Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
Kah Hui Yap,Shahrul Azmin,Suzana Makpol,Hanafi Ahmad Damanhuri,Muzaimi Mustapha,Jemaima Che Hamzah,Norlinah Mohamed Ibrahim,
Abstract Trehalose,a unique nonreducing crystalline disaccharide,is a potential disease-modifying treatment for neurodegenerative diseases associated with protein misfolding and aggregation due to aging,intrinsic mutations,or autophagy dysregulation.This systematic review summarizes the effects of trehalose on its underlying mechanisms in animal models of selected neurodegenerative disorders(tau pathology,synucleinopathy,polyglutamine tract,and motor neuron diseases).All animal studies on neurodegenerative diseases treated with trehalose published in Medline (accessed via EBSCOhost)and Scopus were considered.Of the 2259 studies screened,29 met the eligibility criteria.According to the SYstematic Review Center for Laboratory Animal Experiment (SYRCLE) risk of bias tool,we reported 22 out of 29 studies with a high risk of bias.The present findings support the purported role of trehalose in autophagic flux and protein refolding.This review identified several other lesserknown pathways,including modifying amyloid precursor protein processing,inhibition of reactive gliosis,the integrity of the blood-brain barrier,activation of growth factors,upregulation of the downstream antioxidant signaling pathway,and protection against mitochondrial defects.The absence of adverse events and improvements in the outcome parameters were observed in some studies,which supports the transition to human clinical trials.It is possible to conclude that trehalose exerts its neuroprotective effects through both direct and indirect pathways.However,heterogeneous methodologies and outcome measures across the studies rendered it impossible to derive a definitive conclusion.Translational studies on trehalose would need to clarify three important questions: 1)bioavailability with oral administration,2) optimal time window to confer neuroprotective benefits,and 3) optimal dosage to confer neuroprotection.
Key Words: amyotrophic lateral sclerosis;autophagy;neurodegenerative disease;neuroinflammation;polyglutamine tract;protein refolding;spinocerebellar ataxia;synucleinopathy;tau pathology;trehalose
From the Contents
Introduction 1179
Methods 1179
Results 1180
Discussion 1181
Conclusions 1184
Introduction
Neurodegenerative diseases are heterogeneous disorders characterized by progressive neuronal cell loss within the central nervous system (CNS)(Rubinsztein,2006).Presently,no disease-modifying treatments (DMTs) are neuroprotective or can slow disease progression (Cummings,2017).Current therapies for neurodegenerative diseases provide only symptomatic relief without altering disease progression (Hussain et al.,2018).Furthermore,the availability of pre-symptomatic genetic testing enables the early diagnosis andtimely treatment of neurodegenerative diseases,making the need for DMTs more pressing (Lee et al.,2018).
Trehalose is an omnipotent disaccharide molecule composed of two glucose molecules bound in a highly stable α,α–1,1 glycosidic linkage (Lee et al.,2018).The findings suggest that trehalose’s amorphous and non-reducing properties can be attributed to its high hydrophilicity,chemical stability,and strong resistance to denaturation/breakdown by heat,acid,or enzymes (Lee et al.,2018).
Trehalose has been shown to trigger autophagic flux to clear protein aggregates in neuronal cells by upregulating autophagyin vitro(Yoon et al.,2017),and facilitating the refolding of partially-denatured proteins,thereby stabilizing the protein aggregates of polyglutaminein vivoandin vitro(Olsson et al.,2016).These findings suggest that trehalose may exert its neuroprotective mechanisms through both direct and indirect pathways (Lee et al.,2018;Assoni et al.,2021).Trehalose acts as an autophagic enhancer to clear off protein aggregates by converting autophagosomes to autolysosomes in the direct pathway.Trehalose may also exert indirect neuroprotective effects by enhancing the survival of gut microbiota and increasing microbiota diversity through its non-reducing property,thereby maintaining the integrity of microbiota-gut-brain signaling (Lee et al.,2018;Assoni et al.,2021).At present,evidence of its therapeutic effect on humans is still not convincing.Specifically,the bulk of the evidence on trehalose is derived from animal studies,which have been pivotal in providing data on preliminary efficacy,pharmacokinetics,and safety (Junod,2013;Khalifeh et al.,2021).
We conducted this systematic review to evaluate all original research reports that assessed the effect of trehalose in animal models of neurodegenerative diseases,with their primary or secondary outcomes being to examine the behavioral and physiological changes related to trehalose.Specifically,we focused primarily on the underlying mechanisms of DMT using animal models as a critical source ofin vivoinformation.Subsequently,we summarized the mechanisms involved in the therapeutic effects of trehalose according to the type of neurodegenerative disease.
Methods
Search strategy and study selection
On June 24,2022,we conducted a systematic review to identify all animal studies on neurodegenerative diseases that had used trehalose as a possible DMT,using two electronic databases: Medline (accessed via EBSCOhost) and Scopus.We used the following population,intervention,comparator,and outcome (PICO) criteria: 1) animal models of neurodegenerative diseases,2) trehalose as intervention,3) (non)randomized controlled trials,and 4)physiological (i.e.,biochemical or brain changes) and behavioral (if applicable)changes as outcome measures.We performed the search with Medical Subject Headings (MeSH) terms related to 1) neurodegenerative diseases,such as ‘Alzheimer,’ ‘dementia,’ ‘Parkinson,’ ‘Huntington,’ ‘disease,’ ‘ataxia,’‘sclerosis,’ ‘brain,’ ‘atrophy,’ and ‘neurodegeneration’ and 2) DMT,using‘trehalose.’ Only studies written in the English language were included.Review articles were excluded to avoid reporting overlapping data.Case studies/series were excluded to prevent selection bias.This review treated multiple studies of the same material as a single entry.
Data extraction
We conducted this systematic review according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (Page et al.,2021).The first author performed an initial eligibility screening to determine whether each result’s title and abstract were relevant to the scope of the review.Another reviewer cross-checked the process.Next,the reviewers conducted a full-text appraisal of potentially eligible studies.
The following information was extracted from the included studies: first author,year of publication,country,blinding,intervention design,animal characteristics,outcome measures,clinical outcomes,physiological changes,adverse events (AEs),and conclusion.In addition,we grouped the studies according to the type of neurodegenerative disease.We classified neurodegenerative diseases into the following groups: 1) tau pathology[e.g.,Alzheimer’s disease (AD) and frontotemporal dementia (Goedert and Spillantini,2017)],2) synucleinopathy [e.g.,Parkinson’s disease (PD) and Lewy body dementia (LBD) (Jellinger,2007)],3) polyglutamine tract diseases [e.g.,Huntington’s disease (HD) and spinocerebellar ataxia (SCA) (Lieberman et al.,2019)],and 4) motor neuron diseases (MND) [e.g.,amyotrophic lateral sclerosis (ALS) (Hardiman et al.,2017)].
Methodological quality
We evaluated the risk of bias in the included studies using the SYstematic Review Center for Laboratory Animal Experimentation (SYRCLE) risk of bias tool,which is a variant of the Cochrane Collaboration risk of bias tool adapted for animal intervention studies (Hooijmans et al.,2014).It assesses the high,low,or unclear risk of bias across nine categories: 1) sequence generation,2) baseline characteristics,3) allocation concealment,4) random housing,5)blinding of caregivers and researchers,6) random outcome assessments,7)blinding of assessors,8) incomplete outcome data,and 9) selective outcome reporting.We considered studies that lacked assessor blinding or that did not adhere to intention-to-treat analysis (i.e.,those with a high risk for incomplete outcome data) to have an increased risk of bias.Two reviewers assessed each included study independently,while a senior reviewer resolved any conflicts.
Results
Study selection and methodological quality
We screened 2259 studies,29 of which were finally selected according to the PRISMA guidelines (Figure 1;Page et al.,2021).Eight of the 29 studies did not adopt the intention-to-treat analysis of the SYRCLE risk of bias tool.Twenty-two of the 29 studies had a high risk of bias,as information regarding the blinding of outcome assessors was absent.None of the studies reported randomization status or allocation concealment (Additional Table 1).
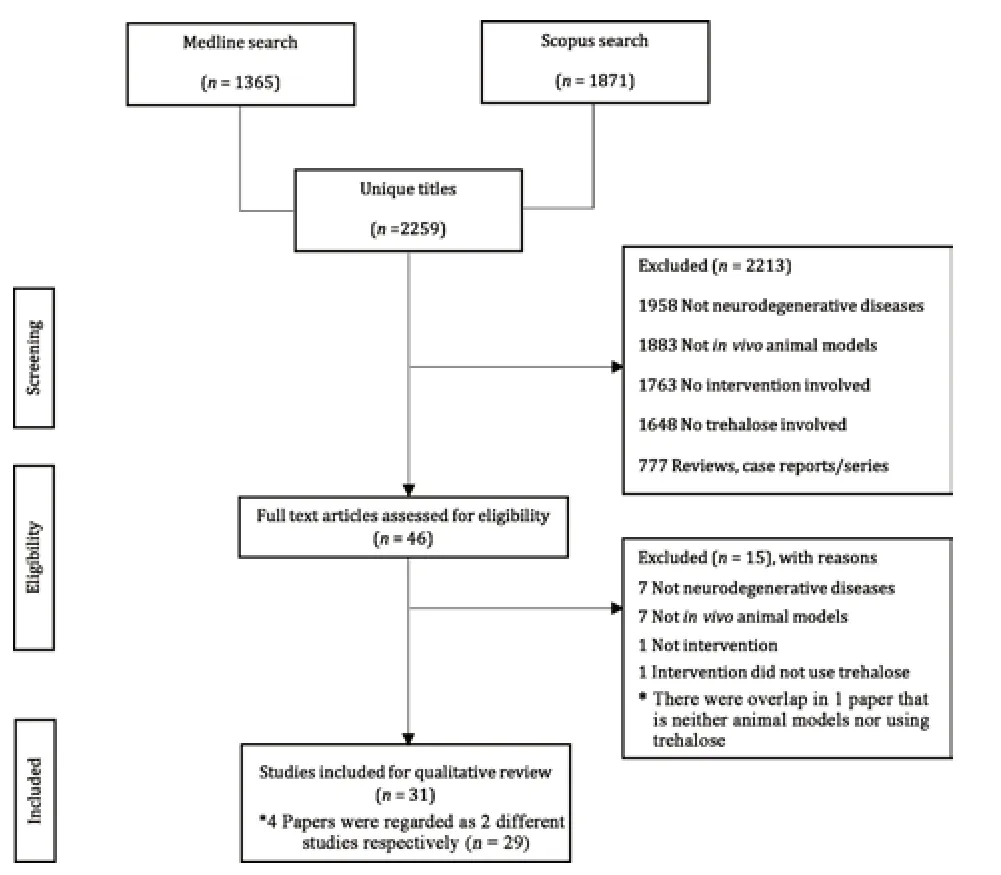
Figure 1|Study search per the PRISMA guideline (Page et al.,2021).
Study characteristics
We classified the studies into four groups based on the underlying pathology leading to neurodegeneration: tau pathology,synucleinopathy,polyglutamine tract diseases,and ALS.We reviewed the effects of trehalose on physiological and behavioral changes,as well as the underlying mechanisms investigated by these studies.None of the studies reported any AEs from trehalose administration.The details of the data extracted from the studies related to the above-mentioned pathology are tabulated inAdditional Tables 2–5,respectively.All studies used rodents,while one study used macaques in addition to rodents (Howson et al.,2019).
Tau pathology
Nine studies investigated tau pathology.While one study did not report the exact numbers of animals used (Pupyshev et al.,2022),the mean number of animals per study for the remainder was approximately 37.38 ± 24.40(range=15–90).Regarding trehalose administration,88.9% of the studies used oral administration,and 11.1% used ventricular injection.In terms of concentration,six studies used 1–2% trehalose for oral administration(Rodríguez-Navarro et al.,2010;Perucho et al.,2012;Schaeffer and Goedert,2012;Schaeffer et al.,2012;Holler et al.,2016;Liu et al.,2020;Pupyshev et al.,2022),whereas one study used 0.5% trehalose for ventricular injection(Du et al.,2013),and another used 0.5–1% trehalose as pre-treatment and its indigestible analogue,lactulose,before disease onset (Lee et al.,2021).
Six studies investigated the effect of trehalose on autophagic flux,and five of these studies demonstrated increased autophagic flux (Rodríguez-Navarro et al.,2010;Perucho et al.,2012;Schaeffer et al.,2012;Holler et al.,2016;Lee et al.,2021;Pupyshev et al.,2022).The remaining study did not show changes in autophagy flux (Portbury et al.,2017).Two studies investigated and demonstrated the beneficial effect of trehalose on protein refolding(Perucho et al.,2012;Lee et al.,2021).One study demonstrated the effect of trehalose in modifying amyloid precursor protein (APP) processing (Liu et al.,2020).Three studies demonstrated that trehalose administration reduced reactive gliosis (Rodríguez-Navarro et al.,2010;Perucho et al.,2012;Lee et al.,2021;Pupyshev et al.,2022),and two studies demonstrated upregulation of growth factors (Holler et al.,2016;Portbury et al.,2017).One study failed to demonstrate the effect of trehalose on the expression of biometals(Portbury et al.,2017).Five studies that investigated autophagic flux (Perucho et al.,2012;Lee et al.,2021;Pupyshev et al.,2022),protein refolding (Perucho et al.,2012;Lee et al.,2021),upregulation of growth factor (Portbury et al.,2017),and reducing reactive gliosis (Rodríguez-Navarro et al.,2010;Pupyshev et al.,2022) reported corresponding improvements in behavioral outcomes,including memory,anxiety,and motor activity,while three studies did not investigate behavioral outcomes (Schaeffer and Goedert,2012;Schaeffer et al.,2012;Holler et al.,2016;Liu et al.,2020).Conversely,one study reported that trehalose improved spatial memory and learning and reduced protein aggregations in the hippocampus without investigating the underlying mechanism (Du et al.,2013).We have summarized the investigated underlying mechanisms inTable 1.
Synucleinopathy
Eleven studies investigated synucleinopathy.While one study did not report exact numbers of the animals used (Moon et al.,2022),the mean number of animals per study for the remainder was approximately 43.30± 16.74 (range=30–64).Regarding trehalose administration,90.9% used oral administration and 9.1% used intraperitoneal injection.Seven studies used trehalose at concentrations ranging from 0.5–5% (Sarkar et al.,2014;Ferguson et al.,2015;Tanji et al.,2015;He et al.,2016;Howson et al.,2019;Pupyshev et al.,2019,2021).For three remaining studies,one used 3% trehalose pre-treatment before disease onset (Darabi et al.,2019),one investigated the effect of 4 g/kg trehalose and its combined effect with sodium butyrate (Kakoty et al.,2021),one investigated the effect of 2 g/kg trehalose for intraperitoneal injection (Moon et al.,2022),and one study compared the effect of 2% trehalose with its indigestible analogues lactulose and melibiose (Lin et al.,2020).
In terms of disease mechanisms,eight of the 11 studies demonstrated the effect of trehalose in terms of activating autophagic flux (Tanji et al.,2015;He et al.,2016;Darabi et al.,2019;Pupyshev et al.,2019,2021;Lin et al.,2020;Kakoty et al.,2021;Moon et al.,2022).One study investigated and showed the beneficial effect of trehalose on protein refolding (Tanji et al.,2015).Two studies reported a reduction in reactive gliosis (Sarkar et al.,2014;Kakoty et al.,2021) and upregulation of the downstream antioxidant signaling pathway(Darabi et al.,2019;Lin et al.,2020).Other studies have investigated and demonstrated the beneficial effect of trehalose in modifying the processing of APP (Lin et al.,2020),expressing growth factors (Kakoty et al.,2021),and maintaining the integrity of the blood-brain barrier (BBB) (Sarkar et al.,2014).Eight studies reported a corresponding improvement in behavioral outcomes,including motor and non-motor (memory,depression,and olfactory function)activities (He et al.,2016;Darabi et al.,2019;Howson et al.,2019;Pupyshev et al.,2019,2021;Lin et al.,2020;Kakoty et al.,2021;Moon et al.,2022),while two studies did not investigate behavioral outcomes (Sarkar et al.,2014;Tanji et al.,2015).Lastly,one study reported that improved dopaminergic deficits in the striatum did not alleviate any behavioral deficits (Ferguson et al.,2015).We have summarized the investigated underlying mechanisms inTable 2.

Table 1|Mechanism of actions of trehalose studied in animal models of tau pathology
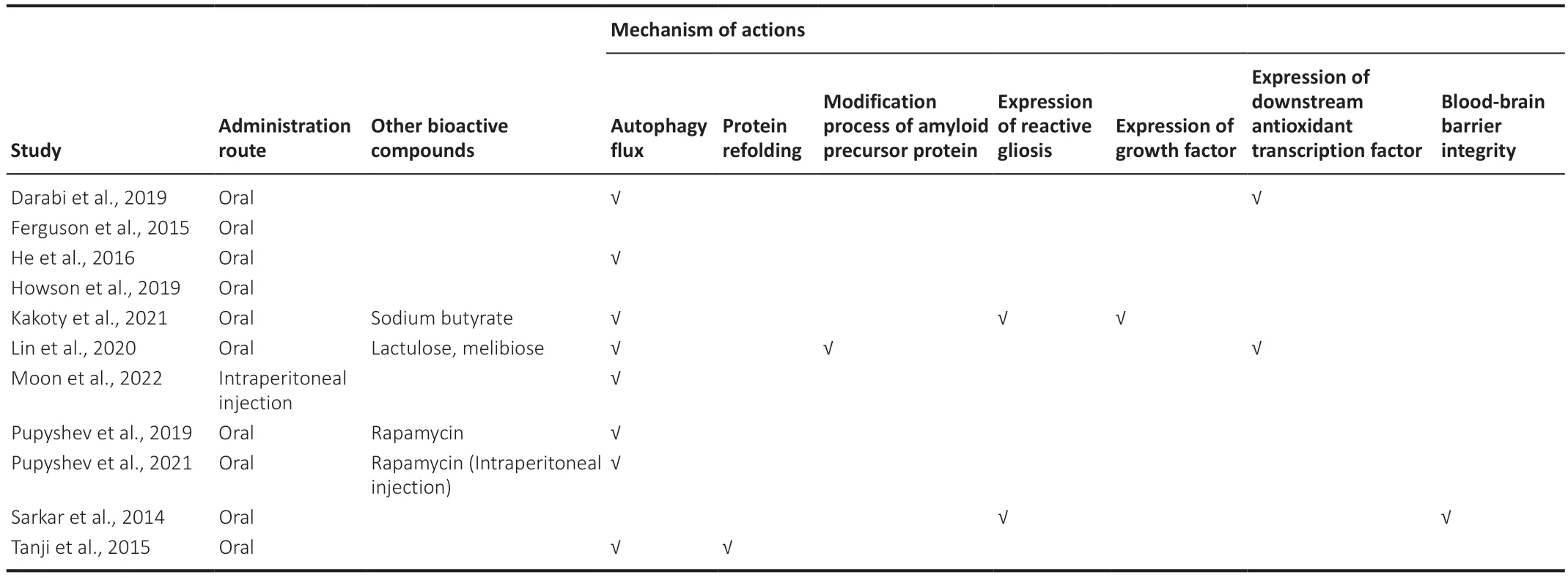
Table 2|Mechanism of actions of trehalose studied in animal models of synucleinopathy
Polyglutamine tract diseases
Six studies investigated polyglutamine tract diseases;it was impossible to determine the number of animals used in the polyglutamine tract disease studies,as three did not report the exact numbers.Regarding trehalose administration,83% of the studies used oral administration,and 17% used nano-injection.The trehalose concentration ranged from 2% to 4% for oral administration (Tanaka et al.,2004;Chen et al.,2015;Santana et al.,2020),one of which was administered in conjunction with C17.2 stem cell transplantation (Yang and Yu,2009).Other studies either orally administered 0.4% of weight for BBB-permeable trehalose derivative (Im et al.,2013) or administered through a 0.4 mg/mL for poly(trehalose) nanoparticle injections(Debnath et al.,2017,2019).
One study investigated,but failed to demonstrate,the effect of trehalose on activating autophagic flux (Santana et al.,2020).Another study was unable to demonstrate the effect of trehalose on protein refolding,but it showed that trehalose reduced the expression of reactive gliosis (Chen et al.,2015).These studies reported a corresponding improvement in motor activity (Chen et al.,2015;Santana et al.,2020).Conversely,three studies reported that trehalose improved memory,anxiety,and motor activity alongside reducing protein aggregates and brain atrophy (if applicable) without investigating the underlying mechanism (Tanaka et al.,2004;Yang and Yu,2009;Im et al.,2013).Lastly,one study reported that poly(trehalose) nanoparticle injections reduced protein aggregates and increased the degree of nanoparticles entering the brain without investigating behavioral outcomes (Debnath et al.,2017,2019).We summarized the investigated underlying mechanisms inTable 3.
Amyotrophic lateral sclerosis
Three studies were conducted on ALS;the mean number of animals per study was approximately 72 ± 21.63 (range=47–90).Regarding trehalose administration,66% had used oral administration,and 33% had used injections.Two studies used 2% trehalose for oral administration (Zhang et al.,2014;Li et al.,2015),whereas the other study used a 2 g/kg concentration for injection (Castillo et al.,2013).
All three studies investigated and demonstrated the effect of trehalose on activating autophagic flux.Two studies showed that trehalose reduced the expression of reactive gliosis (Castillo et al.,2013;Li et al.,2015),while another study reported that trehalose protected against mitochondrial defects (Zhang et al.,2014).We have summarized the investigated underlying mechanisms inTable 4.
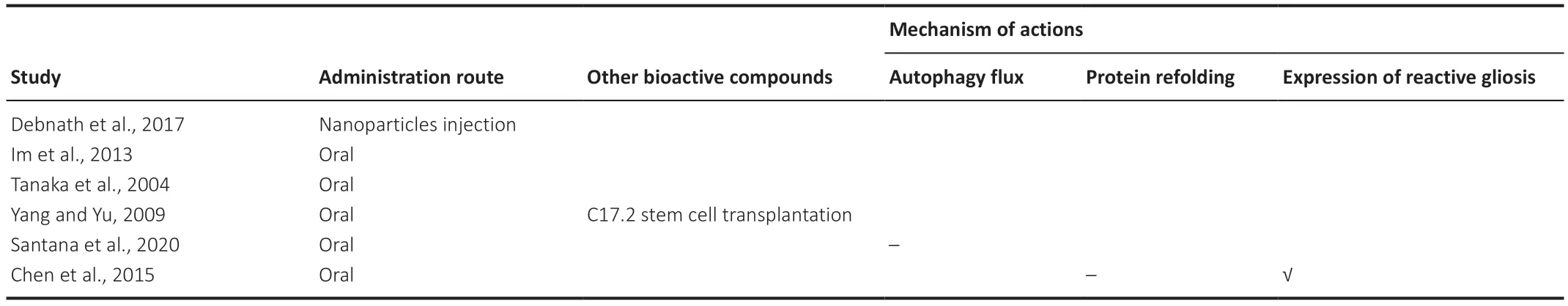
Table 3|Mechanism of actions of trehalose studied in animal models of polyglutamine tract disease
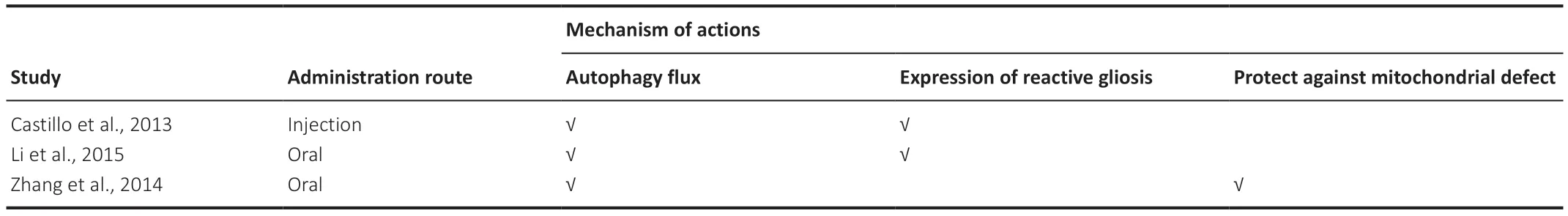
Table 4|Mechanism of actions of trehalose studied in animal models of amyotrophic lateral sclerosis
Discussion
In this review,we have systematically evaluated the therapeutic effects of trehalose in animal models of neurodegenerative diseases.Given that none of the studies reported AEs,this discussion is focused solely on the behavioral changes and mechanisms underlying the therapeutic effect of trehalose.This review aims to outline the fundamental scientific knowledge of trehalose to facilitate the transition from animal studies to human subjects with neurodegenerative diseases.Because different studies employed heterogeneous outcome measures,we did not compare the effects of trehalose across neurodegenerative diseases.
Tau pathology
Neurodegenerative diseases with tau pathology are characterized by the aggregation of tau proteins into neurofibrillary tangles in the human brain(Goedert and Spillantini,2017).This protein aggregation disrupts neural processes,such as axonal transport and neural communication (Wang and Mandelkow,2016),leading to progressive neuronal cell loss within the CNS(Rubinsztein,2006).This process is often coupled with inefficient autophagy mechanisms in removing protein aggregations.The latter is due to mutations in the lysosomes,which hinder the maturation of autolysosomes and,subsequently,the activation of autophagy.This reduction in autophagic flux leads to the net production of protein aggregates (Uddin et al.,2018).
Consistently,trehalose administration was shown to improve anxiety symptoms,spatial memory,and motor activity in mouse models of tau pathology (Rodríguez-Navarro et al.,2010;Perucho et al.,2012;Du et al.,2013;Portbury et al.,2017;Lee et al.,2021;Pupyshev et al.,2022).The studies also reported reduced protein aggregations in the hippocampi of mice treated with trehalose (Rodríguez-Navarro et al.,2010;Perucho et al.,2012;Du et al.,2013;Pupyshev et al.,2022).The hippocampus is a brain region vulnerable to the deposition of protein aggregates in AD pathology(Furcila et al.,2018) and contributes to memory deficits (Eichenbaum,2017).The improvement in spatial memory suggests that trehalose may upregulate the glucose transporter GLUT8 (Narita et al.,2019),a mammalian trehalose transporter required for the signaling of trehalose-induced autophagy(Mayer et al.,2016),which is abundant in the hippocampus (Reagan et al.,2002).Aggregate reduction in the cerebral cortex,limbic system,and striatum explains the improvement in anxiety symptoms and motor function(Rodríguez-Navarro et al.,2010;Perucho et al.,2012;Pupyshev et al.,2022).These observations could be related to the activation of autophagic flux in clearing protein aggregates,which,in turn,improved neuronal survival(Rodríguez-Navarro et al.,2010;Perucho et al.,2012;Schaeffer and Goedert,2012;Schaeffer et al.,2012;Holler et al.,2016;Pupyshev et al.,2022).Two other studies demonstrated that trehalose acts as a chaperone in protein refolding (Perucho et al.,2012;Lee et al.,2021).This mechanism was consistent within vitrofindings that trehalose maintained the protein structure against thermal denaturation and protein aggregation (Olsson et al.,2016),contributing to neuroprotection against neurodegenerative diseases.The findings support the possible role of trehalose in autophagic flux and protein refolding.In addition,given that trehalose reduced the production of neurofibrillary tangles by modifying the processing of APP (Liu et al.,2020),these mechanisms produced a synergistic effect in terms of plaque removal.
Three studies reported that trehalose was effective against reactive gliosis in the hippocampi and cerebral cortexes of mouse models (Rodríguez-Navarro et al.,2010;Perucho et al.,2012;Lee et al.,2021;Pupyshev et al.,2022).Reactive gliosis is an inflammatory response in the CNS that drives the pathogenesis of neurodegenerative diseases (Li et al.,2019).This finding is consistent with the postulated role of trehalose as an indirect antioxidant by enhancing the expression of downstream antioxidant signaling pathways,which,in turn,reduces oxidative stress and neuroinflammation (Mizunoe et al.,2018).In AD,the degree of reactive astrocytes correlates with cognitive decline (Kashon et al.,2004).Therefore,trehalose can potentially slow down cognitive decline via the inhibition of reactive gliosis and the subsequent regulation of neuroinflammation to halt the pathological process of AD.
Two more studies reported that trehalose played a role in increasing synaptic activity,activating neurogenesis,and secreting growth factor progranulin(PGRN) (Holler et al.,2016;Portbury et al.,2017).While trehalose increases synaptophysin and doublecortin expression,reliable neurogenesis markers,it remains unknown how trehalose modulates this mechanism (Portbury et al.,2017).An indigestible analogue,lactulose,further upregulated the expression of synapses,(Lee et al.,2021).Lactulose is highly resistant to hydrolysis,and absorption in the mammalian digestive system confers a greater effect than trehalose on promoting the growth of human gut microbiota (Khangwal and Shukla,2019).This finding is in line with the role of trehalose and its analogues in the indirect neuroprotective pathway on the integrity of microbiota-gut-brain signaling (Lee et al.,2018;Assoni et al.,2021).However,there was no difference in the improvement of behavioral performance between lactulose and trehalose (Lee et al.,2021).Nevertheless,the enhancement of neurogenesis,such as the coordinated proliferation,differentiation,and migration of neural precursor cells,has been regarded as a potential therapy for neurodegenerative diseases rather than explicitly implicating improved cognitive function and enhanced neuroplasticity (Shohayeb et al.,2018).Likewise,it remains unknown how trehalose upregulates the expression of PGRN,although the results indicated increased gene transcription,at least in part (Holler et al.,2016).PGRN offers protection against the neurotoxicity of protein aggregates in AD (Minami et al.,2014),thereby providing an additional pathway for trehalose in alleviating the cognitive impairment caused by AD.
Synucleinopathy
Synucleinopathies are a group of neurodegenerative diseases characterized by the accumulation of α-synuclein aggregates in the human brain (McCann et al.,2014).The misfolding and aggregation of α-synuclein with concomitant cytotoxicity plays a pivotal role in pathogenesis and disease progression(Delenclos et al.,2019).Synucleinopathies primarily share features of parkinsonism and impaired cognition,among other symptoms (Jellinger,2007).These diseases may also overlap with neurodegenerative diseases with tau pathologies due to the interaction between the synuclein and tau proteins(Moussaud et al.,2014).
In concordance with the studies that used mouse models of tau pathology,trehalose may have led to improvements in procedural learning and motor coordination in mouse models of PD and LBD through similar mechanisms[i.e.,activating autophagic flux (Tanji et al.,2015;He et al.,2016;Darabi et al.,2019;Pupyshev et al.,2019;Lin et al.,2020;Kakoty et al.,2021;Pupyshev et al.,2021;Moon et al.,2022),protein refolding (Tanji et al.,2015),modifying the processing of APP (Lin et al.,2020),inhibiting reactive gliosis (Sarkar et al.,2014;Kakoty et al.,2021),and upregulating the expression of growth factor (Kakoty et al.,2021) and the downstream antioxidant-signaling pathway (Darabi et al.,2019;Lin et al.,2020)].These processes may promote the survival of dopaminergic neurons in the striatums of PD animal models(Ferguson et al.,2015;He et al.,2016;Darabi et al.,2019;Howson et al.,2019;Pupyshev et al.,2019,2021;Moon et al.,2022).The striatum has bilateral dopaminergic innervations,with different brain regions responsible for procedural learning and goal-directed actions.The selective loss of dopaminergic neurons in the striatum leads to motoric clinical features of synucleinopathies (Zhai et al.,2018).Therefore,a key therapeutic strategy would be to confer neuroprotection to dopaminergic neurons.
However,the trehalose-induced improvement was dose-dependent.As such,only trehalose concentrations of 2% and 5% demonstrated therapeutic effects (Sarkar et al.,2014;Tanji et al.,2015;He et al.,2016;Darabi et al.,2019;Howson et al.,2019;Pupyshev et al.,2019,2021;Lin et al.,2020).In contrast,concentrations of 0.5% and 1% were insufficient to confer any neuroprotection against alterations in mouse models of PD (Ferguson et al.,2015;He et al.,2016;Kakoty et al.,2021).At present,pretreatment with 2% trehalose effectively confers neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic deficits and reactive gliosis-mediated neuroinflammation in mouse models of PD (Sarkar et al.,2014).In comparison,another study showed that pretreatment with 3% trehalose upregulated the autophagic flux and the expression of the downstream antioxidant signaling pathway (Darabi et al.,2019).However,the absence of therapeutic effects in the macaque model of PD,coupled with the alleviation of dopaminergic deficits (i.e.,survival of dopaminergic neurons),could imply that such clinical improvement is restricted to rodents (Howson et al.,2019).
Trehalose may also confer neuroprotection against microvessels and endothelial damage in maintaining BBB integrity,thereby slowing the onset of PD pathology.Several molecular action pathways have been recorded,including neuroprotection for glucose transporters and endothelial tight junction proteins (Sarkar et al.,2014).Glucose transporters,including GLUT8(Narita et al.,2019),are membrane proteins that transfer sugars,one of the leading energy sources,across cellular membranes (Takata,1996).On the other hand,endothelial tight junction proteins are crucial in forming the BBB.This selective physical barrier prevents solutes in the circulating blood from nonselectively crossing into the CNS (de Vries et al.,1997).Both proteins are essential in transporting nutrients across the BBB (Cabezas et al.,2014;Patching,2017),which,in turn,mitigates MPTP-induced damage to dopaminergic neurons (Sarkar et al.,2014).
Polyglutamine tract diseases
Polyglutamine tract diseases are a group of neurodegenerative diseases resulting from cytosine-adenine-guanine (CAG) trinucleotide repeat expansion within the affected gene.They include diseases such as HD,SCA (subtypes 1,2,3,6,7,17),dentatorubral-pallidoluysian atrophy,and spinal and bulbar muscular atrophy (Takahashi et al.,2010).The translated elongated polyglutamine tracts form protein aggregates that lead to degenerative processes within the affected brain regions,depending on the gene (Shao and Diamond,2007).Longer CAG repeats result in earlier disease onset and a faster rate of progression (Lee et al.,2019).Studies in mouse models of HD showed that trehalose at a concentration of 2% reduced the partially misfolded protein in the motor cortex and striatum,leading to increased brain weight.These changes contributed to improved motor coordination and a prolonged lifespan in mouse models of HD (Tanaka et al.,2004).Combining trehalose with C17.2 stem cell transplantation in mouse models of HD showed greater improvements in dystonia,motor coordination,and learning and reduced protein aggregates in the striatum and the size of lateral ventricles.The synergistic effect is likely attributed to the role of C17.2 stem cell transplantation,which stimulates growth factors and boosts the ability of trehalose to stabilize misfolded proteins (Yang and Yu,2009).
A few studies have also taken innovative approaches to remove protein aggregation,in which the BBB permeability of trehalose was improved (Im et al.,2013;Debnath et al.,2017,2019).A synthetic trehalose derivative improved dystonia,locomotion,motor coordination,and prolonged lifespan by reducing protein aggregation in the brains and livers of mouse models of HD more efficiently (Im et al.,2013).Another study that used mouse HD models reported that trehalose nanoparticles inhibited polyglutamine aggregation through multivalent binding,which worked comparatively better than molecular trehalose (Debnath et al.,2017,2019).While increasing the BBB permeability of trehalose may have improved its ability to reduce protein aggregation and behavioral performances in HD,the exact mechanisms were not investigated in these studies.It is possible that one mechanism,but not the other,may benefit from increasing BBB permeability.
Another polyglutamine tract disease,SCA,is primarily characterized by an increasingly worsening cerebellar function,leading to a lack of coordination of voluntary movements (Bodranghien et al.,2016).By constricting polyglutamine aggregates in the lobule IX of the cerebellum,trehalose also improved motor coordination and gait in mouse models of SCA3 due to neuronal survival and thicker cerebellar layers (Santana et al.,2020).Another study using mouse models of SCA17 reported trehalose-induced enhancement in motor coordination and gait via the inhibition of reactive astrogliosis followed by increased cerebellum weight (Chen et al.,2015).Interestingly,neither study reported evidence suggesting activities relating to autophagic flux (Santana et al.,2020) or protein refolding (Chen et al.,2015),thus warranting further investigation of the possible mechanisms underlying the therapeutic effects of trehalose on SCA.
Notably,only two of six studies investigated trehalose’s neuroprotective mechanism on polyglutamine tract diseases.Therefore,the role of trehalose in polyglutamine tract diseases remains unknown,apart from a study that showed the inhibition of reactive gliosis (Chen et al.,2015).Regardless,these studies consistently reported that trehalose reduced protein aggregations in the brains of mouse models (Tanaka et al.,2004;Yang and Yu,2009;Im et al.,2013;Chen et al.,2015;Debnath et al.,2017,2019;Santana et al.,2020),implying that either autophagic flux,protein refolding,or modifying the processing of APP may all be contributory.Consistent improvement in neuronal survival and behavioral performance (Tanaka et al.,2004;Yang and Yu,2009;Im et al.,2013;Chen et al.,2015;Debnath et al.,2017,2019;Santana et al.,2020) further supports the idea that trehalose could be a promising neuroprotective agent in future clinical trials of polyglutamine tract diseases.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that results in the progressive loss of motor neurons that control voluntary muscles.Like other neurodegenerative diseases,the pathological hallmark of ALS is the aggregation of Bunina bodies in the cytoplasm of motor neurons,with the resulting toxicity leading to skeletal muscle atrophy,motor cortex atrophy,sclerosis of the corticospinal and corticobulbar tracts,thinning of the hypoglossal nerves (which control the tongue),and thinning of the anterior roots of the spinal cord (Hardiman et al.,2017).
Trehalose consistently prolonged the lifespan of mouse models of ALS,suggesting that it could delay disease onset and progression (Castillo et al.,2013;Zhang et al.,2014;Li et al.,2015).The underlying neuroprotective mechanisms were similar to other animal models,including autophagic flux activation (Castillo et al.,2013;Zhang et al.,2014) and the inhibition of reactive astrogliosis (Castillo et al.,2013;Li et al.,2015).Studies have also observed reduced protein aggregates in the frontal cortex and spinal cords of mouse models of ALS (Castillo et al.,2013;Li et al.,2015).In addition,trehalose also arrested skeletal muscle denervation,protected against mitochondrial defects,and inhibited apoptosis in ALS mouse models (Zhang et al.,2014).ALS pathology is characterized by mitochondrial defects,the death of motor neurons,and neuronal apoptosis (Federico et al.,2012).Notably,improvements in motor coordination with trehalose administration in mouse models of ALS occurred only in the early stages of the disease (Li et al.,2015),which was likely dependent on the neuroplasticity of the surviving motor neurons.These observations may imply that trehalose could be an effective adjunctive therapy,albeit only in the early stages of the disease.
Summary
Most studies primarily supported the purported role that trehalose plays in the autophagic flux and protein refolding necessary for the removal of protein aggregates in neurodegenerative diseases.Trehalose also possesses lesserknown neuroprotective mechanisms,including modification of the processing of APP,inhibition of reactive gliosis and regulation of neuroinflammation,integrity of the blood-brain barrier,activation of growth factors,upregulation of the antioxidant signaling pathway,and protection against mitochondrial defects.Either altering the permeability of trehalose through the BBB or using trehalose as adjunct therapy alongside other treatments may enhance its therapeutic effect,both of which remain avenues for future research.While trehalose exerts its neuroprotective mechanisms in both direct and indirect pathways (Lee et al.,2018;Assoni et al.,2021),the link between the indirect pathway (i.e.,the integrity of microbiota-gut-brain signaling)and the neuroprotective mechanisms of trehalose has not been established in animal models of neurodegenerative diseases.It is possible that some neuroprotective mechanisms may be exclusive to the indirect pathway or may activate synergistically through both pathways.Likewise,while different mechanisms of action were identified for trehalose,whether these mechanisms work independently or interactively remains unknown.One finding implied that autophagic flux might activate the secretion of growth factors (Holler et al.,2016).This finding leads to the possibility that other mechanisms that also target the protein aggregates (i.e.,protein refolding and modifying the processing of APP) may be related to and trigger the same response.Notably,the original findings provide explanations for the relationships (i.e.,correlations) between the identified mechanisms and the therapeutic effect rather than direct causation (Figure 2).
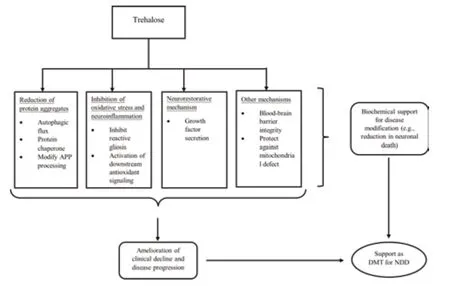
Figure 2|A theoretical framework outlining the neuroprotective mechanisms of trehalose as a DMT for neurodegenerative diseases,based on the framework by Cumming (2017).
The present findings affirm that trehalose activates autophagic flux in 17 of 19 studies involving tau pathology,synucleinopathies,and ALS,representing the mechanism with the most robust evidence.In support,mutations in genes encoding proteins involved at various steps in the autophagy pathway are implicated in these neurodegenerative diseases,including lysosomes and autophagosomes (Fleming et al.,2022).In this case,trehalose may have activated autophagic flux through direct pathways by inducing rapid and transient lysosomal enlargement and membrane permeabilization to facilitate autolysosome formation (Rusmini et al.,2019).The absence of similar evidence in polyglutamine tract disease could perhaps be explained by the fact that one of six studies examined the role of trehalose in autophagic flux (Table 3).On the other hand,trehalose failed to demonstrate autophagic flux in one study using mouse models of tau pathology (Portbury et al.,2017)and another using SCA3 (Santana et al.,2020).These studies used a similar(i.e.,2%) trehalose concentration and a more extended treatment duration than several studies with positive findings on autophagic flux,suggesting that the difference in dosage and treatment duration is unlikely to explain the negative findings.We speculate that not only might one neuroprotective mechanism (i.e.,autophagic flux) activate another (i.e.,secretion of growth factor) (Holler et al.,2016),but the activation of different neuroprotective mechanisms requires a different threshold.For example,two studies showed that trehalose failed to activate autophagic flux but inhibited reactive gliosis(Santana et al.,2020),and the secretion of growth factors (Portbury et al.,2017) may imply that the latter requires a lower threshold for activation.However,what determines the threshold remains unclear and requires vigorous investigation.Alternatively,SCA pathology may hinder the activation of autophagic flux,which inhibits reactive gliosis (Chen et al.,2015).Thus,the differing neuropathologies may determine the activation threshold for neuroprotective mechanisms of trehalose to take effect.Likewise,this speculation needs support from more findings,given that there were no more than three studies from each of the neurodegenerative diseases that investigated mechanisms other than autophagic flux.
In terms of methodological quality,most studies (n=22) had a high risk of bias due to a lack of outcome assessor blinding,except for the studies on ALS (Table 1).Blinding of the outcome assessors allowed for observer bias reduction,contributing to the robustness of the findings (Bello et al.,2014).Following the guidelines for preclinical animal research in ALS/MND,these studies monitored disease onset,progression,and lifespan,which are critical for a full assessment of the therapeutic effects of an agent (Ludolph et al.,2010).Therefore,existing findings on trehalose are substantiated with data from more rigorous scientific methods in ALS studies compared to other neurodegenerative diseases.Apart from blinding of outcome assessors,none of the studies reported randomization status,blinding of caregivers and researchers,random housing,or allocation concealment,which may increase the risk of false alarms,namely activating neuroprotective mechanisms and corresponding behavioral and physiological changes in the treatment group(Bebarta et al.,2003).Future studies should address these shortcomings to provide robust evidence of the different mechanisms of trehalose before translation into human clinical trials.
Implications for potential human translation in future studies
Hypothetically,trehalose maintains the integrity of microbiota-gut-brain signaling by enhancing the survival of gut microbiota (Felice et al.,2016;Lee et al.,2018;Assoni et al.,2021).Consistently,oral intake of trehalose,rather than an intraperitoneal injection,induced autophagic flux in mouse brains,implying that trehalose could exert neuroprotection through the gastrointestinal system (Tanji et al.,2015),which supports the therapeutic effect of oral administration in published human studies on SCA3 (Noorasyikin et al.,2020;Zaltzman et al.,2020).However,one study reported autophagic flux in mouse brains using intraperitoneal injections,possibly through the direct pathway (Moon et al.,2022).While it is expected that indigestible analogues of trehalose,namely lactulose and melibiose,may confer a more remarkable effect through microbiota-gut-brain signaling (Lin et al.,2020;Lee et al.,2021),a randomized controlled trial using oral trehalose on patients with neurodegenerative diseases is lacking and deserves priority in terms of future study.
In addition,trehalose-induced neuroprotection may be effective only in the early stages of neurodegenerative diseases (Li et al.,2015).A window of opportunity may exist for trehalose to exert its neuroprotective effect during these stages.Conversely,the later stages of neurodegenerative diseases have insufficient structural integrity to respond to DMTs due to severe neuronal loss.This finding further emphasizes that trehalose could be implemented as adjunctive therapy in addition to targeted approaches,including pharmacological and non-pharmacological approaches,to improve patients’ quality of life by delaying disease onset and progression,especially in the early stages.This postulation is supported by the additive effect on biochemical parameters when treated in combination with rapamycin,a treatment that slows down age-related diseases in humans (Pupyshev et al.,2019,2021,2022).
Concerning dosage,most of the reviewed studies reported therapeutic effects after administering a 2% concentration of trehalose on mouse models,regardless of the type of neurodegenerative disease.However,this dosage was insufficient for larger animals,such as macaques (Howson et al.,2019).Therefore,an even larger dosage of trehalose may be needed to demonstrate therapeutic effects for humans,which should be considered when planning a clinical trial.An open-label,single-arm pilot study treating 13 SCA3 patients with 20% oral trehalose daily intake improved and stabilized motor function over six months.This particular study,which also monitored biochemical parameters,such as fasting blood sugar,HbA1c levels,and renal and liver function,did not report any AEs other than acute diarrhea or bloating (Noorasyikin et al.,2020).Alternatively,another dose-escalation and dose-controlled randomized controlled trial showed that a 10% intravenous trehalose given weekly in 14 SCA3 patients stabilized ataxia over six months(Zaltzman et al.,2020).These findings indicated that the dosage given was relatively safe for humans and provided a promising avenue for future studies to examine the therapeutic effect of trehalose at similar dosages with a more robust study design.However,it is also important to note that at high concentrations,trehalose may inhibit the reactivation of denatured proteins by molecular chaperones (Olsson et al.,2016).Therefore,a balance of trehalose concentrations is needed to ensure the optimal dosage of trehalose for neuroprotection.
Limitations
The main limitation of this review was the heterogeneous outcome measures employed across the different studies.The lack of well-validated universal outcome measures is apparent.Specifically,the mechanisms of action underlying the neuroprotective effects of trehalose have not been consistently investigated against various types of neurodegenerative diseases.Therefore,it is impossible to identify or make claims (e.g.,autophagic flux is responsible for neuroprotection in neurodegenerative disease “A” but not a neurodegenerative disease “B” or that autophagic flux is accountable for its neuroprotection results in more significant physiological and behavioral changes than other mechanisms).Likewise,disease induction and trehalose administration were heterogeneous and may render irrelevant comparisons of findings across studies.
Conclusions
This review paper discussed the therapeutic effects and possible mechanisms of trehalose as a disease-modifying treatment in animal models from four different clusters of neurodegenerative diseases.Trehalose may exert neuroprotective effects via multiple pathways,thereby possessing a range of molecular targets.Although we are unable to reach a definitive conclusion on the underlying neuroprotective mechanisms of trehalose given the heterogeneous outcome measures used across the reviewed studies,the studies confirmed that trehalose is safe,at least for animals.The transition from animal models to human clinical trials would need to clarify three important questions: 1) the beneficial effect of oral administration,2)the window of opportunity to administer trehalose at the early stage of neurodegenerative disease,and 3) the sufficient dosage required to confer neuroprotection.
Author contributions:Conceptualization: NMI;formal analysis: KHY;funding acquisition: NMI and SA;investigation: KHY and SA;methodology: KHY and JCH;project administration: KHY and NMI;supervision: NMI,SA,SM,HAD,and JCH;visualization: KHY;writing– original draft: KHY;writing– review &editing: SA,SM,HAD,MM and NMI.All authors approved the final version of this manuscript.
Conflicts of interest:The authors declare no conflict of interest.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Table 1:Risk of bias assessment of the animal intervention studies based on SYRCLE’s risk of bias tool.
Additional Table 2:Characteristics of studies on trehalose intervention for animal models of tau pathology.
Additional Table 3:Characteristics of studies on trehalose intervention for animal models of synucleinopathy.
Additional Table 4:Characteristics of studies on trehalose intervention for animal models of polyglutamine tract diseases.
Additional Table 5:Characteristics of studies on trehalose intervention for animal models of amyotrophic lateral sclerosis.
- 中国神经再生研究(英文版)的其它文章
- Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update
- Do tau-synaptic long-term depression interactions in the hippocampus play a pivotal role in the progression of Alzheimer’s disease?