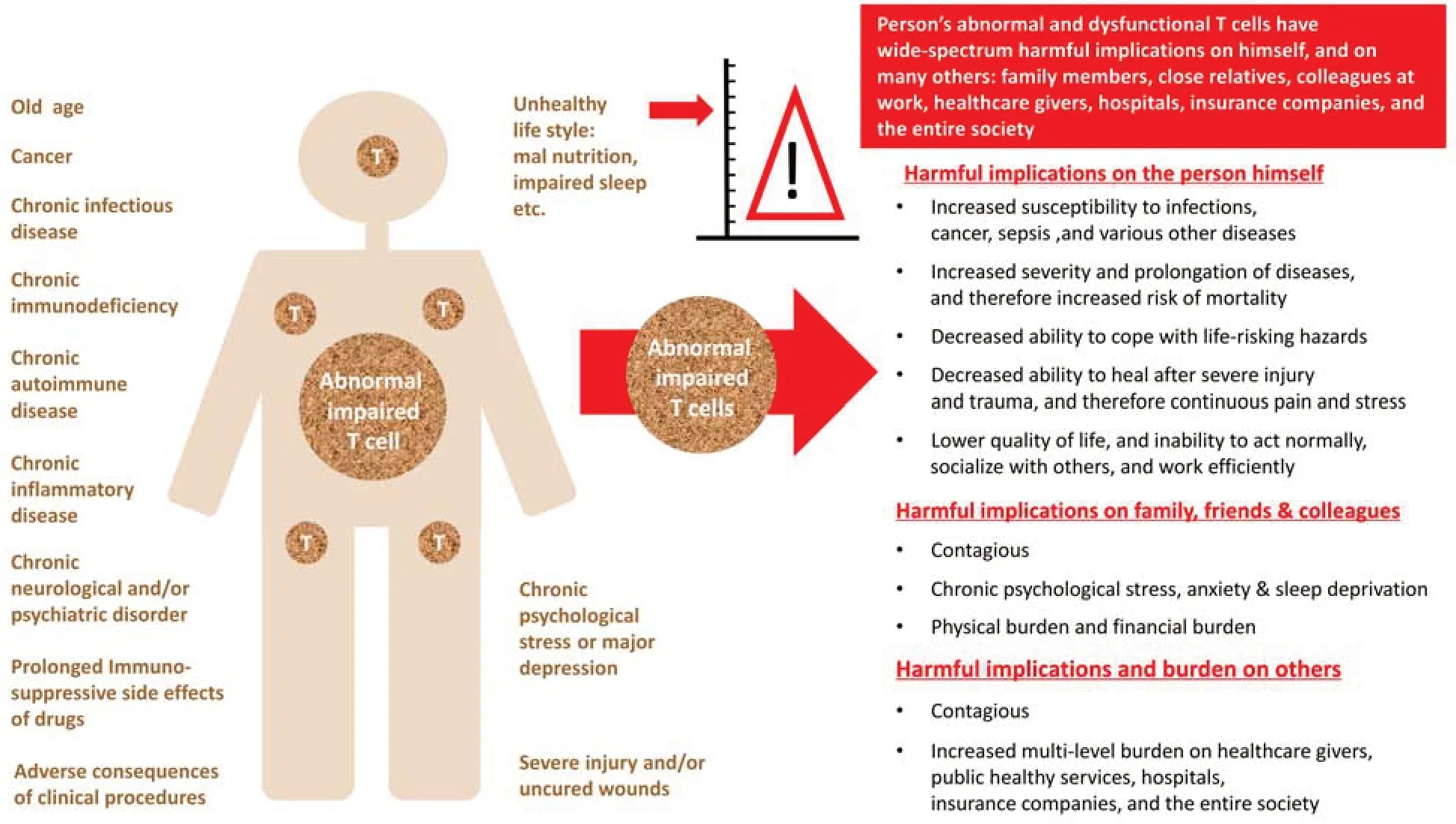
Figure 4|Broad and multilevel harmful implications of abnormal and dysfunctional T cells.
People with dysfunctional T cells are at a much higher risk for developing severe infectious diseases due to various viruses (e.g.,acute coronavirus disease 2019 (COVID-19)),bacteria,fungi,and parasites.T cell dysfunction also puts others at risk,since the people with impaired T cells carry the infectious organisms without being able to eradicate them properly,and are often contagious.And worst of all: people with dysfunctional T cells are at much higher risk of developing cancer and dying from cancer.
In addition to all this,low numbers of T cells and/or abnormal T cell function can lead directly or indirectly to ongoing chronic stress,depression,fear,and anxiety,which are high-risk factors.The opposite direction is also true,as chronic stress and major depression weaken and impair proper T cell function.
On top of all the above mentioned harsh implications,people whose T cells are scarce and/or dysfunctional,and who become increasingly and chronically ill,can become a physical,physiological,and economical burden on close family members,friends,hospitals,in-hospital medical staff,emergency and intensive care units,critical medical instruments,out-of-hospital healthcare providers,insurance companies,and other people and organizations.Taken together,the T cell abnormalities of many people can adversely affect large circles of other persons and organizations in the population and society.
6.T Cells Are Abnormal and Dysfunctional in Aging and Various Neurological Diseases and Injuries
Because of the limitations of this article,this paper reports only some of the neuropathological conditions in which T cells were found to be abnormal,and provides only some of the evidences for the abnormal numbers,ratios,conditions,and functions of specific T cell subpopulations.Interested readers can find many interesting and important findings related to this topic,which were discovered by various research groups worldwide,in articles cited in this paper,and by conducting a literature search.
6A.T cells deteriorate and become senescent in old age
Aging causes deterioration of T cells,and T cells of elderly people share some similarities with senescent cells (Childs et al.,2015;Pangrazzi and Weinberger,2020).The similarities between T cells of elderly people and senescent cells include shorter telomeres,accumulated DNA damage,and metabolic changes(Childs et al.,2015;Pangrazzi and Weinberger,2020).
T cell impairments in elderly people are due to decreased output of lymphoid cells from the bone marrow,and involution of the thymus (Pangrazzi and Weinberger,2020).Concomitantly,there is an accumulation of highly differentiated T cells that previously encountered antigens,and this leads to diminished TCR repertoire.
The deteriorated T cells of older adult people have multiple harsh detrimental implications on their lives: they increase their susceptibility to infectious diseases and cancer;lead to impaired,delayed,and incomplete recovery after injuries,surgeries,and transplantations;decrease their effective responses to vaccines,and lead to various other problems that reduce their quality of life(Pangrazzi and Weinberger,2020).
6B.T cells are severely impaired and dysfunctional in malignant brain tumors
Malignant brain tumors,primarily glioblastoma (GBM) and anaplastic astrocytoma,have a poor prognosis,despite aggressive surgical and medical care.People with GBM,the most common form of malignant brain tumors,have a five-year survival rate of only 5%.These brain tumors frequently invade and infiltrate the surrounding brain loci,which make them difficult to treat,and lead frequently to fatality.The standard treatment for malignant gliomas includes microsurgical resection,postoperative external beam radiation therapy with a total dose of 54–60 Gy,and different protocols of chemotherapy.In addition,in recent years a novel treatment method called tumor treating fields (TTFields) has been proposed as adjuvant therapy after irradiation.Even so,75% of patients with GBM will die within 18 months from diagnosis.Therefore,there is a great need and ambition for the continuous exploration of new research trajectories for the treatment of high-grade gliomas.
The brain is considered an “immunologically privileged site”,and brain tumors are considered “cold tumors”– tumors which are not penetrated and eradiated sufficiently by T cells.The blood-brain barrier and absence of lymphatic drainage restrict the entry of blood-borne immune and inflammatory cells into the CNS,leading to an exclusion of the brain from systemic immune surveillance.This may explain the paucity of successful immunotherapies for brain tumors.
A functional and complete T cell repertoire is an integral component of adequate immune surveillance,and needed for the initiation and maintenance of productive antitumor immune responses.GBM does not allow such beneficial T cell responses to take place,as it elicits severe T cell dysfunction that is both qualitative and quantitative (Goods et al.,2017;Mohme et al.,2018;Woroniecka et al.,2018a,b).T cell dysfunction is a hallmark of GBM,as proven and discussed in many original papers and reviews,among them those cited herein and others.Newer immunologic frameworks allowed the recent reclassification of T cell deficits elicited by GBM into five relevant categories: senescence,tolerance,anergy,exhaustion,and ignorance.Categorization is made according to the molecular bases of the T cell dysfunction (Woroniecka et al.,2018a,b).
Recent findings also reveal that GBM elicits a particularly severe T cell exhaustion signature among infiltrating T cells,characterized by the following events: (1) prominent upregulation of multiple immune checkpoints,(2)stereotyped T cell transcriptional programs,matching classical virus-induced exhaustion,and (3) notable T cell hyporesponsiveness in tumor-specific T cells.Exhaustion signatures differ predictably with tumor identity but remain stable across manipulated tumor locations (Woroniecka et al.,2018a).
T cell exhaustion is a known contributor to failures of T cell immunotherapy via immune checkpoint blockade in GBM (Goods et al.,2017).Other immunotherapeutic strategies,including adoptive T cell therapies and various other immunotherapies,are under investigation in clinical trials in GBM (Choi et al.,2019),while others are being developed and considered (Goods et al.,2017).On top of all these,additional innovative different immunotherapies for GBM should be designed.Some of them may hopefully be found successful in overcoming the primary immunologic challenges in this disease,which include T cell exhaustion,antigenic heterogeneity,and immune suppression.
Two reviews,Sampson et al.(2017) on immunotherapy for brain tumors,and Choi et al.(2019) on adoptive T cell strategies in GBM,report and discuss recent findings on these topics,providing a basis for new approaches of cellular therapies for tumors that involve the CNS,and discuss pioneering clinical trials of CAR T-cell therapy in GBM.
6C.T cells are essential for the eradication of viruses,but dysfunctional in multiple viral infections that harm the brain directly or indirectly,including COVID-19
Viral diseases that harm the brain
Many viruses can harm the brain directly or indirectly,via various mechanisms(Berth et al.,2009;Ramamurthy et al.,2016;Nath and Johnson,2021).In general,virus infections of the brain are rare in individuals with intact and efficient immune system.However,neurotropic viruses have developed mechanisms to exploit weaknesses in immunological defense mechanisms that eventually allow them to reach and infect CNS neurons.Once in the CNS,these viruses can induce significant neuronal dysfunction and degeneration of specific neuronal populations,sometimes leading to devastating,lifethreatening consequences for the affected individuals (Berth et al.,2009).
Several published reviews summarize and discuss the current knowledge with regard to how viruses enter the CNS and infect neuronal cells,the cellular and molecular alterations they induce in these cells,and the resulting pathologies(Berth et al.,2009;Ramamurthy et al.,2016).
Multiple viruses can infect the CNS at one or more sites,and different viruses seem to have a tendency to infect different areas of the brain,although the association of certain viruses with given CNS sites is not fixed (Ramamurthy et al.,2016).
An important fact to remember is that neurotropic virus infections do not necessarily lead to clinical disease,and many of them are asymptomatic.
T cells are essential for the eradication of viruses,but exhausted and dysfunctional in many persistent viral infections
Cytotoxic CD8+T cells are critically important for the protective immunity against intracellular pathogens.Yet,various chronic infections cause severe T cell exhaustion (Crespo et al.,2013;McKinney et al.,2015;McKinney and Smith,2016;Fisicaro et al.,2017;Hashimoto et al.,2018;Saeidi et al.,2018;Stelekati et al.,2018;Kurachi,2019;McLane et al.,2019;Beltra et al.,2020;Gonzalez et al.,2021).T cell exhaustion,evident by dysfunction or physical elimination of antigen-specific T cells,occurs in various chronic infections,for example diseases caused by human immunodeficiency virus (HIV),hepatitis B virus (HBV),hepatitis C virus,and other viruses (Crespo et al.,2013;McKinney et al.,2015;McKinney and Smith,2016;Fisicaro et al.,2017;Hashimoto et al.,2018;Saeidi et al.,2018;Stelekati et al.,2018;Kurachi,2019;McLane et al.,2019;Beltra et al.,2020;Gonzalez et al.,2021).
In these viral diseases,the exposure of CD8+T cells to persistent and excessive amounts of viral antigens and inflammatory signals can lead to gradual deterioration of CD8+T cell function;i.e.,T cell exhaustion.The exhausted T cells are characterized by the following abnormal processes: progressive loss of effector functions-cytokine production,killing,and others;expression of multiple inhibitory receptors-PD-1,LAG3,Tim-3,TIGIT,CTLA-4,CD160 and others;dysregulated metabolism;poor memory recall;homeostatic self-renewal and proliferation;and distinct transcriptional and epigenetic programs (Crespo et al.,2013;McKinney et al.,2015;McKinney and Smith,2016;Hashimoto et al.,2018;Saeidi et al.,2018;Stelekati et al.,2018;Kurachi,2019;McLane et al.,2019;Beltra et al.,2020).
The exhausted T cells in viral diseases and cancer are heterogeneous,and include progenitor and terminal subsets with unique characteristics and responses to checkpoint blockade.These altered T cell functions are closely related to altered transcriptional programs and epigenetic landscapes that clearly distinguish them from normal effector and memory T cells.
T cell exhaustion,which represents a continuous spectrum of cellular dysfunction induced during chronic viral infections,facilitates viral persistence and is associated with poor clinical outcomes.Several types of modulations can restore the function of exhausted T cells,promoting viral clearance
HBV-specific CD8+T cells are functionally exhausted in HBV infection,and this condition can be corrected only partially through the modulation of inhibitory pathways.The exhausted HBV-specific CD8+T cells are markedly impaired at multiple levels and show substantial downregulation of various cellular processes centered on extensive mitochondrial alterations (Fisicaro et al.,2017).
SARS-Cov2 infection causes multiple neurological and immunological complications and exhausts T cells,which are required to eliminate the virus and overcome COVID-19
SARS-Cov2 infection,leading to COVID-19,is associated with multiple neurological complications (Ghannam et al.,2020;Montalvan et al.,2020;Farhadian et al.,2021;Hosseini et al.,2021;Mussa et al.,2021;Nath and Johnson,2021;Ritschel et al.,2021;Soltani et al.,2021).
Neurological symptoms,such as anosmia,agnosia,stroke,paralysis,cranial nerve deficits,encephalopathy,meningitis,delirium and seizures,are reported as common complications affecting the course of the disease and its treatment.The CNS and peripheral nervous system complications in SARSCov2-infected patients,and the pathophysiology of neurological abnormalities in COVID-19,are described and discussed in many studies,among them those cited above and others.
A previous study shows that severe SARS-CoV2 infection also induces multiple immune abnormalities,including lymphopenia;higher neutrophil-lymphocyte ratios;reduced percentages of CD4+,CD8+,natural killer (NK) cells,and B cells;exhausted lymphocytes with the compromised functional response;and cytokine storm (Poonia and Kottilil,2020).
T cells are critically important for immune protection from SARS-Cov2,and for preventing very severe COVID-19 (Braun et al.,2020;Le Bert et al.,2020).
Focusing on T cells in COVID-19,few recent studies report on a dramatic reduction in a total number of T cells,both helper CD4+and cytotoxic CD8+T cells,and functional exhaustion of T cells in SARS-CoV2-infected patients,especially in patients requiring intensive care (Le Bert et al.,2020).
Counts of total T cells,CD8+T cells,or CD4+T cells lower than 800,and 300 or 400 per milliliter,respectively,negatively correlate with the survival of SARSCoV-2-infected patients (Diao et al.,2020).
T cell numbers in COVID-19 negatively correlate with serum IL-6,IL-10,and tumor necrosis factor-alpha (TNFα) concentration,with patients in the disease resolution period showing reduced concentrations and restored T cell counts (Diao et al.,2020).Moreover,T cells from COVID-19 patients have significantly higher levels of the exhausted marker PD-1.Increasing PD-1 and Tim-3 expression on T cells was seen as COVID-19 patients progressed from prodromal to overtly symptomatic stages.
In addition to the above described T cell abnormalities caused by SARS-CoV-2,a recent study discovered an imbalance of regulatory and cytotoxic SARS-CoV-2-reactive CD4+T cells in patients with COVID-19 (Meckiff et al.,2020).
6D.T cells are abnormal in Chronic Stress
Chronic psychological stress is closely related to immune dysfunction in humans and animal models,and various peripheral T cell abnormalities have been documented (Glaser et al.,2001;Kim et al.,2012;Schmidt et al.,2016;Rudak et al.,2021).These T cell impairments include,but are not limited to:increased frequency and suppressive function of CD4+CD25+and CD4+FoxP3+Tregs;a synergistic decreased function of Teffs and Antigen Presenting Cells(APCs);and a shift in the Th1 to Th2 cytokine response (Glaser et al.,2001;Kim et al.,2012;Schmidt et al.,2016).Moreover,recently published evidence show that long-term stress abrogates both Th1 and Th2-type responses orchestrated by invariant natural killer T (iNKT) cells.Activated iNKT cells in stressed mice exhibited a “split” inflammatory signature that triggered sudden spikes in the serum levels of the following cytokines: IL-10,IL-23,and IL-27.The iNKT cell dysregulation was mediated by cell-autonomous glucocorticoid receptor signaling,and corrected upon habituation to predictable stressors.Importantly,under stress,iNKT cells failed to potentiate cytotoxicity against lymphoma,or to reduce the burden of metastatic melanoma (Rudak et al.,2021).Together,these T cell abnormalities can actively contribute to the immune dysfunction during chronic stress,and can be responsible for increased susceptibility to infectious diseases and cancer in chronically stressed individuals (Glaser et al.,2001;Kim et al.,2012;Schmidt et al.,2016).
Interestingly,in agreement with the known ability of psychological factors to influence susceptibility to viral infections,several recent studies have revealed the effects of stress and depression on susceptibility to SARS-CoV-2 infection,and on the levels of antibodies produced against the virus (Ayling et al.,2022;Vedhara et al.,2022).In one study (Vedhara et al.,2022),102 participants completed measures of anxiety,depression,positive mood,and loneliness,and provided a blood sample for the measurement of antibodies to the SARS-CoV-2 spike and nucleocapsid proteins.SARS-CoV-2 seropositivity was significantly associated with anxiety,and significantly positively associated with depression.These findings show that psychological factors may influence susceptibility to SARS-CoV-2 infection.In another study,performed during the early phase of the pandemic,Ayling et al.(2022) found that greater psychological distress was significantly associated with subsequent self-reported SARS-CoV-2 infection,and experiences of a greater number of,and more,severe symptoms.
Thus,COVID-19 infection and its symptoms may be more common among those experiencing elevated psychological distress,and it is logical to assume that stress and the infection together can impair beneficial T cells even more than any factor alone.
6E.T cells are abnormal in Major Depression
A subset of patients with major depressive disorder (MDD) has impaired adaptive immunity,characterized by a greater vulnerability to viral infections,and a deficient response to vaccinations,along with a decrease in the number and/or activity of T cells and NK cells (reviewed in (Suzuki et al.,2017),and shown by studies cited therein).
Indeed,altered populations of cytotoxic T cells,Tregs and NK cells are found in MDD patients,and these T cell abnormalities associate with sleep disturbance (Suzuki et al.,2017).
In specific,Suzuki et al.(2017) found that MDD patients have a significantly increased percentage of CD127low/CCR4+Tregcells,memory Tregcells,as well as a reduction in CD56+CD16–(putative immunoregulatory) NK cell counts,compared to healthy controls.
There is also a trend towards higher effector memory CD8+cell counts in MDD patients,as compared to healthy controls.Further,within the MDD group,self-reported sleep disturbance is associated with an increased percentage of effector memory CD8+cells,but a lower percentage of CD56+CD16–NK cells.Suzuki et al.(2017) provided novel evidence that MDD and associated sleep disturbance increased effector memory CD8+and Tregpathways.Accordingly,they proposed that targeting sleep disturbance may have implications as a therapeutic strategy to normalize NKC and memory CD8+cells in MDD (Suzuki et al.,2017).
Patas et al.(2018) studied T cells from antidepressant-free patients with MDD and without any somatic or psychiatric comorbidities,and found they had significantly lower surface expression of the chemokine receptors CXCR3 and CCR6,which are central to T cell differentiation and trafficking.Moreover,purified CD4+T cells of MDD patients were found to have a higher frequency of CD4+CD25highCD127low/–cells,greater FOXP3 mRNA expression,and a less diverse TCR Vβ repertoire.
6F.T cell impairments in Parkinson’s Disease
PD is an age-related degenerative movement disorder of the CNS resulting from the death of dopamine-containing cells in the substantia nigra,a region of the midbrain.Various studies show disturbed cellular and humoral immune functions in peripheral blood of patients with PD (Bas et al.,2001;Fiszer,2001;Hisanaga et al.,2001;Schonhoff et al.,2020).
Recently,Schonhoff et al.(2020) reviewed dysfunctional immune systems in both human PD and animal models of PD.They discussed associations between genetic risk factors and risk modifiers with pro-inflammatory immune responses,emphasizing evidence that the immune response drives the pathogenesis and progression of PD,and raising key questions that remain to be investigated in order to identify suitable immunomodulatory therapies.Some of the specific T cell abnormalities found in patients with PD are summarized in the coming paragraphs and reviewed in (Schonhoff et al.,2020).
Patients with PD were reported to have reduced frequency of helper CD4+T cells and reduced CD4+:CD8+ratio,due to decreased proportions of helper CD4+T cells,and an increased proportion of cytotoxic CD8+T cells (Bas et al.,2001;Baba et al.,2005;Saunders et al.,2012;Stevens et al.,2012).
A shift toward a Th1 immune response with increased IFN-γ,reduced numbers and suppressive capacity of Tregs,reduced B cells,and an increase in NK cells were also reported in patients with PD (Bas et al.,2001;Baba et al.,2005;Niwa et al.,2012;Saunders et al.,2012).CD8+and CD4+T cells infiltrate the brain during PD,and several evidence obtained in mouse models of PD support T cell-mediated dopaminergic toxicity in PD,which is almost exclusively mediated by CD4+T cells IFN-γ (Brochard et al.,2009).
Fiszer et al.(1994a,b,2001) showed in several studies that PD patients have decreased percentage of CD45RA+naive T cells,and increased percentage of CD45RO+T cells in their peripheral blood,and increased HLA-DR expression on monocytes in cerebrospinal fluid (CSF).In contrast to these alterations,the proportions of CD8+CD11b+high suppressor T cells remained unchanged.The researchers concluded there is a selective loss of CD4+CD45RA+cells in PD as compared with healthy controls,and suggested this may be a common immunological abnormality in several neurological disorders,since it was previously observed also in MS and Down’s syndrome.
Hisanaga et al.(2001) found that patients with PD displayed a significantly greater population of circulating CD3+CD4 bright+CD8 dull+T cells than agematched control subjects or patients with cerebrovascular disease (Hisanaga et al.,2001).This increase in T cells appeared to continue for at least 17 months.The T cells also expressed CD45RO and Fas-markers for activated T cells,while CD1a,a marker for thymic T cells,was negative,suggesting that these are mature T cells with immune activities.As CD4+CD8+T cells increase after some specific viral infections,the continuous increase in CD4 bright+CD8 dull+T cells shown in this study may indicate post-infectious immune abnormalities that are possibly associated with the pathogenesis of this slowly progressive,multifactorial neurodegenerative disease.
Bas et al.(2001) discovered that patients with PD have a numeric decrease in helper T cells (higher in CD4+CD45RA+than in CD4+CD29+) and B cells,and an increase in activated CD4+CD25+T cells that correlate with lymphocyte depletion.They also found these alterations were independent of levodopa treatment,and that MPP+but not 6-OHDA,can increase CD4+CD25+T cells when striatal dopamine depletion was performed in rats.
Patients with PD have been found to have lower lymphocyte numbers in their blood as compared to controls,mostly driven by a decrease in subsets of CD4+T cells,specifically naıve and memory subsets,whereas the numbers of CD8+cytotoxic T cells are unchanged (Bas et al.,2001;Schonhoff et al.,2020).Although naıve and memory subsets decrease,there is an overall increase in the number of activated antigen-experienced CD4+T cells (CD4+CD25+) (Bas et al.,2001).These findings indicate an overall shift to an activated antigenexperienced T cell phenotype in the blood (Bas et al.,2001).
Some of the additional abnormalities detected in PD include: elevated levels of gamma delta+T cells,increased IgG immunity against heat shock proteins in CSF,and elevated cytokines and apoptosis-related proteins in the striatum of patients with PD and others (Fiszer,2001).
6G.T cell impairments in Alzheimer’s Disease
AD is a neurodegenerative disease,characterized by the loss of neurons and synapses primarily in the cerebral cortex.This neurodegeneration causes memory loss,and in later stages can also cause impairment in other capacities such as language,emotions,and behavior (Burns and Iliffe,2009).In addition,in their recent review,Lei et al.(2021) discuss several other neurochemical abnormalities that occur in the AD brain,among them elementomic signatures of iron,copper,zinc,and selenium.
The few studies cited below,among the many performed so far,summarize various T cell impairments in AD (Schindowski et al.,2006;Richartz-Salzburger et al.,2007;Bonotis et al.,2008;Larbi et al.,2009;Evans et al.,2019;Gate et al.,2020).
Schindowski et al.(2006) found increased apoptosis in CD4+T and NK cells in patients with AD,while during aging all subsets were affected.The expression of anti-apoptotic Bcl2 correlated with observed cell death in helper T cells and B cells,and the levels of Bcl2 in T cells were significantly increased in mild AD.Apoptosis and Bcl2 levels was also elevated in the APP (751SL)xPS1 (M146L)transgenic mouse model of AD.
Richartz-Salzburger et al.(2007) found a significant decrease in CD3+T and CD19+B cells in patients with AD.In addition,a slight increase of CD4+and a decrease of CD8+T cells could be observed,without a significant change in the CD4+/CD8+ratio.The researchers claimed their findings of decreased T cell and B cell numbers in AD sustain the hypothesis of a general decline of immune activity in AD.
Bonotis et al.(2008) found a significant decrease in CD4+T cell levels in patients in severe AD stages,as compared to mild-moderate stage patients and controls.In addition,the researchers found a significant increase in TNFα in the serum of AD patients in the severe stage,as compared to controls.No significant differences were observed between mild-moderate and severe stage patients in the levels of the following cytokines: IL-1 alpha,IL-2,IL-6,IL-8,and IL-10;or in the levels of CD8+and CD4+/CD8+T cells between patients and controls.CD4+T cells and IL-2 were revealed as having a significant relationship (positive and negative respectively) with patients’ MMSE scores,suggesting the existence of detectable changes in the peripheral immune system in AD patients.
Larbi et al.(2009) found dramatic alterations in naïve and memory subsets of CD4+cells in patients with mild AD: greatly decreased percentages of naïve cells,elevated memory cells,and increased proportions of CD4+T cells (but not CD8+T cells) lacking the costimulatory receptor CD28.CD4+CD25+(high)potentially Tregs with a naïve phenotype were also reduced in AD patients(Larbi et al.,2009).The authors claim that their findings provide strong evidence for more highly differentiated CD4+and CD8+T cells in AD patients,consistent with an adaptive immune system undergoing persistent antigenic challenge,and possibly manifesting dysregulation as a result.
Speciale et al.(2007) found that AD patients had a significant decrease in circulating CD8+CD28–T cells and B cells,and an increase in CD8+T cells expressing CD71+and CD28+.A significant decrease in IL-10 production was also found after stimulation of PMBC with the beta-amyloid fragment 1–40.The decreased IL-10 production was not related to disease severity.The authors claim that the observed imbalance of immune peripheral cell subpopulations and decreased IL-10 production indicate a reduction of suppressor cell function in AD patients.
Gate et al.(2020) report in their recentNaturepaper,the discovery of clonally expanded CD8+T cells that patrol the CSF in AD.Specifically,the researchers found increased numbers of CD8+T effector memory CD45RA+(TEMRA) cells in AD,and that these cells were negatively associated with cognition.The TCR signaling was enhanced in these cells.The researchers further found that the TCR of the clonally expanded CD8+TEMRA cells in the CSF of patients with AD were specific for two separate Epstein-Barr virus antigens.These results revealed an adaptive immune response in the blood and CSF of AD patients,and provide evidence of clonal,antigen-experienced T cells patrolling the intrathecal space of brains affected by age-related neurodegeneration.
Xu et al.(2021) recently published a study in which they profiled 36,849 peripheral blood mononuclear cells of AD patients with amyloid-positive status,and of normal controls with amyloid-negative status,by 5′ singlecell transcriptome and immune repertoire sequencing,using the cell ranger standard analysis procedure.The researchers revealed five immune cell subsets: CD4+T cells,CD8+T cells,B cells,NK cells,and monocytesmacrophages cells,and disentangled the characteristic alterations of cell subset proportion and gene expression patterns in AD.Thirty one cell-typespecific key genes,comprising abundant human leukocyte antigen genes,and multiple immune-related pathways were identified by protein-protein interaction network and pathway enrichment analysis.The researchers further found high-frequency amplification clonotypes in T cells and B cells,and decreased diversity in T cells in AD.As the clone amplification suggested the activation of an adaptive immune response against specific antigens,the researchers speculated that the peripheral adaptive immune response,especially mediated by T cells,may have a role in the pathogenesis of AD.
6H.T cell impairments in Schizophrenia
Schizophrenia is a severe mental disorder with high heritability.Although its pathophysiology is mainly unknown,dysregulated immune activation and inflammation have recently emerged as possible underlying candidates (Zhang et al.,2002;Steiner et al.,2010;Brito-Melo et al.,2012;Kelly et al.,2018;Akkouh et al.,2020;Sahbaz et al.,2020).
The paragraphs below specify some of the findings published on T cell impairments in schizophrenia,and others can be found through a literature search.
Zhang et al.(2002) found that blood CD4+cells,mitogen-induced IL-2 secreting cells,and IL-2 production were significantly lower in schizophrenic subjects than in the normal controls.There was a significantly positive correlation between CD4+cells and IL-2 production in healthy controls but not in patients.Based on their findings the researchers suggest that immune disturbance may be present in schizophrenic patients,and that the lowerin vitroIL-2 production is probably related to the decreased number of T cells that secret IL-2,as well as to the intrinsic disorder of the patients’ T cells.
Drexhage et al.(2011) detected the following immune abnormalities in patients with schizophrenia: higher percentages of activated CD3+CD25+T cells,pro-inflammatory Th17 cells,anti-inflammatory CD4+CD25highFoxP3+Tregs,IL-4+lymphocytes,and pro-inflammatory-prone monocytes.They further found that: the activated T cell set point was reflected in significantly raised serum levels of sCD25;the up-regulation of IL-4+-containing lymphocytes was predominantly found in patients characterized by a monocyte pro-inflammatory set point;the numbers of Tregs and Th17 cells were higher in patients irrespective of the pro-inflammatory state of their monocytes.
Brito-Melo et al.(2012) determined by flow cytometry the expression pattern of: dopamine receptors D2R and D4R;serotonin receptors SR1A,SR1C,SR2A,and SR2B;and serotonin transporter,in CD4+and CD8+T cell subsets of schizophrenic patients.They found increased percentages of CD4+D4R+and CD8+D4R+in schizophrenic patients as compared to controls.They also found increased percentages of CD8+D2R+T cells in schizophrenic patients,albeit this population revealed fewer CD4+D2R+T cells in comparison to controls.Interestingly,a relationship between clinical symptoms and immunological parameters was discovered.Furthermore,they found that the Brief Psychiatric Rating Scale,Positive and Negative Syndrome Scale,and Abnormal Involuntary Movement Scale were positively related to CD8+D2R+T cells,though Abnormal Involuntary Movement Scale was inversely related to CD4+D4R+cells.In contrast to the increase of DRs in T cells of schizophrenic patients,the expression of serotonin receptors and serotonin transporter in T cells of schizophrenic patients was not different from controls.
Steiner et al.(2010) found significant abnormalities during acute psychosis:lower level of T helper cells,reduced CD4/CD8 ratio,and elevated B cells.After 6 weeks of medication,an inverse pattern was observed in initially drugfree patients: total T cells,T helper cells,and T suppressor/cytotoxic cells increased,while B cell counts declined.The researchers concluded that acute paranoid schizophrenia may be accompanied by a reduced T cell defense and shift towards B cell immunity,which normalizes in response to treatment.They also found that disease stage or subtype and medications,cigarette smoking,and stress were important co-factors.
Kelly et al.(2018) found a significantly increased proportion of Tregs in patients with schizophrenia as compared to healthy controls.No differences were observed in total lymphocyte counts or CD3+and CD4+T cells,confirming a specific effect for Tregs.Interestingly,the elevated Tregs in schizophrenia correlated with fewer negative symptoms,a core domain of the illness.This suggests that Tregs may contribute to improved negative symptoms in schizophrenia,possibly by counteracting ongoing inflammatory processes.
Sahbaz et al.(2020) found that the percentages of activated T cells were higher in patients with schizophrenia as compared to healthy controls,under both unstimulated and stimulated conditions.They also found that the percentages of Treg in patients with schizophrenia were higher in unstimulated conditions,but lower in the stimulated condition.With regards to cytokine levels,patients had higher levels of plasma levels of IL-6 and IL-17A,as compared to healthy controls.
Akkouh et al.(2020) found significantly decreased mRNA expression of the Treg-specific marker FOXP3 in the blood of a large cohort of schizophrenia patients (n=484),as compared to controls (n=472) (Akkouh et al.,2020).The researchers also found significant differences in the expression of HILPDA and CCL20 genes in astrocytes,both of which had reduced up-regulation upon IL-1beta treatment in schizophrenia astrocytes,as compared to control astrocytes.Since CCL20 is a specific chemoattractant for CD4+CD25+CCR6+Tregs,which are crucially involved in anti-inflammatory responses during brain (auto) inflammation,the researchers hypothesized a plausible role for an altered astroglia-CCL20-CCR6-Treg axis in schizophrenia pathophysiology.
6I.T cell impairments in Amyotrophic Lateral Sclerosis
Lymphocytes are found in the majority of ALS patients’ spinal cords,and along the vessels in the precentral gyrus,extending into the areas of neuronal injury(Engelhardt et al.,1993).Perivascular and intraparenchymal CD4+T cells are evident in the proximity of degenerating corticospinal tracts and ventral horns in two-thirds of patients with ALS (Engelhardt et al.,1993).
Multiple T cells impairments have been discovered in the blood of ALS patients,and in ALS animal models,as compared with controls (Zhang et al.,2005;Shi et al.,2007;Mantovani et al.,2009;Lincecum et al.,2010;Seksenyan et al.,2010;Beers et al.,2017).
The paragraphs below specify a few of the published findings on T cell impairments in ALS,and others can be found by a literature search.
ALS patients’ Tregs are dysfunctional,have impaired suppressive function,and correlate with disease progression rate and severity (Beers et al.,2017).Treg numbers and FOXP3 expression are also decreased in rapidly progressing ALS patients.Both slowly and rapidly progressing ALS patients have dysfunctional Tregs;yet,Treg dysfunction is worse the greater the clinically assessed ALS burden,or the more rapidly the patient progresses.
Afterin vitroexpansion,ALS Tregs regained suppressive abilities to the levels of control Tregs,suggesting that autologous passive transfer of expanded Tregs might offer a novel cellular therapy to slow disease progression(Beers et al.,2017).In ALS mouse models,decreased Tregs exacerbated the neuroinflammatory process,leading to accelerated death of motor neurons and shortened survival.Passive transfer of Tregs suppressed the neuroinflammation and prolonged survival (Beers et al.,2017).
6J.T cell impairments in Multiple Sclerosis
Numerous studies in MS patients and MS animal models,primarily experimental autoimmune encephalitis,have shown that MS is mediated by pathogenic autoimmune T cells that attack cells in the myelin-the protective sheath that surrounds nerves in the brain and spinal cord.In addition,MS patients have several impairments in T cell numbers,ratios,and activity(Viglietta et al.,2004;Haas et al.,2005;Huan et al.,2005;Feger et al.,2007;Arneth,2016;van Nierop et al.,2017).
In the paragraphs below,only a tiny fraction of the evidence of T cell impairments in MS is reviewed.Multiple other studies can be found through a literature search.
Evidence pooled from several different studies revealed in MS lower levels of CD4+T cells (van Nierop et al.,2017) (Arneth,2016),significantly lower levels of CD4+central memory T cells,and CD8+effector memory T cells (Haegele et al.,2007),but significantly higher levels of NK T cells (CD3+CD16+CD56+)(Arneth,2016).In addition,several studies showed impairments in Tregs in MS: significantly lower numbers of Tregs (Feger et al.,2007),and Tregs with reduced suppressive capacity and lower levels of FoxP3 expression (Viglietta et al.,2004;Haas et al.,2005;Huan et al.,2005;Feger et al.,2007).
Another study found that MS patients with relapsing-remitting disease having an expanded disability status score of > 3 or higher,and those not receiving disease-modifying therapy,have significantly higher numbers of CD4+IFN-γ+T cells and CD8+IFN-γ+T cells in peripheral blood,as compared to healthy controls and MS patients with an expanded disability status score < 3 (Sepulcre et al.,2005).
7.Specific Normal Beneficial T Cell Subpopulations Are Therapeutic in Some Neurological Diseases,According to Findings in Animal Models
The pathogenic effects of detrimental autoimmune and cytotoxic T cells in a growing number of CNS disorders are already well documented.Discoveries over the past two decades have elucidated the autoimmune basis of several,previously poorly understood,neurological disorders.Autoimmune disorders of the nervous system may affect any part of the nervous system,including the CNS and peripheral nerves,neuromuscular junctions,and skeletal muscles (peripheral nervous system).A recent review addresses the pathophysiological basis and clinical features of autoimmune diseases of the nervous system (Bhagavati,2021).
While autoimmune and cytotoxic T cells in the CNS are detrimental in most cases,the presence of normal helper CD4+T cells and CD4` CD25+Tregs in the CNS seem to be needed and even therapeutic in several neurological diseases(Evans et al.,2019).Indeed,recent studies in animal models of a variety of CNS abnormalities and diseases show a large body of convincing evidence of beneficial therapeutic effects of normal T cells in various neuropathologies,ranging from tissue protection to regeneration.This topic is reviewed and discussed in depth by Evans et al.(2019).
The paragraphs below present only very few evidences of the beneficial and therapeutic effects of T cells in animal models of various CNS impairments and diseases.Additional ones can be found by a literature search.Figure 1 illustrates schematically the beneficial effects of normal T cells,as opposed to the pathogenic effects of autoimmune and cytotoxic T cells,in the healthy and diseased brain,respectively.Especially compelling evidences are of the beneficial therapeutic effects of helper CD4+T cells and CD4+CD25+Tregs in a variety of neuroinflammatory and neurodegenerative diseases including: MS,AD,PDALS,stroke,and CNS trauma.Both T cell-secreted molecules,e.g.,brain-derived neurotrophic factor,and direct cell-cell contacts deliver neuroprotective,neuroregenerative,and immunomodulatory effects in these diseases.
7A.Normal T cells can induce beneficial effects in Alzheimer’s Disease
On the one hand,AD is associated with multiple T cell impairments,some already specified in Part 6G of this paper,while on the other hand specific T cell types can confer beneficial effects in AD (Ethell et al.,2006;Cao et al.,2009;Marsh et al.,2016).For example,Th2 cells specific for β-amyloid can reverse cognitive decline and synaptic loss in AD-like pathology in AD mice(Ethell et al.,2006;Cao et al.,2009).In other studies,the adaptive immune system was found to restrain AD pathogenesis by modulating microglial function (Marsh et al.,2016).Supporting evidence for the importance of beneficial T cells and other immune cells in AD originates also from the finding that immunodeficient AD mouse model– “Rag-5xfAD” mice,that lack T cells,B cells,and NK cells,exhibited a greater than 2-fold increase in β-amyloid pathology (Marsh et al.,2016).Neuroinflammation is also greatly exacerbated in ‘Rag-5xfAD’ mice,as indicated by a shift in microglial phenotype,increased cytokine production,and reduced phagocytic capacity.Strikingly,bone marrow transplantation studies in Rag-5xfAD mice revealed that replacement of the missing adaptive immune populations can dramatically reduce AD pathology (Marsh et al.,2016).
7B.Normal T cells can induce beneficial effects in Amyotrophic Lateral Sclerosis
Patients with ALS have several T cell abnormalities,some of which already specified in Part 6I of this paper.Independent of the studies showing these T cell impairments in human ALS,others,primarily performed on SOD1 mutant mice (SOD1mt)-a model of familial ALS,revealed that T cells may play a neuroprotective role in ALS,through a variety of potential mechanisms(Alexianu et al.,2001;Beers et al.,2008;Chiu et al.,2008).
Tregs in ALS were reported to be dysfunctional and correlated with disease progression rate and severity of ALS (Beers et al.,2017).Convincing evidence from studies in human ALS and in ALS animal models indicate that Tregs seem to induce a neuroprotective effect and slow ALS progression (Beers et al.,2011;Sheean et al.,2018).For example,Sheean et al.(2018) reported an inverse correlation between levels of Tregs and ALS disease progression,and that Treg expansion in the SOD1G93A mouse slowed disease progression and augmented survival duration.Since increased Treg levels are associated with slow progression in ALS patients,several approaches to improve,expand,or generate Tregs have been tested on ALS patients (Alsuliman et al.,2016;Mandrioli et al.,2018;Thonhoff et al.,2018).
7C.Normal T cells can induce beneficial effects in Multiple Sclerosis
MS is mediated by pathogenic autoimmune T cells.In addition to having autoimmune T cells,MS patients have several impairments in T cell numbers,ratios,and activity (Viglietta et al.,2004;Haas et al.,2005;Huan et al.,2005;Feger et al.,2007;Arneth,2016;van Nierop et al.,2017).Some of the specific findings on this topic were specified in Part 6J of this paper.Independent of the T cells abnormalities in MS,several studies showed that subsets of normal healthy T cells can induce beneficial regenerative and re-myelination effects in MS (Dombrowski et al.,2017;El Behi et al.,2017;McIntyre et al.,2020).This topic is reviewed in a paper by Evans et al.(2019).In summary,evidence from MS experimental models shows that the adaptive immune system is essential for efficient remyelination in general,and that Tregs have a pro-regenerative beneficial role in remyelination in particular.In contrast,Th17 and Th1 seem to inhibit remyelination and oligodendrocyte differentiation,respectively(Evans et al.,2019)
7D.Normal T cells can induce beneficial effects in Parkinson’s Disease
Human PD is associated with numerous T cells impairments,some of which are specified in Part 6F of this paper.Independent of these findings,several studies have shown that adoptive transfer of normal T cells,especially Tregs,can induce beneficial effects in animal models of PD (Reynolds et al.,2007,2009;Huang et al.,2017).For example,the adoptive transfer of activated Tregs into the MPTP mouse model of PD caused increased protection against neurotoxicity,and prevented the microglial release of reactive oxygen speciesin vitro,leading to the prevention of neuronal damage (Reynolds et al.,2007,2009).Tregs are able to protect nigrostriatal neurons via cell-to-cell contact between Tregs and dopaminergic neurons,by a CD47-SIRPa interaction,which triggers Rac1/Akt signalingin vitro(Huang et al.,2017).In addition,the adoptive transfer of Tregs protects dopaminergic neurons in PD mice by a cellto-cell contact mechanism underlying CD45-galectin-1 interaction (Huang et al.,2020).Taken together,Tregs seem to be decreased in their numbers and suppressive capacity in patients with PD,and increasing their numbers may be beneficial for preserving dopaminergic neurons in PD.
Another study showing beneficial effects of adoptively transferred T cells on PD involves anti-Copolymer-1 T cells adoptively transferred into MPTPintoxicated mice– a PD animal model.The adoptive transfer of these T cells led to the accumulation of T cells in the substantia nigra,suppression of microglial activation through secretion of IL-4 and IL-10 by T cells,and increased astrocyte-associated glial cell line-derived neurotrophic factor.This immunization strategy resulted in significant protection of nigrostriatal neurons against MPTP-induced neurodegeneration,that was abrogated by depletion of donor T cells (Benner et al.,2004).
7E.Normal T cells can induce beneficial effects in chronic,comorbid inflammatory pain and depression-like behavior
T cells appear to be an emerging target for chronic pain therapy,based on evidence identified and discussed by Laumet et al.(2020).Especially interesting and encouraging is that CD3+T cells seem to be essential for the resolution of comorbid inflammatory pain and depression-like behavior,as revealed in a model of peripheral inflammation.Specifically,it was found in a model of peripheral inflammation that normal beneficial T cells are required for resolution of the comorbid persistent mechanical allodynia,spontaneous pain,and depression,via specific T cell subsets,releasing cytokines and endogenous opioid peptides,which can either promote or suppress,or even resolve pain (Laumet et al.,2020).
Based on these evidences,it was suggested that the pro-resolution effects of T cells may have a major impact on treating patients with comorbid persistent pain and depression.
However,the relationship between T cells and pain is more complicated,since pain studies on animal models show that T cells play a double opposite role in acute or chronic pain.Thus,depending on the type of injury,T cells subset,and animal sex in the tested animal models,T cells may contribute both to the onset and resolution of pain.It is therefore suggested that inhibiting the pain-promoting functions of T cells,and/or enhancing the beneficial effects of pro-resolution T cells,may offer new disease-modifying strategies for the treatment of chronic pain (Laumet et al.,2020).
8.Nerve-Driven Immunity: Neurotransmitters and Neuropeptides Induce Direct,Powerful,and Beneficial Effects on T Cells,via Their Own Functional Receptors
8A.Neurotransmitters and neuropeptides can transmit accurate messages within the nervous and immune systems,and between these two body systems
Neurotransmitters,neuropeptides,and the many types and subtypes of specific receptors each of them has,are all natural molecules,which are constantly being used in the body to transmit information of paramount importance in health and disease.
The neurotransmitters are the size of a single amino acid,and include:glutamate,GABA,dopamine,adrenaline,noradrenaline,serotonin,and acetylcholine.
The neuropeptides and neurohormones,which are peptides of 10–40 amino acids,consist of dozens of peptides,among them: GnRH-I,GnRHII,Neuropeptide Y,Calcitonin gene-related peptide (CGRP),Substance P,Somatostatin,VIP,Galanin,Cholecystokinin,TRH,CRH,FSH,LH,ACTH,PHI,Enkephalin,β-Endorphin,Angiotensin,Neurotensin,Oxytocin,Vasopressin,and many others.
The main characteristic properties and the most fundamental differences between neurotransmitters and neuropeptides are summarized in a Table in a recent study (Levite,2021),and are also described in many textbooks,reviews,and educational websites.
Traditionally,neurotransmitters and neuropeptides are viewed as endogenous chemical substances used primarily by the nervous system,to transmit messages within the nervous system,as well as to deliver information from the nervous system to several peripheral organs and tissues.In addition,plentiful of evidence that accumulated in the last two decades has shown that neurotransmitters and neuropeptides have multiple direct effects on immune cells in general,and on T cells in particular,and as such play a very important role in the immune system and neuroimmune dialogues.
Scientific discoveries and evidences for the direct effects on neurotransmitters and neuropeptides on T cells can be found in many publications on “Nerve-Driven Immunity”,among them the following ones,papers cited in them,and others (Levite,2000,2012a,b,2021;Ganor and Levite,2012,2014).In addition,a recent review discusses the role of critical neurotransmitters in neuroimmune networks in the body (Hodo et al.,2020).
Based on pooled evidence discovered thus far on the effects,receptors,production,release,and mechanisms of action of neurotransmitters and neuropeptides in the nervous system and immune system,I humbly propose that neurotransmitters and neuropeptides can induce and transmit direct,potent,and accurate effects in four major pathways of information transmission,within and between the immune system and the nervous system.These four communication pathways are specified below,and illustrated schematically inFigure 5.They include the following information transfer routes: (1) from and to different cells in the nervous system,in paracrine and autocrine mechanisms of action,(2) from cells of the nervous system to cells of the immune system,(3) from cells of the immune system to cells in the nervous system,and (4) from and to cells of the immune system,in paracrine and autocrine mechanisms of action.
I assume that in principle,fast,direct,potent,specific,and tightly-regulated information transfer in these four information transfer routes can take place thanks to the following facts (Levite,2021): (1) some neurotransmitters and neuropeptides are produced and can be secreted by cells of both the nervous system and the immune system;(2) receptors for neurotransmitters and neuropeptides are expressed and functional in cells of both the nervous system and the immune system;(3) nerve fibers,at the ends of which neurotransmitters and neuropeptides are secreted,reach immune cells in multiple body organs in which T cells and other immune cells are present,including all organs of the lymphatic system,skin,blood,and various others;(4) specific immune cells of various types can “talk” to themselves (in autocrine manner),and to other immune cells (in autocrine manner),via neurotransmitters and neuropeptides they produce and secrete,and via neurotransmitter/neuropeptide receptors which are expressed on their cell surface;and (5) T cells are present in the CNS,and can communicate with neural cells in multiple conditions,for better or for worse.The beneficial or rather detrimental effects of the T cells in the CNS,are dictated by their type,normality or not,and the overall context.Thus,if the T cells in the CNS are normal in their numbers,types,ratios,condition,and function,they can most probably induce beneficial,protective,and even regenerative effects in the CNS.But if the T cells in the CNS are abnormal,especially if they are autoimmune and cytotoxic,they are harmful (Figure 1).
8B.Neurotransmitters and neuropeptides independently induce multiple,direct,rapid,potent,and beneficial effects on naïve resting human T cells
Specific neurotransmitters and neuropeptides,among them Dopamine,Glutamate,GnRH-I,GnRH-II,Neuropeptide Y,Calcitonin Gene Related Peptide(CGRP),and Somatostatin,can induce multiple,direct,potent,activating and beneficial effects on normal naïve human T cells,via their own receptors expressed in T cells,as we discovered and described in many published papers,some of them are specified herein (Levite,1998,2000,2008,2012a,2015,2021;Levite and Chowers,2001;Chen et al.,2002;Ganor et al.,2003,2009;Besser et al.,2005;Ganor and Levite,2012,2014;Saussez et al.,2014;Levite et al.,1998,2001,2021).In addition,we discovered various findings which are still unpublished,and experiments are ongoing in the scientific field of “Nerve-Driven Immunity”.
The main direct effects that neurotransmitters and neuropeptides induce in normal naïve T cells that we found are listed inFigure 6and below.
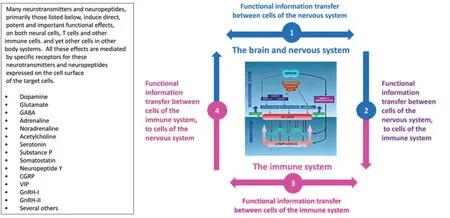
Figure 5|Neurotransmitters and neuropeptides can convey very important messages in and between the nervous and immune systems,in four information transmission routes,since they reach,have receptors in,and are secreted by,cells in both of these body systems.
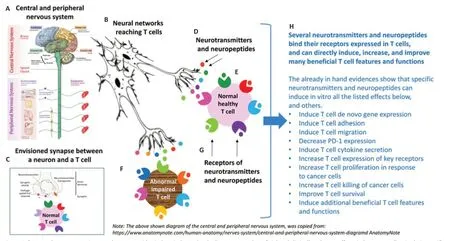
Figure 6|Several neurotransmitters and neuropeptides induce independently direct,potent,beneficial and clinically-relevant effects in human T cells,via their specific receptors expressed in T cells.
In summary,we found that specific neurotransmitters and neuropeptides can induce all the followingin vitroeffects (i.e.,in tissue culture conditions),or at least some of them,on normal naïve and resting human T cells,via their receptors in T cells.(1) Decrease PD-1 expression (Levite et al.,2021).A decrease in PD-1 expression in T cells is expected to,and indeed has been found to,be associated with,a decrease in PD-1-mediated checkpoint inhibition of T cells,leading to their augmented function;(2) Improve T cell survival (Levite et al.,2021);(3) Increase T proliferationin vitroin response to human cancer cells (Levite et al.,2021);(4) Increase T cell killing of human cancer cells (Levite et al.,2021);(5) Induce and increase integrin-mediated T cell adhesion to extracellular glycoproteins: fibronectin and laminin (Levite et al.,1998,2001);(6) Induce and increase T cell migration– spontaneous migration (Saussez et al.,2014),chemotactic migration (Ganor et al.,2003;Saussez et al.,2014),and migration towards autologous cancer cells and factors they secrete to the extracellular medium (Saussez et al.,2014);(7)Increase T cell penetration of solid organsin vivo[(Chen et al.,2002) and unpublished evidence];(8) Induce unique T cell de novo gene expression[(Chen et al.,2002) and multiple unpublished evidence];(9) Induce typical and atypical T cell cytokine secretion (Levite,1998;Levite and Chowers,2001;Besser et al.,2005);(10) Induce rapid shifts in T cells’ membrane potential[see for example (Keren et al.,2019);and found also in our still unpublished studies].
These effects,or at least some of them,were discovered in T cells of healthy human subjects (Levite,1998,2001,2008,2015;Levite et al.,2001,2021;Ganor et al.,2003;Besser et al.,2005;Ganor and Levite,2014),in T cells of several cancer patients,suffering from either Head and Neck Squamous Cell Carcinoma (HNSCC) (Saussez et al.,2014) or Hepatocellular Carcinoma (HCC)(Levite et al.,2021),and in mouse T cell lines and clones (see for example(Levite,1998).The optimal concentration range in which neurotransmitters and neuropeptides inducein vitrovarious direct effects on normal T cells is very low: 10–7–10–8M (0.1 μM–10 nM).
Furthermore,it is very important to consider that the neurotransmitters and neuropeptides induce their own direct and potent activating effects on T cells,in the complete absence of any other molecule (e.g.,antigen,mitogen,cytokine,chemokine,growth factor,or other).Thus,the effects of neurotransmitters and neuropeptides on T cells,via their own receptors,are by far more than just immunomodulating,minor,or secondary effects.Some of the T cell effects triggered by neurotransmitters and neuropeptides are evident within seconds to minutes.
8C.Normal beneficial T cells can benefit tremendously from the direct effects of neurotransmitters and neuropeptides,and from their receptors
Normal T cells can benefit greatly in a number of ways and levels from neurotransmitters,neuropeptides,and their receptors expressed in T cells(Levite,2021),especially as neurotransmitters and neuropeptides can deliver to T cells “messages” which are different in their timing,type,location,and overall context from classical immunological T cell signaling– via specific foreign antigens that bind and activate the TCR.This is especially true and important in view of the many types and subtypes of receptors each neurotransmitter and neuropeptide has,and the richness of the very rapid,specific,accurate,coordinated,tightly-regulated,well coordinated,and context-dependent signals they can deliver.
The envisioned advantages for T cells from neurotransmitters,neuropeptides,and their receptors are specified below.
Neurotransmitters and neuropeptides are natural physiological molecules that can be secreted in the vicinity of T cells in real time,as needed,bind to their receptors in T cells,and deliver to the T cells very rapid,accurate,transient,potent and beneficial effects.Such rapid and specific effects can be dictated to T cells by the brain and other parts of the nervous system,in a coordinated and orchestrated manner.Thus,different T cells subsets,present at a given moment in different body locations,could be influenced simultaneously or sequentially by neurotransmitters and neuropeptides secreted close to them from different nerve terminals of the very rich and branched neural networks.
In addition,the language in which neurotransmitters and neuropeptides“speak” to T cells has a very high degree of freedom,flexibility,potential,and precise adaptation to any given situation,in any given location,in the body.This is due to: (1) A large number of natural neurotransmitters and neuropeptides,(2) A broad family of natural receptor types and subtypes for each neurotransmitter and neuropeptide,which are expressed in multiple target cells throughout the body,and (3) The neurotransmitters and neuropeptides can be delivered to T cells by an enormous number of very branched neuronal networks.
Thanks to all these facts and unique characteristics,the effects of neurotransmitters and neuropeptides on T cells are fundamentally different from the ‘classical’ and most potent and specific T cell signaling via the TCR.The TCR-mediated T cell activation depends on specific foreign antigens,and such activation is essential and operational primarily in cases of danger:infections and cancer.Moreover,unlike some of the rapid T cells effects induced by neurotransmitters and neuropeptides,the functional T effects induced by antigens,via the TCR,are classically evident only after a very longtime of hours-days from the time of the initial antigenic stimulation,and they typically take placein vivoinside organs of the immune system.
Therefore,classic antigen-specific TCR-mediated T cell effectscannotconstitute the language via which T cells are activated within seconds,at any needed time and location in everyday life,even in the absence of danger.Furthermore,the TCR-mediated signaling by itself doesnotafford simultaneous communication between T cells and other cells in other body locations,and doesnotallow real time coordinated T cell activity in various peripheral tissues and organs.In addition and of primary importance to keep in mind: activation of T cells by antigens,via their TCR,cannotbe the “language” by which T cells speak to the brain.Neurotransmitters and neuropeptides can serve this important purpose.
The effects of neurotransmitters and neuropeptides on T cells are also different in various aspects from the T cell effects induced by cytokines and chemokines.
9.“Personalized Adoptive Neuro-Immunotherapy”– A Novel Adoptive T Cell Immunotherapy,Based on Ex Vivo Activation and Improvement of a Person’s T Cells by Neurotransmitters and Neuropeptides,and Their Subsequent Repeated Delivery into the Body
The “Personalized Adoptive Neuro-Immunotherapy” is a novel mode of personalized cellular immunotherapy that aims to rejuvenate,activate,and improve the function of abnormal and dysfunctional T cells in any person having such T cells,and who is in need of immunotherapy.It may be especially attractive to test the safety and beneficial effects of this novel adoptive immunotherapy,not yet tested in humans,in people with cancer,severe chronic infectious diseases,aging,and a few other neurological diseases in which the T cells are exhausted,senescent,and dysfunctional.
The “Personalized Adoptive Neuro-Immunotherapy” is drawn schematically inFigure 7.This novel type of adoptive T cell immunotherapy translates into the therapeutic method the successful and encouraging experimental “Nerve-Driven Immunity” findings,i.e.the direct beneficial and therapeuticallyrelevant effects of specific neurotransmitters and neuropeptides on human T cells.These effects are summarized in Part 8,Figure 6,and described in many publications,some of which are cited herein (Levite,1998,2000,2008,2012a,2015,2021;Levite et al.,1998,2001,2021;Levite and Chowers,2001;Chen et al.,2002;Ganor et al.,2003,2009;Besser et al.,2005;Ganor and Levite,2012,2014;Saussez et al.,2014).
The “Personalized Adoptive Neuro-Immunotherapy” was invented and patented by M.Levite (author of this paper),with the vision,aim and hope that this novel therapeutic method will enable rejuvenation,activation,and improvement of scarce,exhausted,senescent,and dysfunctional T cells of any person having such T cells,and at any given time,even repeatedly for many months and years.
The “Personalized Adoptive Neuro-Immunotherapy” was designed to meet twenty specific criteria and aims (defined by the inventor-the author of this paper),and these criteria are specified in (Levite,2021).In practice,this therapeutic method has been designed to contain two stages and corresponding protocols: personalized diagnostic protocol-ex vivoonly,and personalized therapeutic protocol-ex vivoandin vivo,both summarized below,and in (Levite,2021).
The initial diagnostic process of the “Personalized Adoptive Neuro-Immunotherapy”
The initial diagnostic tests of the “Personalized Adoptive Neuro-Immunotherapy” can be performed for any given individual,at any desired time point,and even periodically.The diagnostic pre-clinical tests are performedin vitro,on T cells separated from a small quantity of person’s own blood,and can be completed within few days.
The first major aim of these tests is to examine,in vitro,the individual’s T cells,purified from a small,fresh blood sample (30–50 mL),and to reveal their present condition,namely their number,viability,and general functional status (normal,or rather exhausted,senescent or dysfunctional) in the body.The results of these tests will reveal whether the individual’s T cells are suboptimal and impaired,and if the tested individual requires a therapeutic procedure that will revive and improve his/her T cells.The second major aim of the diagnostic tests is to examinein vitrothe responsiveness of the individual’s T cells to selected neurotransmitters and neuropeptides.The T cell responsiveness to each of the neurotransmitters and neuropeptides will be examined and judged by the beneficial increase or decrease in the expression level of several very important T cell proteins serving as biomarkers,as well as by the increased and improved T cell’s functional responses,primarily:adhesion,migration,secretion,proliferation in response to cancer cells,and killing of cancer cells (the latter tests performed only when possible and relevant).
The results of these functional tests inform whether the person’s peripheral T cells,at that specific time point and condition,can be rejuvenated,improved,activated,or augmentedex vivoby transient incubation with natural neurotransmitters and neuropeptides,and if so;which neurotransmitter and/or neuropeptide induce the best effects on the patient’s own T cells.
Only if a person’s T cells respondin vitroin a positive and quantitativelysignificant manner to some of the tested physiological neurotransmitters and/or neuropeptides,will it be decided to proceed with this specific person,at this specific time point,to the therapeutic phase of “Personalized Adoptive Neuro-Immunotherapy” (explained below),because the person may benefit from it.
Of note,we have already successfully performed many such diagnostic tests,on both normal naïve T cells of healthy subjects,and on scarce and abnormal T cells of a small number of cancer patients,suffering from either HNSCC or HCC.Tests of T cells of patients with few other types of cancer are ongoing.
The therapeutic process of the “Personalized Adoptive Neuro-Immunotherapy”
The therapeutic procedure of the “Personalized Adoptive Neuro-Immunotherapy” (Figure 7) will be administered only to patients whose T cells require it,and are found to be responsive,at least to some of the neurotransmitters and neuropeptides tested in the preceding pre-clinical diagnostic tests.
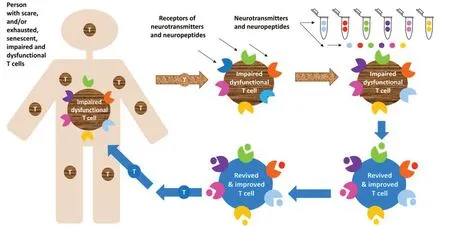
Figure 7|Novel proposed “Personalized Adoptive Neuro-Immunotherapy”.
These needy and responsive people will undergo an initial procedure of leukapheresis,in order to obtain a very large number of their white blood cells.Thereafter,T cells will be separated from the white blood cells,
subsequently divided into dozens of aliquots,and then deep-frozen in liquid nitrogen.This procedure will create a novel precious “Personalized T cell Bank” for each individual who will undergo this procedure.
From that time on,during the therapeutic period,a few ampoules of the patient’s frozen T cells will be thawed.Then the T cells will be treatedex vivoonce with the best neurotransmitter and/or neuropeptide,i.e.the specific one/s that induced the best significant effect on the patient’s own T cells at the initial personalized diagnostic stage.
After each single and transient incubation of the T cells with the selected neurotransmitters and/or neuropeptides,the T cells will be washed quickly and then injected immediately into the patient’s body.This planned procedure will be performed weekly over a few months,using the patient’s frozen T cells.
Importantly,the patient’s T cells willnotbe exposed to,or activated by,any other molecule besides the neurotransmitters and/or neuropeptides,and willnotundergo any otherin vitroprocess aimed to activate them,increase their number,or manipulate them genetically.
Furthermore,the “Personalized Adoptive Neuro-Immunotherapy” doesnotrequire that patients undergo any further process,either before,during,or after the injection of their T cells,following rejuvenation,activation,and improvement of these T cells by neurotransmitters and neuropeptides.This therapeutic procedure will alsonotrequire hospitalization of the patient.Another very valuable benefit,of immense importance,is that the“Personalized Adoptive Neuro-Immunotherapy” isnotexpected to cause adverse negative side effects,since it is the patient’s own T cells,activatedex vivoonly by physiological neurotransmitters and neuropeptides,and each time only once,without any unnatural process of activation,expansion,genetic manipulation or other,and without prolongedin vitroparking of the T cells in tissue culture conditions,known to change various advantageous T cell features (e.g.T cell migration,homing and various others).
In fact,I envision that the “Personalized Adoptive Neuro-Immunotherapy”may cause only positive side effects,thanks to the repeated administration into the body of revived and improved T cells,that would subsequently perform various beneficial T cell tasksin vivoin an improved manner,from which multiple organs and the entire body would benefit.
Based on all the above,and on all the envisioned advantages of the“Personalized Adoptive Neuro-Immunotherapy” (specified in (Levite,2021),this novel therapeutic procedure should be tested for its safety and efficacy in clinical trials.
The “Personalized Adoptive Neuro-Immunotherapy” is anticipated to be especially attractive for people with cancer,or chronic diseases caused by certain infectious organisms,and for elderly people,because all of them have severely impaired and dysfunctional T cells,and the exhausted and/or senescent T cells can lead to multiple life-threatening consequences.
That being said,the “Personalized Adoptive Neuro-Immunotherapy”,does not in any way diminish the value of,criticize,or deny any other type of immunotherapy,or any other treatment that can benefit the patient.In addition,combined or alternating therapies may be most effective in some situations.Of greatest importance is that the patients are treated by all means that may completely defeat their disease,or at least weaken it and keep it under control,over a long period,and provide a good quality of life.
10.Concluding Remarks
This paper discusses the neuro “faces” of normal beneficial T cells from several angles,and under different conditions and contexts.The most important messages are written in the following six points,without citing the relevant papers.
First,normal beneficial T cells are constantly needed for the brain,for proper functioning,protection,and coping with injuries and diseases of various kinds.Second,normal beneficial T cells,or at least some specific T cell subpopulations,become abnormal and dysfunctional in several pathological conditions that involve and affect the CNS.Indeed,T cell impairments of various types and severity are evident in aging,brain tumors,chronic viral infections that affect the brain,chronic stress,major depression,PD,AD,ALS,Schizophrenia,MS,and other diseases.T cells of elderly subjects,and of patients with brain tumors or severe viral infectious diseases,are especially exhausted,senescent,and severely dysfunctional.
Third,T cell impairments and dysfunction have multiple detrimental implications on the person himself,as well as on his/her family members,close relatives,and many unrelated people and health-related organizations.Since many people have various problems in their T cells,many parts of the population,and even entire countries,can be adversely affected.
Fourth,normal beneficial T cells of specific subpopulations,mainly CD4+CD25+Tregs,were shown to have protective and regenerative roles in several neuroinflammatory and neurodegenerative diseases.These include cognitive deficits,impaired emotion,and behavioral abnormalities,stroke/ischemia,chronic pain,severe trauma and injury,major depression,PD,AD,Schizophrenia,ALS,MS,and others.
Fifth,specific physiological neurotransmitters and neuropeptides,primarily:
Dopamine,Glutamate,GnRH-II,Neuropeptide Y,CGRP,and Somatostatin,induce independently multiple direct,potent,beneficial,and therapeuticallyrelevant effects on normal human T cells,even on scarce and impaired T cells of aging cancer patients.These effects are mediated by functional neurotransmitter receptors and neuropeptide receptors,which human T cells express on their cell surface.
Sixth,a novel and unique type of adoptive T cells therapy named: “Personalized Adoptive Neuro-Immunotherapy” was invented and patented by the author of this paper.This therapeutic method is based on the directex vivoactivation and improvement of person’s T cells,by physiological neurotransmitters and neuropeptides,and subsequent injection of the improved T cells into the patient’s body.This novel immunotherapy aims to safely,efficiently,and repeatedly rejuvenate,activate,and improve abnormal and dysfunctional T cells of any person in need,at any given time.The “Personalized Adoptive Neuro-Immunotherapy” has multiple unique advantages that call for validation of safety and efficacy in clinical trials.
Author contributions:ML performed literature search,analyzed the data,wrote the manuscript,drew the figures,raised suggestions,and invented and patented the “Personalized Adoptive Neuro-Immunotherapy”,described in the paper.The author approved the final version of the manuscript.
Conflicts of interest:The author declares no conflicts of interest.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update
- Do tau-synaptic long-term depression interactions in the hippocampus play a pivotal role in the progression of Alzheimer’s disease?

