Ketamine,a trauma analgesic with sex-specific immunomodulatory function
Haley F.Spencer,Rina Y.Berman,Martin Boese,Kwang H.Choi
Ketamine,a multimodal dissociative anesthetic,produces powerful analgesia at subanesthetic doses in traumatically injured patients.As ketamine does not induce respiratory depression or hemodynamic instability,the Committee on Tactical Combat Casualty Care for the US military recommends the use of subanesthetic doses of ketamine for acute pain management(Butler et al.,2014).Additionally,ketamine may have immunomodulatory effects after injury at subanesthetic doses,mediating the balance of proand anti-inflammatory processes (Loix et al.,2011;De Kock et al.,2013).The majority of preclinical studies have examined the immunomodulatory effects of ketamine using only male animals,leaving the issue of sex as a biological variable unanswered.Therefore,in a recent study,we investigated the effects of subanesthetic doses of an intravenous (IV) ketamine infusion (0,10,and 40 mg/kg,2 hours) on inflammatory cytokine levels in both male and female Sprague-Dawley rats (Spencer et al.,2022).Using rats with indwelling jugular venous catheters,we were able to measure time-dependent changes in plasma cytokine levels following the IV ketamine infusion.This is a significant contribution to the scientific community due to the growing interest in how sex-related differences may play a role in immune function.Moreover,we tested IV ketamine in a non-inflammatory condition,which has not been well characterized previously.Due to the basal immune response differences between males and females (Klein and Flanagan,2016),examining ketamine effects in a non-inflammatory condition lays the groundwork for future studies utilizing injured or inflammatory conditions with ketamine administration in male and female animals.
Our findings suggest heterogeneous sex-related changes in cytokine levels following an IV ketamine infusion in rats.The anti-inflammatory cytokine interleukin (IL)-10 and the pro-inflammatory cytokines IL-6 and tumor necrosis factor alpha(TNF-α) showed sex-,dose-,and time-dependent changes after the IV ketamine infusion.The time course of plasma cytokine changes following a single IV ketamine infusion revealed that while most cytokine reduction occurred at 2 hours,IL-6 and TNF-α showed alterations at 2 and 4 hours with sex-specific differences in their time courses.In male rats receiving ketamine,IL-6 levels were normal at 2 hours but decreased at 4 hours,while the opposite trend was observed in female rats.In contrast,reduction of TNF-α at 2 hours in male and female rats was recovered to control levels at 4 hours in males,but not in females.The doseresponse study with 10 and 40 mg/kg IV ketamine infusions revealed that IL-10 was reduced in male rats with 10 mg/kg ketamine,IL-6 in female rats at both doses with a significant sex difference with 40 mg/kg ketamine,and TNF-α with both infusion doses in both sexes.These differences were not associated with estrous cycle phases in female rats,suggesting that they are not dependent on gonadal hormones.These sex-specific and time course differences may have clinical implications for differential timing and dosing of IV ketamine in order to maximize its immunomodulatory properties in men and women.
Although there is an agreement in the clinical and preclinical literature that ketamine reduces inflammation,the role of ketamine on the balance of pro-and anti-inflammatory processes is contested.A review article suggested that ketamine mostly alters pro-inflammatory processes(Loix et al.,2011),while another review proposed that ketamine may promote balance between pro-and anti-inflammatory factors to maintain homeostasis (De Kock et al.,2013).Thus,the term“immunomodulatory” is used by the authors to differentiate from the term “immunosuppressant”,which would imply anti-inflammatory effects as opposed to contributing to both pro-and antiinflammatory processes (Loix et al.,2011;De Kock et al.,2013).This is in line with our findings as both pro-inflammatory cytokines,namely IL-6 and TNF-α,and an anti-inflammatory cytokine,IL-10,were modulated in a sex-specific manner following the IV ketamine infusion.Interestingly,the aforementioned reviews suggested that ketamine may not produce immunomodulatory effects unless it is given in the presence of an inflammatory condition such as lipopolysaccharide stimulation,injury,or surgical manipulation (Loix et al.,2011;De Kock et al.,2013).Our findings disagree with this claim,as we did not use an inflammatory or injury condition in our study in order to assess the inherent immunomodulatory properties of ketamine.Previous ketamine studies (Taniguchi et al.,2003;Yu et al.,2007)on inflammation seldom examined sex as a biological variable;therefore,an examination of ketamine’s immunomodulatory effects in male and female rodents in both inflammatory and noninflammatory conditions is warranted.
Emerging evidence suggests that inflammation may underlie ketamine’s clinical and behavioral effects,such as its antidepressant properties.In a chronic restraint stress model of depression in mice,ketamine reduced immobility and anhedonia while concurrently reducing serum levels of IL-1β,TNF-α,and IL-6 (Tan et al.,2017).The exact nature of the interaction between the effects of IV ketamine on neuroinflammation and corresponding behavioral and functional changes is largely unknown,but there is reason to believe that sex plays a significant role.An extensive review details the interplay between ketamine,sex,neuroinflammation,and major depressive disorder,which has implications for the sex-specific treatment of mood disorders and inflammatory conditions as a whole (Gagne et al.,2021).In depressed patients receiving ketamine,changes in levels of the pro-inflammatory cytokine IL-8 after ketamine treatment interacted with sex to influence the Hamilton Depression Rating Scale score;specifically,decreasing IL-8 levels were correlated with depressive symptom improvement in men,but not in women (Kruse et al.,2021).This indicates that antidepressant doses of ketamine are capable of modulating cytokines to influence clinical outcomes in a sex-related manner.Because of complex interactions between ketamine,sex,inflammation,and behaviors,future research should consider ketamine’s effects in the context of an inflammatory insult in both males and females.
One example of an inflammatory insult is a traumatic brain injury (TBI).Similar to the example of major depressive disorder,inflammatory cytokines may be useful predictors of clinical outcomes in TBI patients.Serum IL-10 has been associated with TBI severity as measured by the Glasgow Coma Scale and higher mortality in severe TBI patients (Schneider Soares et al.,2012).Additionally,serum IL-13 and TNF-α levels were associated with improved recovery 12 months after severe TBI (Wu et al.,2021).Considering the clinical predictive value of cytokines as biomarkers,the sex-specific differences in inflammatory and behavioral profiles following TBI (Späni et al.,2018),and ketamine’s modulation of cytokines to produce sex-specific clinical outcomes (Kruse et al.,2021),it stands to reason that ketamine may sex-dependently modulate inflammation in TBI patients to influence clinical outcome in much the same way.It is important to understand the effects of ketamine on sex-related differences with respect to functional outcomes after TBI,and therefore,studies on ketamine’s effects on inflammation after TBI in male and female subjects are warranted.
Our study utilized both male and female rats in assessing ketamine’s effects on inflammation(Spencer et al.,2022),which remains a gap in the preclinical literature.Additionally,the use of indwelling IV jugular venous catheters in rats enhances clinical translation by enabling repeated blood sampling from the same animals without causing stress or anesthesia during the procedure.The heterogeneous effects that IV ketamine produces on male and female subjects provide critical insight for translational research and clinical practice for many applications ranging from acute pain management to treatmentresistant major depressive disorder.In particular,TBI may present an important and promising application for ketamine as a trauma analgesic with immunomodulatory properties,as there are no Food and Drug Administration-approved treatments for TBI currently despite its status as a major cause of death and disability.Ketamine was recently shown not to increase intracranial pressure as was previously feared (Zeiler et al.,2014),and it is no longer contraindicated for TBI due to intracranial pressure concerns.Furthermore,ketamine is already a staple of battlefield and emergency medicine,allowing for seamless implementation as a standard of care.The need for more research on sex differences is particularly important in this context,as women are going to be involved in combat roles and therefore may comprise a larger portion of the TBI population than in the past,but the literature has not caught up to this shift.Additionally,the inclusion of sex as a biological variable is particularly important as females may have more responsive immune systems,and thus,may be more susceptible to inflammatory conditions (Klein and Flanagan,2016).Figure 1illustrates a potential mechanism by which ketamine,a multimodal trauma analgesic,produces immunomodulatory effects in a sex-specific manner.
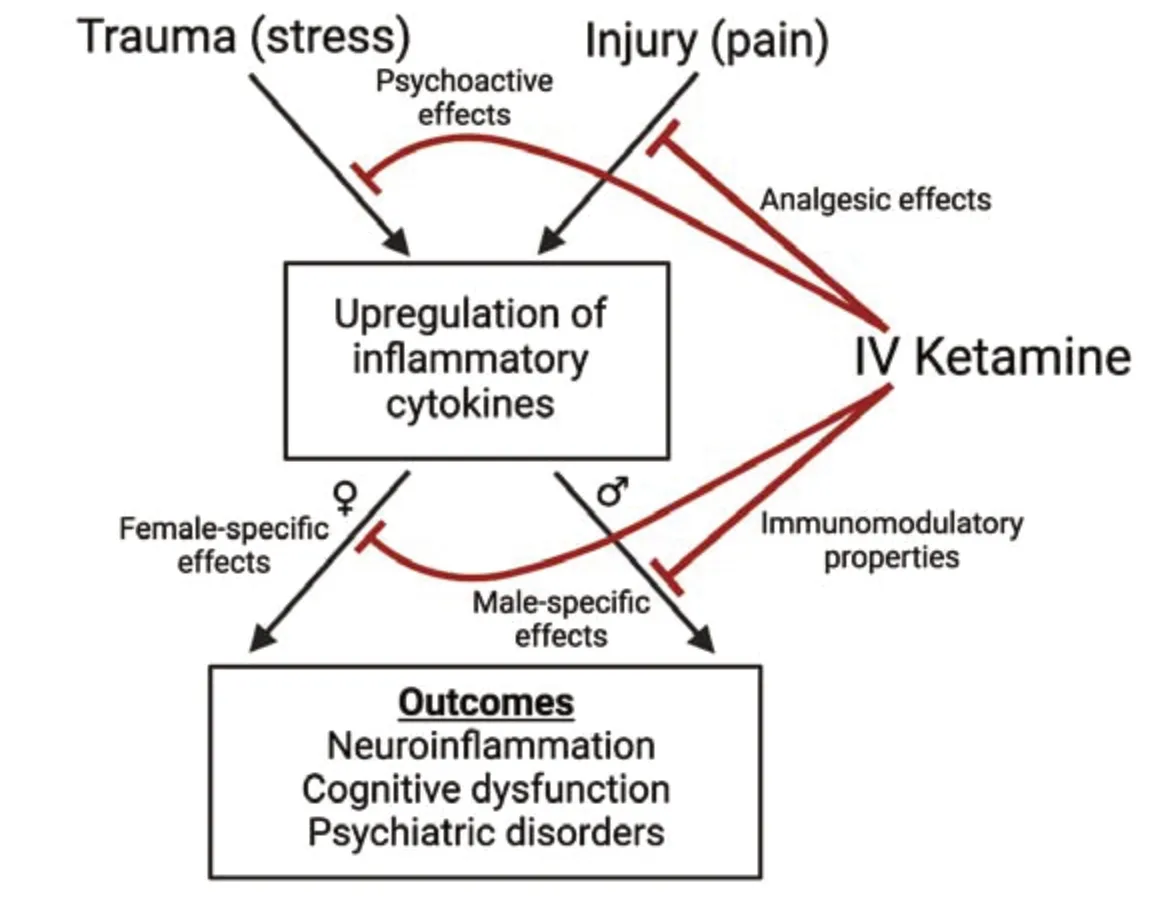
Figure 1|A potential mechanism by which ketamine produces immunomodulatory effects.
In conclusion,future research should investigate injury and inflammatory conditions such as TBI utilizing IV ketamine administration,and it is imperative that these studies examine both males and females in preclinical and clinical contexts.It is clear that the route,timing,and dosage of ketamine administration in male and female subjects are crucial when considering the future goal of personalized medicine.
Haley F.Spencer,Rina Y.Berman,Martin Boese,Kwang H.Choi*
Program in Neuroscience,Uniformed Services University,Bethesda,MD,USA (Spencer HF,Choi KH)
Center for the Study of Traumatic Stress,Uniformed Services University,Bethesda,MD,USA(Berman RY,Choi KH)
Daniel K.Inouye Graduate School of Nursing,Uniformed Services University,Bethesda,MD,USA(Boese M,Choi KH)
Department of Psychiatry,F.E.Hébert School of Medicine,Uniformed Services University,Bethesda,MD,USA (Choi KH)
*Correspondence to:Kwang H.Choi,PhD,kwang.choi@usuhs.edu.
https://orcid.org/0000-0003-2884-4996(Kwang H.Choi)
Date of submission:June 16,2022
Date of decision:August 25,2022
Date of acceptance:September 20,2022
Date of web publication:October 24,2022
https://doi.org/10.4103/1673-5374.358617
How to cite this article:Spencer HF,Berman RY,Boese M,Choi KH (2023) Ketamine,a trauma analgesic with sex-specific immunomodulatory function.Neural Regen Res 18(6):1263-1264.
Author statement:The views expressed are solely those of the authors and do not reflect the official policy or position of the US Army,US Navy,US Air Force,the Department of Defense,US Government,or the Uniformed Services University.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
- Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update

