Neurotrophic factor-based pharmacological approaches in neurological disorders
Margherita Alfonsetti,Michele d’Angelo,Vanessa Castelli
Abstract Aging is a physiological event dependent on multiple pathways that are linked to lifespan and processes leading to cognitive decline.This process represents the major risk factor for aging-related diseases such as Alzheimer’s disease,Parkinson’s disease,and ischemic stroke.The incidence of all these pathologies increases exponentially with age.Research on aging biology has currently focused on elucidating molecular mechanisms leading to the development of those pathologies.Cognitive deficit and neurodegeneration,common features of aging-related pathologies,are related to the alteration of the activity and levels of neurotrophic factors,such as brain-derived neurotrophic factor,nerve growth factor,and glial cell-derived neurotrophic factor.For this reason,treatments that modulate neurotrophin levels have acquired a great deal of interest in preventing neurodegeneration and promoting neural regeneration in several neurological diseases.Those treatments include both the direct administration of neurotrophic factors and the induced expression with viral vectors,neurotrophins’ binding with biomaterials or other molecules to increase their bioavailability but also cell-based therapies.Considering neurotrophins’ crucial role in aging pathologies,here we discuss the involvement of several neurotrophic factors in the most common brain aging-related diseases and the most recent therapeutic approaches that provide direct and sustained neurotrophic support.
Key Words: Alzheimer’s disease;brain;brain-derived neurotrophic factor;glial cell-derived neurotrophic factor;nerve growth factor;neurotrophins;neurturin;Parkinson’s disease;stroke;tropomyosin receptor kinase receptors
From the Contents
Introduction 1220
Data Sources 1222
Neurotrophic Factors and Alzheimer’s Disease 1222
Neurotrophic Factors and Parkinson’s Disease 1223
Neurotrophic Factors and Stroke 1224
Conclusions 1225
Introduction
Neurotrophic factors and their receptors
Neurotrophins (NTs) exert a pivotal role in the central and peripheral nervous systems in controlling homeostasis and neuronal survival and also synaptic plasticity processes (Duan et al.,2022).
NTs are first synthesized as proforms (pro-neurotrophins) that are processed by proteolytic cleavage to the mature secreted forms.For example,pro-brain derived neurotrophic factor (proBDNF) and pro-nerve growth factor (proNGF)can be cleaved either intracellularly by the action of furin or proconvertase,or extracellularly by the action of plasmin and matrix metalloproteinases.Notably,pro-neurotrophins elicit activities opposite to those of their mature counterparts.
Nerve growth factor (NGF) is the first discovered neurotrophin (Levi-Montalcini,1964;Lorenzini et al.,2021).Moreover,the family includes brain derived neurotrophic factor (BDNF),neurotrophin-3 (NT3),and neurotrophin 4/5 (NT4/5) (Skaper,2018).Neurotrophins were described for the first time for their activity in neuronal development,but it has been shown that these molecules can exert multiple functions such as participating in neuronal differentiation,growth of axons and dendrites,and synaptic plasticity(Kowiański et al.,2018).
Neurotrophins are a part of the neurotrophic factors that include also neurokines and glial cell-derived neurotrophic factor (GDNF) family ligands.GDNF is expressed by neurons and,interestingly,it has been shown that it can also derive from a single neuronal subpopulation.Belonging to the same GDNF family,neurturin (NRTN),artemin (ARTN),and persephin (PSPN) are other crucial factors that regulate neuronal survival and function (Saarma and Sariola,1999;Duarte Azevedo et al.,2020).NRTN together with ARTN and PSPN acts to support multiple neuronal populations in the central nervous system (CNS) such as midbrain dopamine neurons and motoneurons(Sariola and Saarma,2003).Additionally,GDNF,NRTN,and ARTN support the survival and regulate the differentiation of several peripheral neurons,such as sympathetic,parasympathetic,sensory,and enteric neurons (Airaksinen and Saarma,2002).Furthermore,NRTN,ARTN,and PSPN have domains that are very similar in sequence to those of the transforming growth factor beta family,dimerize through the equivalent cysteine,and have similar structures(Wang et al.,2006;Morel et al.,2020).
NTs bind with different receptors: a related member of the tropomyosin receptor kinase (Trk) receptor tyrosine kinase family and the mutual p75 neurotrophin receptor (p75NTR) part of the tumor necrosis factor receptors(Sajanti et al.,2020),which regulates cell survival/death but it can also regulate neurite outgrowth.Moreover,p75NTRcan also contribute to neuronal differentiation and cell cycle exit (Underwood and Coulson,2008).
Trk receptors represent a family of receptor tyrosine kinases that present an extracellular domain for NT binding,a unique transmembrane domain,and an intracellular domain with tyrosine kinase action.In mammals,there are three different Trks: TrkA,TrkB,and TrkC.NTs bind with the same affinity to p75NTR,nevertheless,the NTs are more specific for their interaction with the Trk receptors;NGF usually binds TrkA,BDNF and NT4/5 are favored ligand for TrkB and NT3 for TrkC.This specification is not total and NT3 can also bind TrkA and TrkB.Moreover,the activity of slice variants receptors containing deletions in the extracellular domain and truncations in the intracellular region (kinase domain included) has been described.TrkB splice variants,described as T1 and T2,are mostly present in the mature brain (Cao et al.,2020).
NTs,in their mature form,generate homodimers that can bind together two receptor molecules.Moreover,Trk receptors can form homodimers that can concurrently bind two ligands or can associate with p75NTR(Figure 1).For this reason,a neurotrophic dimer can concurrently bind a Trk dimer or a Trk:p75NTRcomplex (Conroy and Coulson,2022).The presence of Trk:p75NTRcomplex allows lower concentrations of NTs to start signaling via the Trk pathway than in Trk receptors alone (Conroy and Coulson,2022).Sortilin,a co-receptor able to bind the Trk receptors,is involved in TrkA anterograde transport along axons enhancing neurotrophins signals.Moreover,in multiple human cell lines,low sortilin levels might affect neurotrophin trafficking and release.Sortilin interacts also with p75NTRforming a heteromeric complex at cell surface levels then triggering the pro-neurotrophin cell death signals(Bothwell,2019).
However,p75NTRsignaling inhibits axon growth and dendritic complexity via the GTPase RhoA (Schmidt et al.,2022).Furthermore,whereas Trk receptors bind only mature NTs,p75NTRcan also bind pro-neurotrophins generating more elaborate signaling.NGF pro form can induce cell death through a p75NTRand sortilin complex (Figure 1).Moreover,pro-BDNF produces axon pruning of cultured neurons from the hippocampus (Meeker and Williams,2015;Camuso et al.,2022).Trk signaling comprises survival and differentiation pathways while p75NTRactivity depends on the cells state and the formation of complexes in association with several co-receptors and ligands,such as sortilin,as mentioned above,for apoptosis and Nogo/Lingo-1/NgR for axonal pruning (Vilar and Mira,2016).
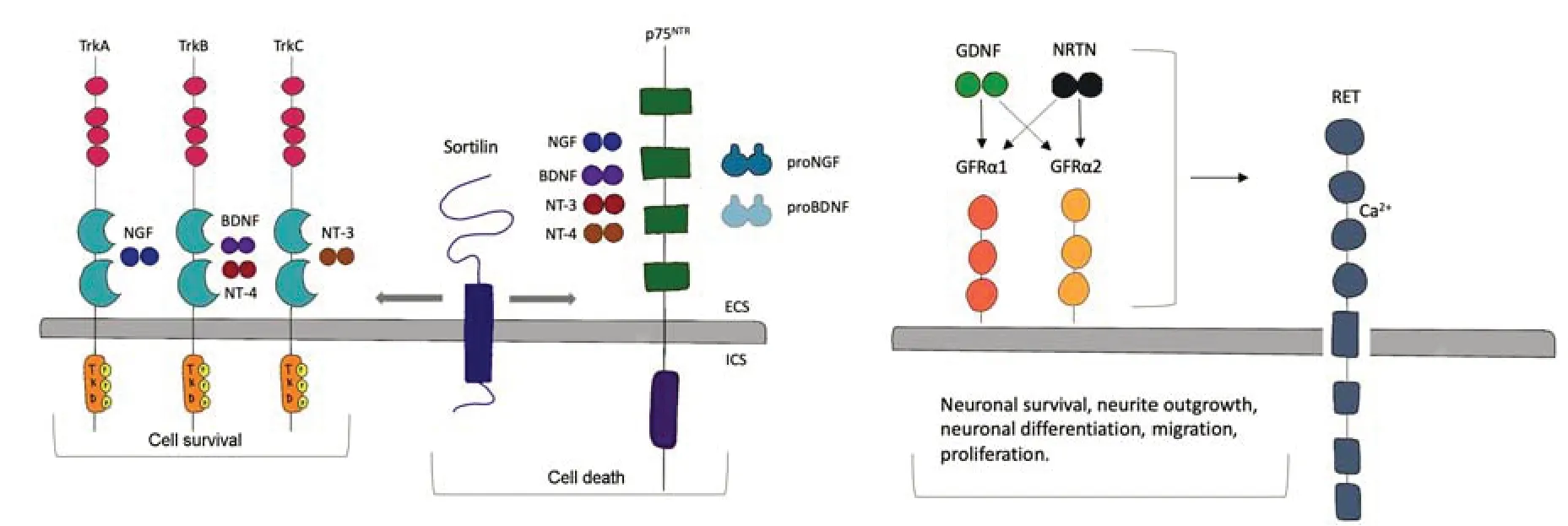
Figure 1|Neurotrophic factors and receptors: neurotrophic factors interaction with receptors,the implication of co-receptors,and their main activities in the brain.
For what concerns p75NTR,after shedding and receptor intramembrane proteolysis,the intracellular domain is released,and it can perform intracellular activities relevant to migration,proliferation,and transcriptional modulation (Wislet et al.,2018).
p75NTR-dependent death signaling is mediated via one or more of the receptor’s intracellular co-receptors,leading to c-jun kinase activation,and then p53,Bax-like proteins,and caspases activation.Activation of these pathways has been shown in multiple experimental conditions to be liganddependent.p75NTRregulation of neurite outgrowth is Rho A-dependent,in some cases following activation by myelin proteins together with the Nogo66 receptor.Moreover,it can also facilitate neuronal differentiation and cell cycle exit (Becker et al.,2018).
Regarding GDNF family ligands,all of them bind to the receptor tyrosine kinase RET that acts as their common signaling receptor.The factors bindingspecificity is due to GDNF family receptor (GFR) α proteins.Specifically,GDNF family ligands form a high-affinity complex with each GFRα protein.GDNF,NRTN,ARTN,and PSPN bind to GFRα1,GFRα2,GFRα3,and GFRα4,respectively.The complex formation leads to the transphosphorylation of two RET molecules at the specific tyrosine residues in their tyrosine kinase domains triggering intracellular signaling (Donnelly and Pierchala,2020).At this point,RET can activate multiple pathways such as the mitogenactivated protein kinase pathway,which regulates neurite outgrowth and the phosphoinositide 3-kinase (PI3K) pathway that modulate cell survival.The phospholipase Cγ signaling affects the intracellular level of calcium ions by increasing the level of inositol (1,4,5)-trisphosphate.GDNF signaling activates Src-family kinases,which also lead to neurite outgrowth and neuronal survival(Cintrón-Colón et al.,2020;Figure 1).
The therapeutic potential of neurotrophic factors has been shown for years,with clinical trials in neurodegenerative pathologies extending back at least 25 years.
Neurotrophic factors were able to enhance the activity of the dopamine system improving innervation and increasing cell survival in Parkinson’s disease (PD) individuals (Chmielarz and Saarma,2020).
For example,the long-term effects of adeno-associated virus (AAV)-NRTN injections in a post-mortem study after 8–10 years from the virus injection in the putamen and substantia nigra were recently described.Notably,in the areas of NRTN expression,dopaminergic innervation was ameliorated and dopamine cell markers were increased,thus showing the long-term benefits of this neurotrophic factor-based treatment (Chu et al.,2020).
The firstNGF-gene therapy trial for AD was performed on mild diagnosis patients (Tuszynski et al.,2005).In this study,theNGFgene was administered through genetically manipulated autologous fibroblasts and then introduced into the basal forebrain.Results showed a significant increase in glucose uptake in multiple brain regions and improved cognitive tests (Tuszynski et al.,2015).Moreover,also for AD,AAV-based gene delivery was used to treat symptoms and progression of this pathology (Rafii et al.,2014).Recently,a phase II clinical trial using AAV vectors expressing human NGF showed that this treatment was well-tolerated over 2 years but with no effects on clinical outcomes (Rafii et al.,2018).
Another technique used to deliver neurotrophic factors directly to the brain regions of interest is the encapsulated cell biodelivery system.In encapsulated cell biodelivery-NGF clinical studies,AD patients were treated at the sametime with cholinesterase inhibitors for the long term (Hampel et al.,2018).
Currently,there is only one clinical study on Stroke for the evaluation of the effects of the central administration of neurotrophic factors (NCT03686163).In this trial,106 patients were daily treated with intranasal NGF for 2 weeks beginning at least 72 hours post-stroke.Moreover,in different clinical trials the effects of agents that have the potential to influence BDNF levels such as memantine in stroke recovery were evaluated (NCT02144584).In addition to pharmacological treatments,different interventions comprising exercise or motor therapy were able to increase neurotrophin levels (Małczyńska-Sims et al.,2020).In the next paragraphs,we will describe the role of neurotrophic factors in a healthy brain and in age-related disorders such as PD,AD,and stroke and the therapeutic potential of neurotrophin-based approaches in these diseases.
The role of neurotrophic factors in the adult brain
Recent evidence showed that NTs and their receptors represent crucial regulators in adult neurogenesis (Leal-Galicia et al.,2021).Notably,due to the presence of a niche of neural stem cells (NSCs) in the subependymal or subventricular zone (SVZ) near the lateral ventricles,and the subgranular zone(SGZ) of the dentate gyrus in the hippocampus,that maintain the ability to proliferate and differentiate in neurons through intermediate progenitor cells,neurons production continues throughout life (Leal-Galicia et al.,2021).
It is now clear the involvement of BDNF/TrkB in adult hippocampal neurogenesis.It has been shown that neurogenesis is attenuated by BDNF knockdownin vivoin the dentate gyrus using lentiviral-mediated RNAi but increased after BDNF administration (Taliaz et al.,2010).TrkB receptor is crucial for normal proliferation and neurogenesis in the SGZ even though conflicting evidence has been reported (Shohayeb et al.,2018).
Conditional deletion of TrkB in neural stem cells in the hippocampus decreased SGZ proliferation in post-natal day 15 animals and in adults,without any effect on cell survival (Li et al.,2008).An alteration in TrkB activationin vivo(TrkB-T1-overexpressing mice) showed an increased proliferation and a decrease in cell survival (Sairanen,2005).Moreover,BDNF promotes the proliferation of hippocampal neural progenitor cell culture via TrkB (Li et al.,2008).
The p75NTRis also involved in the regulation of adult neurogenesis (Meier et al.,2019).In the knock-out animals for p75NTRthat only express the short p75NTRisoform,a reduction in the number of neuroblasts and newborn neurons was observed together with an increase in neurodegeneration and altered hippocampal-mediated behavior (Catts et al.,2008).Nevertheless,in knockout mice that lack both the long and short isoforms of p75NTR,an increase in the number and complexity of cholinergic newborn neurons and a decrease in cell death were reported (Poser et al.,2015).
The post-natal neurogenesis in the mammalian brain represents a unique form of plasticity that can have the potential to create new connections and modify existing neuronal circuits.Consequently,adult neurogenesis is implicated in hippocampal modulation in terms of both structure and function.High neurogenesis events positively impact processes such as learning,memory,and cognitive flexibility (Cushman et al.,2021).
Moreover,NSCs can also generate glial cells (Doetsch,2003) and,in turn,they are modulated by signals from different cell types for example astrocytes and endothelial cells in both the SVZ and SGZ (David-Bercholz et al.,2021).Moreover,due to SVZ-NSCs contact with the ventricular lumen,their activity is controlled by molecules inside the cerebral spinal fluid,and by signals from ependymal cells (Alonso and Gato,2018;Kaneko and Sawamoto,2018).
Overall,neurotrophins have a crucial role in the adult CNS,generating trophic signals that preserve target innervation,sustain cell survival,plasticity mechanisms,and axonal pruning,modulate neurotransmitter levels and neuronal excitability,and facilitate regeneration and sprouting as a consequence of neuronal damage.
It has been widely shown bothin vivoandin vitrothat a neurotrophin reduction can determine cell alterations,and the treatment with neurotrophins can modulate these events (Miranda et al.,2019).For example,humans with a BDNF-gene polymorphism showed a decreased volume in the hippocampus due to a decreased wiring of the neurons (Miranda et al.,2019).Low levels of functionalNGF,BDNF,andNT3genes resulted in severe neuronal deficits and in early post-natal death.BDNF is one of the crucial factors leading to the increase of dendrites length and spine densities in the CA1 area in the hippocampus (De Vincenti et al.,2019).Long-term depression can decrease spine densities and this event is modulated by stress,environment,age,and neurotrophic support (Pinar et al.,2017;Kaul et al.,2020).It has been shown that treatment with BDNF can decrease longterm depression mechanisms in the hippocampus (von Bohlen und Halbach and von Bohlen und Halbach,2018).
BDNF is a crucial player involved in the modulation of long-lasting effects induced by neuronal plasticity.This would further imply that BDNF action can be implicated in antidepressant action (Castrén and Monteggia,2021).
The specific action of neurotrophins in different subsets of neurons,always suggested a connection with selective neuronal alterations in specific diseases,explaining the possibility that a particular neurotrophin can be used therapeutically to target a specific neuronal subset under pathological conditions (Bahlakeh et al.,2021;Nordvall et al,2022).
The aim of this review is to present the most recent advances in neurotrophinbased pharmacological approaches in the most common aging-related CNS diseases.
Neurotrophic factors administration and half-life
When neurotrophic factors are used in pre-clinical as well as in clinical studies,the biodistribution features of these molecules must be taken into account.
For example,to stimulate BDNF-TrkB signaling as a treatment to translate in clinics,the main problem is to overpass the blood-brain barrier (BBB) but also the modest bioavailability of BDNF due to its chemical characteristics(Houlton et al.,2019).It has a plasma half-life of a few minutes in rats and a few hours in sheep.BDNF metabolisms occur primarily in the liver due to its basic isoelectric pH.For these reasons,chemical modifications of the BDNF molecule can be considered to obtain mutants with higher stability,neutral isoelectric pH,and enhanced brain penetration (Wang et al.,2019).Interestingly,the stimulation mediated by physical exercise can increase its bioavailability (Małczyńska-Sims et al.,2020;Palasz et al.,2020).
Also,NGF does not normally pass through the BBB (Liu et al.,2014).Moreover,it must be considered that its direct injection can have serious peripheral side effects in patients (Barker et al.,2020).
Invasive administration procedures are more effective such as using a catheter,pumps,or through treatment with biodegradable polyethylene glycol hydrogel inclosing poly(lactic-co-glycolic acid) particles BDNF-enriched(López-Cano et al.,2021).However,these technologies are not applicable to chronic neurological disease patients.Non-invasive approaches comprise nanoparticle,Trojan horse technology,and nose-mediated delivery.Trojan horse technology consists of binding BDNF to molecules that can easily cross the BBB (Boado et al.,2007).These molecules can be for example ligands that bind endothelial cell receptors or antibodies targeting those receptors (Kim et al.,2021).Notably,it has been proven that BDNF can be conjugated to a monoclonal antibody against insulin receptors resulting in ten-fold growth in BDNF brain levels and a hundred-fold increase in the mean residence time of BDNF in the blood without modifying the glycemia.Comparable effects were obtained by binding BDNF to an antibody anti-transferrin receptor,lipoprotein receptor-related protein 1,or diphtheria toxin receptor (Jones and Shusta,2007).A different delivery method for neurotrophins is the intranasal delivery(IND) method.The intranasal route is another way to carry macromolecules into the brain parenchyma (Erdő et al.,2018).
IND is a non-invasive method that allows a rapid absorption delivering the drug directly into the brain overpassing the obstacles of systemic delivery of both oral and parenteral drug routes (Tashima,2020;Crowe and Hsu,2022).Moreover,it allows not only a rapid transport to the brain but also slight general exposure to the drug and the opportunity for recurring treatments.IND of BDNF determined both a growth in brain tissue levels in~30 minutes and triggered the activity of TrkB and its associated PI3K-AKT signaling (Chen et al.,2016).
Data Sources
Extensive bibliographic research was conducted using the PubMed National Library of Medicine (NIH),Web of Science platform,Google Scholar,and Clinical Key databases.Examples of the search terms used were ‘‘Parkinson’s disease’’,‘‘Alzheimer’s disease’’,‘‘Stroke’’,‘‘neurotrophic factors’’,“BDNF”,“NGF”,“GDNF”,“therapeutic approach”,‘‘in vitro’’,‘‘in vivo,’’,‘‘clinics’’,“experimental studies”.For screening,a restriction was made to those papers published in the last 10 years and preferably in English.Priority was given to prospective studies and reviews with adequate methodological quality.In addition,a secondary search of the bibliography of the papers finally selected was carried out to detect possible omissions.For the analysis of all relevant publications,consensus meetings were held with all the authors.
Neurotrophic Factors and Alzheimer’s Disease
AD is characterized by structural changes in the CNS,in particular a loss of synapses that contact the hippocampus and cortex was reported (Kocahan and Doğan,2017;Kashyap et al.,2019).AD is diagnosed by the formation of amyloid-β protein aggregates (formed by β-cleavage of amyloid precursor protein) generating extracellular and over-phosphorylated aggregates of tau protein (Chen et al.,2017).Neuronal alterations and degeneration occur early in the pathogenesis and mostly in the cholinergic neurons of the basal forebrain (ChBF),together with a reduced choline acetyltransferase activity(Henjum et al.,2022).Choline acetyltransferase activity impairment leads to the development of dementia and it is the main cause of AD memory loss.In 1981,it has been proposed a hypothesis for a common cause of amyotrophic lateral sclerosis,Parkinsonism,and AD (Appel,1981).The hypothesis is that these pathologies were caused by the lack of a specific neurotrophic hormone.The two candidates for this neuroprotective role were NGF and BDNF.Regarding AD,the lack of these specific factors involved in cholinergic survival seems to be involved,which led to an insufficient release by target areas such as the hippocampus,resulting in neuronal loss.Following Appel hypothesis (Appel,1981),it has been shown that in the region innervated by ChBF of rat brains,such as the hippocampus,and in the regions containing the soma of these neurons,high levels of NGF are expressed,thus suggesting that NGF is a crucial trophic factor for cholinergic neurons (Korsching et al.,1985).Moreover,upon the inoculation of radiolabelled NGF into rats’ hippocampus or cerebral cortex,NGF is retrogradely transported from the terminals of ChBF to their somas (Seiler and Schwab,1984).NGF binds both with p75NTRand TrkA on the surface of the cholinergic neurons of mice,rats,and humans(Fahnestock et al.,2001).Notably,over 80% of cholinergic neurons are p75NTRpositive.A reduction in the number of TrkA-expressing neurons in the basal forebrain in AD patients (around 56%) concerning normal brain (Mufson et al.,2000) has been associated with cognitive impairment (Ginsberg et al.,2006a,b).Moreover,through single-cell mRNA analysis in ChBF neurons,a decrease in TrkA,TrkB,and TrkC levels during the development of AD was reported.In contrast,p75NTRmRNA levels stayed stable even in the last stage of the pathology.Overall,the cholinergic system is damaged by multiple mechanisms including impaired NGF maturation,altered TrkA/p75 receptor fraction,ineffective axonal transport and signaling,amyloid-β-dependent modulation of NGF receptors,suboptimal Acetylcholine innervation-mediated inflammatory reaction,and amyloid-β-induced cytotoxicity.During the initial state and development of AD,the decreased activity of cholinergic synapse formation in the cortex and hippocampus is dependent on an ineffective maturation of NGF.Moreover,these effects are accompanied by a lack of plasmin machinery and increased matrix metallopeptidase 9 activity,leading to elevated brain levels of proNGF (Fahnestock et al.,2001;Fabbro and Seeds,2009;Mroczko et al.,2013).Increased proNGF levels determine the instauration of a pro-apoptotic signaling and also affect both the mNGF binding with its receptor and its axonal transport.Thus resulting in retrograde degeneration of ChBF (Ioannou and Fahnestock,2017;Allard et al.,2018).Pro-NGF-induced apoptosis is modulated by the relative expression of TrkA and p75 and is fostered by a reduced ratio of TrkA/p75 that is another feature of AD (Masoudi et al.,2009).These changes in AD rodent models were observed (Tiveron et al.,2013).Notably,in AD11 transgenic mice (expressing a recombinant version of the monoclonal antibody mAb αD11 that specifically recognizes and neutralizes NGF only in the brain) the reduction of mNGF determined primary inflammation and AD-associated neuronal death (Capsoni et al.,2011).Interestingly,NGF was observed to directly modulate microglial cells dampening inflammation (Rizzi et al.,2018).
Neurotrophin-based approaches for Alzheimer’s disease
Currently,the only available treatments for AD are cholinesterase inhibitors such as donepezil,rivastigmine,and galantamine and glutamate receptor antagonists such as memantine.Unfortunately,these drugs are effective only on AD symptoms and can only momentarily stabilize cognitive functions,with no effect on disease evolution or gravity (Deardorff et al.,2015;Marucci et al.,2021).
Among neurotrophins,BDNF has been considered a treatment for AD because of its role in regulating synapse formation.Emergingin vivoandin vitroevidence supported the pro-survival activity of BDNF underlies numerous pathologies.In AD,it has also been observed a specific impairment of GFRα1,which can play a role in glutamatergic neurotransmission,thus suggesting the development of therapeutics for AD that can modulate GFRα1 expression.In normal control human neurons in culture,GDNF was able to enhance the expression of GFRα1,but not the other three subtypes (GFRα2,GFRα3,and GFRα4),whereas GDNF failed to induce GFRα1 expression in AD patientderived cultured cortical neurons (Konishi et al.,2014).In contrast,in anin vivostudy (Gdnf hypermorphic mice,Gdnfwt/hyper),a two-fold increase in endogenous GDNF counteracted the overall age-associated decline in the cholinergic index observed in the brain of control animals.Moreover,the biochemical analysis showed increased levels of NFG in several brain areas of old Gdnfwt/hyper mice.These results suggesting the involvement of GDNF in the modulation of cholinergic pathways upon aging were also confirmed by gene expression analysis (Mitra et al.,2021).Furthermore,it has been shown that some compounds that are commonly used to treat AD can modulate both BDNF expression and the activation of BDNF signaling (Miranda et al.,2019).For example,memantine can increase BDNF levels in the limbic cortex inin vivomodels.Nevertheless,the rise in BDNF levels was only detected at a high dose (50 mg/kg).However,this dose is expected to be clinically toxic,and the effect was minimal at a lower dose (Marvanová et al.,2001).Notably,it has been reported that acetylcholinesterase inhibitors such as donepezil and galantamine,commonly used to treat cognitive impairment in early AD,can increase serum BDNF levels,while it remains uncertain if they can increase BDNF brain levels (Ng et al.,2019).Overall,these molecules can activate the AKT pathway,however without affecting the mitogen-activated protein kinase pathway that is essential in promoting synaptic growth.Consequently,numerous factors must be taken into consideration when administrating molecules that can trigger endogenous BDNF production.In particular,the molecule must work at a non-toxic dose and generate a sufficient concentration of extracellular BDNF to activate TrkB signaling (Santos TB dos et al.,2021).The activation of TrkB signaling is important in the induction of synaptic growth.Ultimately,the molecule should have synaptogenic effects:specifically,facilitating long-term potentiation,stimulating dendritic spine pruning,and improving synaptic protein levels.Every existing pharmacological agent with these characteristics could be taken into account for AD treatment(Zagrebelsky et al.,2020).
Among the neurotrophins,also NGF has been included in clinical trials for multiple diseases such as peripheral neuropathies,human immunodeficiency virus infections,and AD (Aloe et al.,2012).
For example,in a preclinical study on Sprague-Dawley rat model,intracerebroventricular injection of NGF into the adult brain was shown to determine axonal sprouting of mature,intact axons in association with the intradural segment of the internal carotid artery (Isaacson et al.,1990).Notably,in treated-animals,after NGF treatment,a three-fold increase in the overall number of axons accompanying the vessel wall was observed when in comparison to control animals (Isaacson et al.,1990).
To administer NGF into rodents’ brains,IND method was used (Cattaneo et al.,2008).Furthermore,the efficiency of this administration route has been shown in the AD11 anti-NGF transgenic mice model,determining a decreased cognitive loss in AD11 mice (De Rosa et al.,2005).Another way of administration to target cholinergic markers in the basal forebrain in rats is the ocular surface route (Lambiase et al.,2007).Moreover,ocular administration of NGF in adult rats was able to induce cell proliferation in the sub-ventricular zone shown by an increased number of Ki67 expressing cells(indicating cells that can proliferate) which co-express also p75NTR(Tirassa,2011).
Another tool to target tissues and organs of interest is using stem cells as carriers of therapies.Nowadays,cell therapy represents the essential core of regenerative medicine.These cells can deliver neurotrophins in damaged areas of the brain,modulating events such as synaptic plasticity and increasing neuronal survival.For example,NSCs can express high levels of BDNF and NGF (Marsh and Blurton-Jones,2017).Notably,in transgenic animal models of AD,increased synaptic density in the hippocampus,memory impairment,and neurodegeneration after NSCs transplantation were reported (Blurton-Jones et al.,2009;Kim et al.,2010).Furthermore,viral vector-mediated gene transfer procedures (i.e.,those mediated by lentiviruses) showed some useful features in association with cell therapy applications.Recently,it has been shown that infection by a lentiviral vector overexpressing NGF led to effective production of NGF in rat monocytes (Hohsfield et al.,2013).
Nagahara et al.(2009) successfully delivered the lentivirus NGF gene into the cholinergic basal forebrain for a 1-year period in adult monkeys without any systemic leakage of NGF or development of anti-NGF antibodies,nor the presence of brain inflammatory molecules,the development of pain or weight loss.
The first clinical study was performed in individuals with mild AD,treated with genetically manipulated autologous fibroblasts expressing the NGF gene.Fibroblasts were implanted into the patient’s basal forebrain (Tuszynski et al.,2005).This study demonstrated a huge increase in glucose uptake in multiple cortical regions and cognitive tests produced data that were alleviated or weakened at a slower percentage compared with normal pathological conditions (Tuszynski et al.,2005).Moreover,also AAV-based NGF gene intracerebral supply was applied clinically to AD patients (Rafii et al.,2014).In this case,AAV-NGF delivery was well-accepted for over 2 years but without clinical consequences (Rafii et al.,2018).The main limitation of gene therapy is the permanent genetic modification of patients’ neurons.To overcome this problem,self-inactivating viral vectors can be used to express transgenes with minimum invasiveness and can also be switched off (Schambach et al.,2007;Thornhill et al.,2008).
On the other hand,among available technological tools,the use of a simian immunodeficiency virus for long-term delivery of proteins for therapeutic purposes would be challenging (Munis,2020).
Neurotrophic Factors and Parkinson’s Disease
PD is an age-associated neurodegenerative pathology,as a consequence of the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc).Dopaminergic neuronal loss and the consequent reduction of striatal dopamine levels lead to the major symptoms of PD that are tremor,rigidity,and slowness of movements.Another PD feature is the presence of eosinophilic cytoplasmic intracellular inclusions called Lewy bodies,whose main constituent is aberrantly folded α-synuclein protein clusters (Pramanik et al.,2017;Hayes,2019).
Emerging evidence showed that neurotrophins can play a role in PD pathogenesis.It has been suggested that Lewy bodies formation can modulate GDNF and BDNF levels resulting in the lack of BDNF expression and altering neuronal BDNF transport (Pramanik et al.,2017;Miller et al.,2021).Furthermore,since 1999,several post-mortem studies indicated decreased BDNF levels in SNc and striatal cell bodies (caudate and putamen) of PDaffected individuals (Nagatsu and Sawada,2007).Notably,in situ hybridization studies showed a strong reduction of BDNF expression in dopaminergic neurons in PD patients’ substantia nigra in contrast with healthy subjects that express high levels of this neurotrophin (Howells et al.,2000).The interplay between low BDNF levels and PD development was confirmed also by a(123) I-PE2I single-photon emission computer tomography study explaining a positive association between circulating BDNF levels and striatal dopaminergic transporters accessibility in PD individuals (Ziebell et al.,2012).
GDNF was also connected to dopaminergic depletion during PD pathogenesis.Indeed,in PD patients,a reduction of GDNF in SNc compared to other neurotrophins was revealed,thus suggesting GDNF is the most vulnerable factor and the earliest affected in survived substantia nigra neurons (Mesa-Infante et al.,2022).
In vitrostudies suggested that GDNF exerts its effect on dopaminergic neurons through the activation of mitogen-activated protein kinase and PI3K intracellular pathways (Onyango et al.,2005).GDNF signaling also involves Proto-oncogene tyrosine-protein kinase c-Src to promote neurite outgrowth.Although the GFRα1/Ret complex is the most studied GDNF receptor,it is known that this trophic factor can also bind to the alternative signaling system such as neural cell adhesion molecules (Paratcha et al.,2003).
Mice that present a partial deletion of Gfrα1 (heterozygous) showed decreased tyrosine hydroxylase (TH) fiber density in the striatum (STR)together with a lower number of TH+neurons in the substantia nigra.Furthermore,these mice showed increased sensitivity of nigrostriatal dopaminergic neurons to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity (Boger et al.,2008).Overall,these observations suggested that GDNF exerts a crucial role in trophic protection and in counteracting toxic damage(d’Anglemont de Tassigny et al.,2015).
Interestingly,numerous PDin vivomodels showed lower BDNF levels explaining a downregulation of neurotrophin expression with results that were not constant in whole agreement,maybe due to different experimental conditions.Notably,BDNF gene silencing led to the loss of dopaminergic neurons and motor signs,confirming the association of BDNF with motor and cognitive impairment in PD,safeguarding neurodegeneration (Baker et al.,2005;Baquet,2005).Nevertheless,divisive results were obtained from multiplein vivoPD models.
Neurotrophin-based approaches for Parkinson’s disease
To date,there are only treatments that can act in alleviating PD-associated symptoms such as levodopa preparations,dopamine agonists,and monoamine oxidase-B inhibitors but unfortunately,there is no cure to counteract the onset and progression of this pathology (Carrarini et al.,2019).Advanced treatments comprise deep brain stimulation,and MRI-guided focused ultrasound for patients that show medication-resistant tremors and dyskinesias (Ito et al.,2018).
Therapeutic effects of neurotrophins in PD were widely investigated in neurotoxin-inducedin vivo models of PD [6-hydroxydopamine (6-OHDA) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine] both in mice and rats and in non-human primates (Tenenbaum and Humbert-Claude,2017).
Numerousin vitrostudies on rat mesencephalic cultures showed the potential of BDNF in rescuing dopaminergic neurons,promoting their rescue in the ventral tegmental area and medial SNc and supporting their differentiation into dopaminergic neurons (Murer et al.,1999;Baquet,2005).BDNF is crucial not only for its neuroprotective properties but also can counteract apoptosis,oxidation mechanisms,and autophagy,thus leading to mitochondrial alteration (Zuccato and Cattaneo,2009;Miller et al.,2021).
For example,Hernandez-Chan and his group (Hernandez et al.,2000),used a delivery technology called neurotensin-polyplex that uses neurotensin receptors for the incorporation of nanovesicles exclusively in dopaminergic cells.BDNF treatment after 6-OHDA injectionin vivogenerated a substantial improvement in PD symptoms due to the protection of striatal dopaminergic neurons.Furthermore,the sprouting of dopaminergic fibers without the rescue of dopamine levels and the increased amount of TH-positive cells were reported (Hernandez et al.,2000).
Overall,these results suggest that BDNF in the substantia nigra was not able to stimulate neurogenesis but it could improve neurogenesis both in the substantia nigra and STR,in line with preceding works (Somoza et al.,2010).
As described for AD,numerous studies in PD reported that some treatments exert neuroprotective effects modulating BDNF expression in the brain (Palasz et al.,2020).These approaches include the use of probiotic formulations,such as SLAB51 that significantly increased BDNF levels in a 6-OHDA model of PD bothin vitroandin vivo(Castelli et al.,2020).
In another work,mesenchymal stem cells’ secretome was injected into the SNc and STR of 6-OHDA rats,characterizing the behavioral functioning and influencing histological parameters of inoculated animals versus control groups.Secretome injection increased the number of TH-positive cells and neuronal terminals in SNc and STR respectively,thus ameliorating motor performance (Teixeira et al.,2020).Moreover,from the proteomic analysis,it has been observed that these cells can release BDNF (Teixeira et al.,2020).
Cerri et al.(2015) supported the secretome effect,demonstrating that after the injection of mesenchymal stem cells,functional activity of the dopaminergic system was restored and the authors attributed those effects to an in situ release of BDNF by mesenchymal stem cells.
Apart from BDNF,the role of other neurotrophins was also widely explored,including GDNF and its family member NRTN.GDNF is present only in the dorsal and ventral STR,the anteroventral nucleus of the thalamus,septum,and subcommissural organ (Pascual et al.,2011).Notably,GDNF receptors are not expressed in the STR,but they are widely present in the substantia nigra cells (Trupp et al.,1997),thus proposing a specific activity on SNc neurons.
Moreover,physical exercise (Palasz et al.,2019a) was able to mobilize neurotrophic factors such as BDNF and GDNF leading to a neuroprotective and anti-inflammatory action in animal models of parkinsonism (Palasz et al.,2019b).
Notably,GDNF was more effective compared to BDNF in counteracting dopaminergic neurodegeneration of SNc in the brain of lesioned PDin vivomodels (Rosenblad et al.,2000;Sun et al.,2005).It has been reported that GDNF can also trigger axonal sprouting of damaged SNc neurons,but it is less effective in inducing reinnervation in the STR or dopaminergic neurons activity rescue in the 6-OHDA model (Rosenblad et al.,2000).
In vitrostudies indicated that GDNF was able to counteract cell death of embryonic and post-natal dopaminergic neurons.Furthermore,in vivo,repeated injection of GDNF into the intrastriatal nigral grafts increased the survival and fiber outgrowth of dopaminergic neurons.It has also been observed that repeated infusions of GDNF using an osmotic pump for 2 weeks or from implanted polymer-encapsulated genetically modified cells for 6 weeks resulted in an increased survival from 200 % to 1300% (Brundin et al.,2000;Ridet et al.,2000).
NRTN showed a neuroprotective effect both on the neurodegeneration of SNc dopaminergic neurons inin vivomodel of PD and on cultured ventral mesencephalic dopaminergic neurons (Fjord-Larsen et al.,2005).NRTN biodistribution is less efficient compared with GDNF due to its high affinity with heparin (Hadaczek et al.,2010;Runeberg-Roos et al.,2016).For this reason,modified NRTNs with a lower affinity to heparin were produced and resulted to have increased chemical stability and biodistribution as well as to be more effective in a 6-OHDA rat model (Runeberg-Roos et al.,2016).
As previously described for NGF,the injection of genetically modified human fibroblasts,protected by a semipermeable polymer capsule,generated the release of GDNF in the STR of a 6-OHDA-lesioned rodent model,leading to a significant progress of motor signs related to the reinnervation of TH-positive axons in STR (Sajadi et al.,2006).
An innovative strategy is to transplant hematopoietic stem cells taking advantage of the propensity of these cells to migrate to sites of neurodegeneration.A research group observed macrophage-mediated GDNF delivery rescued dopaminergic neurodegeneration and safeguarded against both motor and non-motor symptoms in a rodent PD model (Chen et al.,2020)
Furthermore,the use of viral vectors allows a specific expression only in selected cells mimicking a more natural tissue ligand distribution.Notably,for PD,GDNF expression through viral vectors allows a temporal and amount control of GDNF expression to inhibit compensatory activities on dopamine homeostasis such as impairments in TH production (Georgievska,2002).
Depression is another common aspect of PD patients and impacts numerous other clinical aspects of the pathology.Moreover,depressive disorders negatively impact the quality of life,motor and cognitive deficits,functional disability,and other psychiatric comorbidities.Recently,it has been demonstrated that sigma receptors,especially the sigma-1 receptor subtype can regulate ER stress sensors,the activation of transcription factors,and BDNF expression.The combination of these processes could explain the activity of sigma-1 receptors-targeting drugs in the regulation of neuronal survival in target areas of the brain and the development of antidepressant action (Voronin et al.,2020;Ren et al.,2022).Underlying this link,pharmacological stimulation of sigma receptors in particular sigma 1 showed neurorestorative and protective effects in experimental models of PD,possibly increasing levels of BDNF or other neurotrophic factors that are compromised in neurological disorders (Francardo et al.,2014;Nguyen et al.,2017).
Neurotrophic Factors and Stroke
Stroke,the most common serious consequence of the cerebrovascular disease,is the major cause of severe disability and of hospitalization for neurological disease.It represents a destructive disease that it is generated when a blood vessel that targets the brain either bursts or it is blocked by a clot (Herpich and Rincon,2020).Nowadays,the only Food and Drug Administration-approved treatment for stroke is the tissue plasminogen activator administered within 3 hours of an acute attack.For this reason,it represents a treatment for only a small group of patients (2–5%) (Barthels and Das,2020).
Thus,so far,current therapeutics mostly focused on rescuing neurons from the injury at the acute phase after the stroke,which is both a challenging method to improve stroke signs and represents a problem for the affected individuals to obtain actual treatments early enough.Unfortunately,these neuroprotective treatments were not successful in humans,probably due to the contribution of intricate mechanisms leading to neurodegeneration during a stroke (Barthels and Das,2020;Liu et al.,2020).
BDNF is crucial in the prognosis,pathogenesis,and rehabilitation of stroke.It is now well known that a small amount of systemic BDNF is connected with an elevated risk of stroke and reduced rescue (Eyileten et al.,2021).
Neuroplasticity is the main event in post-stroke rehabilitation and the crucial role of BDNF in this mechanism has been studied through works that focused on the post-stroke treatment of aphasia and motor activities impairment (Su and Xu,2020).Those are events that are mostly regulated by the process of neuroplasticity.Emerging evidence on rat ischemia models showed that when BDNF synthesis was ablated,the benefits on the recovery were predominantly blocked,while intravenous injection of BDNF increased the functional motor recovery in contrast with untreated individuals (Ploughman et al.,2009).
Other kinds of therapies both behavioral and physical,for example,physical exercise,transcranial direct current stimulation,and extremely low-frequency electromagnetic field therapy are all able to impact both BDNF systemic and brain amount (Moya Gómez et al.,2021;Zettin et al.,2021).Furthermore,BDNF-dependent learning memory can also have a role in the post-stroke restoration of motor function and language relearning (Ploughman et al.,2009;Szelenberger et al.,2020).
Cerebral ischemia can modulate the expression of factors involved in GDNF signaling.Both expression and protein levels of GDNF are upregulated inin vivomodels of transient focal and global ischemia.Furthermore,increased expression of GFRα1 was detected in injured brain spots after transient middle cerebral artery occlusion (MCAO),anin vivomodel of focal ischemia(Mokhtari et al.,2017;Zhang et al.,2021).
Neurotrophin-based approaches for stroke
In clinics,promising results have been shown by introducing multiple stroke treatments modulating BDNF levels,such as the administration of hormones and neurotransmitter-modulating molecules,stem cell injection,and modulation of different correlated genes (Miranda et al.,2019).
Due to the CNS’s limited capability to regenerate,usually stroke patients recover poorly.For this reason,research is focused on investigating neuronal mechanisms of regeneration and repair to restore and improve lost function after a stroke event.Most of the work has been focused on the research of exogenous-administered neurotrophins to protect injured brain tissue through its ability to modulate neuronal growth and survival (Houlton et al.,2019).
Among all neurotrophins,BDNF has been defined as a principal factor participating in rehabilitation-mediated recovery after stroke (Ploughman et al.,2009).Furthermore,high levels of BDNF induced by activity can accelerate motor recuperation after stroke (Fritsch et al.,2010;Clarkson et al.,2011).
As mentioned above,the recovering action of neurotrophin-based treatments was already proved inin vivomodels of neurodegenerative pathologies.Notably,it has been shown that the direct infusion of BDNF,NGF,and NT-3 can promote neurite outgrowth,neurogenesis,and functional rescue inin vivomodels of stroke (Schäbitz et al.,2004,2007).
Nevertheless,the effects of BDNF and other neurotrophins have been previously studied in several stroke models showing a solid regenerative activity (Berretta et al.,2014).BDNF effects can be explained through several mechanismsin vivosuch as the beneficial effects against acute ischemic injury(Schäbitz et al.,2004),improved angiogenesis (Kermani and Hempstead,2007),neurogenesis (Schäbitz et al.,2007),and neural repair (Mamounas et al.,2000),together with boosted synaptic plasticity (Waterhouse and Xu,2009;Clarkson et al.,2011).In stroke patients,positive results after the administration of several treatments that influence BDNF levels were observed,such as hormones and neurotransmitter-targeting molecules,stem cell transplants,and the regulation of BDNF-related genes (Liu et al.,2020).
Nowadays,the use of biomaterials represents an effective delivery tool that modifies the pharmacological properties of neurotrophins offering an instrument to cross the BBB and target the ischemic brain with an effective concentration of the specific compound (González-Nieto et al.,2020).
In particular,hydrogels and their property to expand and fill uneven damaged spots in circumstances such as stroke were recognized as enormously useful for neurotrophin delivery.
The use of BDNF-embedded hydrogels showed great results in repairing the brain after ischemia and supporting functional rescue (Clarkson et al.,2015;Cook et al.,2017).Notably,it has been investigated in two mouse models of photothrombotic stroke (strains C57Bl/6,DBA) the effect after the administration of a BDNF-enriched hyaluronan-based hydrogel that was crosslinked with polyethylene glycol (Cook et al.,2017).In bothin vivomodels,this post-stroke treatment was able to lead to significant axonal sprouting inside cortical and cortico-striatal regions.Moreover,those animals showed improved neuroblasts migration in the region of the peri-infarct cortex and ameliorated functional rescue of forelimb activity.Interestingly,this BDNFloaded hydrogel regenerative activity was reported also in aged mice after photothrombotic strokes,however,the level of recovery was minor than the one observed in younger animals (Clarkson et al.,2015).
Notably,in a recent study on rats,the IND of NGF ameliorated the neurological outcome and decreased infarct volume after 7 days following the injury.NGF treatment improved angiogenesis in the peri-infarct regions,elevated the circulating amount of the vascular-endothelial growth factor and stromal cell-derived factor 1,and increased the number of serum levels of endothelial progenitor cells (Li et al.,2018).Furthermore,NGF increased capillary-like tube creation by rat brain microvascular endothelial cells in culture,additionally validating its angiogenic influence.This angiogenic effect probably occurs through PI3K/Akt pathway (Li et al.,2018).
PI3K/Akt pathway and mitogen-activated protein kinase/extracellular signalregulated kinase pathway participate in neuronal protection from apoptosis mediated by BDNF.Apoptosis is recognized as the main event leading to neuronal cell death and brain damage during stroke.Interestingly,the neuroprotective activities exerted by sex hormones and antioxidants in stroke may be triggered through these two pathways.In a rodent model of MCAO,post-stroke treatment with progesterone improved BDNF production,mitigated apoptosis,and attenuated neuronal injury via the PI3K/Akt pathway(Jiang et al.,2016).Notably,it has been observed that progesterone and vitamin D administered together safeguarded neuronal cells from ischemia/reperfusion-mediated cell death by rising B-cell leukemia/lymphoma 2 production and inhibiting caspase-3 cleavage through the BDNF-TrkBextracellular signal-regulated kinase pathway (Atif et al.,2013).
BDNF-based therapies for stroke were the most studied,mainly in rodent models.In an MCAO rodent model of stroke,pre-treatment with intraventricular or intravenous BDNF resulted in an important reduction of infarct size and the amount of neurodegeneration.This treatment was also able to stimulate neurogenesis,promote sensorimotor recovery,and induce plasticity mechanisms (Zhang et al.,2018).
Cell-based approaches resulted efficient also for stroke treatment.In a recent study,the effects of human amniotic fluid stem cells-derived secretome on an ischemia-reperfusionin vitromodel were reported (Castelli et al.,2021).Human amniotic fluid stem cells-derived conditioned media,containing high levels of mature BDNF,was able to activate pro-survival and anti-apoptotic pathways (Castelli et al.,2021).
The implantation of BDNF-overproducing fibroblasts in the medial part of the somatosensory cortexin vivoreduced the amount of DNA damage and increased the amount of mature TrkB in the penumbra.In contrast,the inhibition of BDNF expression impaired post-ischemia recovery (Deng et al.,2016).
Furthermore,for immuno-mediated cell-based therapies against stroke,again,BDNF resulted an optimal target.Intravenous injection of a human microglial cell line into rodent brains 48 hours after MCAO determined the increase of multiple neurotrophins,including BDNF,and anti-inflammatory mediators,which lead to the post-ischemic functional rescue (Kurozumi et al.,2004).
Ultimately,GDNF intrastriatal infusion after stroke promoted striatal neurogenesis in adult rats.The GDNF treatment determined an increase in SVZ cell proliferation and neuroblast recruitment in the striatum.When administered for 2 weeks after the formation of striatal neuroblasts,GDNF promotes an increase in the number of new mature neurons (Kobayashi et al.,2006).
GDNF can positively act also as a pre-treatment before MCAO when administered intracerebroventricularly or intraparenchymally,topically to the cortical surface,or into the hippocampus reducing cerebral infarction.Systemic administration of TAT proteins linked to GDNF (able to cross the BBB)reduced caspase-3 and DNA fragmentation and increased neuronal survival in adult stroke animals.This protective effect was also observed in neonatal rats reducing the incidence and severity of brain injury induced by hypoxia/ischemia (Kilic et al.,2003).
Conclusions
Neurodegenerative diseases such as AD,PD,and stroke represent the leading cause of elderly death.With the increase in the aging population,these pathologies are more frequent and nowadays there is a growing interest in the development of pharmacological approaches that can counteract neurological degeneration and ameliorate functional recovery (Erkkinen et al.,2018).
Neurotrophins are molecules able to not only regulate the development and maintenance of the vertebrate nervous system but also have a great influence on the adult nervous system affecting crucial processes such as neuronal survival,synaptic function,and plasticity.Neurotrophins can bind two different receptors.All these molecules can bind p75NTR,and each also binds to one of a family of Trk receptors (Haddad et al.,2017).Neurotrophininduced cellular pathways are activated by Trk receptors dimerization and consequent trans-phosphorylation of the intracellular domain.Our deep understanding of neurotrophins and their receptor’s activity should now be translated into the development of disease-modifying therapies for agerelated disorders of the nervous system (Cai et al.,2014;Wang et al.,2020).
In this review,we discussed neurotrophic factors’ role in three major agerelated pathologies and how neurotrophin-based treatments can counteract the progression of those pathologies that at present are still incurable.Neurotrophic factors resulted great modulators of the adult injured brain by promoting functional recovery in preclinical models of neurodegenerative diseases,such as AD and PD but also in stroke-induced models (Figure 2).
Nevertheless,the clinical efficacy of these treatments was not already completely observed because of the difficulties of neurotrophins in crossing the BBB,their short half-life,and the lack of sufficient amount for clinical use (Bahlakeh et al.,2021).Thus,researchers worldwide are focusing their attention on finding optimal delivery systems that can modify the neurotrophins pharmacokinetic profile leading to a better penetration across the BBB and the delivery of the drug at a proper concentration in the target area.With the recent findings in neurotrophin structure and their receptors binding,it is now possible to develop molecules that mimic their activities with much ameliorated pharmacotherapeutic profiles (Teleanu et al.,2022).Moreover,recent preclinical studies showed great promise using several delivery systems such as micropumps,viral vectors,conjugatedneurotrophins,and biomaterials.Moreover,cells such as genetically modified fibroblasts for the expression of neurotrophins as well as NSCs can express high levels of BDNF and NGF (Wang et al.,2021).Finally,the efficacy of some pharmacological approaches for neurodegenerative diseases can be explained by the modulation of neurotrophin levels such as BDNF (Camuso and Canterini,2023).However,further studies devoted to testing neurotrophins in clinics are necessary to better define their potential in neurological disorders and age-related diseases.
Author contributions:MA and MdA wrote the manuscript with support from VC.MA prepared all the figures.All authors approved and contributed to the final manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
- Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update

