cGMP signaling: a potential therapeutic target for neurodegeneration in glaucoma?
Joseph M.Holden,Lauren K.Wareham
Neurodegeneration of the central nervous system (CNS) underscores many of humanity’s most debilitating diseases,including Alzheimer’s disease,Parkinson’s disease,and multiple sclerosis.Recently,the nitric oxide-guanylate cyclase-cyclic guanosine monophosphate (NOGC-cGMP) signaling pathway has gained traction as a neuroprotective pathway in the CNS.As an extension of the CNS,the retina is also susceptible to neurodegeneration with age.The most prevalent optic neuropathy is glaucoma,the world’s leading cause of irreversible blindness,predicted to affect more than 112 million people worldwide by 2040 (Calkins,2021).In glaucoma,vision loss occurs due to the progressive degeneration of retinal ganglion cells (RGCs),the output neurons of the retina,along with their axons which form the optic nerve (Wareham et al.,2022).Degeneration of RGCs leads to a characteristic pattern of scotopic visual field deficiencies that spread from one retinotopic sector to the next (Elze et al.,2015).Visual deficits are linked to increasing age and the sensitivity of RGCs to intraocular pressure (Calkins,2021).
Despite treatment to lower intraocular pressure in the clinic,a large proportion of glaucoma patients still progress to vision loss (Wareham et al.,2022).Furthermore,around 30% of patients are normotensive,suggesting that intraocular pressure-independent pathophysiological mechanisms exist (Wareham and Calkins,2020).Besides age and intraocular pressure,other risk factors for the disease include severe myopia,central corneal thickness,race,and inherited genes associated with disease onset and progression (Wareham et al.,2022).The diverse range of risk factors speaks to the complex etiology of the disease and the need for novel therapeutics to prevent vision loss.Neurodegenerative pathophysiology in glaucoma shares many commonalities with other neurodegenerative diseases of the CNS,suggesting that there may be shared cellular mechanisms of neurodegeneration(Wareham et al.,2022).Understanding the early,intraocular pressure-independent disease mechanisms is critical to the development of novel therapeutics for glaucoma.Here we highlight recent work that supports a multifaceted role for dysfunctional cGMP signaling in RGC degeneration.
Glaucomatous neurovascular pathophysiology:chicken or the egg?The critical site of pathology in glaucoma is the optic nerve head,where unmyelinated RGC axons converge to exit the globe to form the optic nerve proper.The increased vulnerability of RGC axons in glaucoma is partially due to changes in the structure and physiology of ocular tissues such as the sclera and optic nerve head,rendering axons more sensitive to intraocular pressure-induced damage (Downs,2015;Stowell et al.,2017).However,vascular mechanisms of the disease have been long argued to play a role in RGC degeneration (Wareham and Calkins,2020).Like its counterpart the brain,the retina is metabolically demanding and relies on sufficient blood flow for the delivery of metabolites and removal of waste products.As such,the optic nerve head and retina have evolved as highly vascularized tissue structures.Glaucoma patients often share comorbidity with other systemic vascular diseases such as migraine,hypotension,hypertension,and stroke (Wareham and Calkins,2020).Vascular dysfunction is evident in glaucoma patients at all stages of disease progression,presenting as low regional ocular blood flow,microhemorrhages at the optic disk,and altered vascular morphology.Vascular dysfunction in any capacity at the optic nerve head and throughout the retina can lead to localized ischemic events,triggering RGC degeneration (Hayreh et al.,1970,1999).Minor changes in retinal vascular function that predicate RGC degeneration may go unnoticed in the clinic due to limitations with detection systems and our understanding of the underlying vascular mechanisms of the disease(Wareham et al.,2022).
The pattern of RGC loss in glaucoma is distinctive;visual field loss generally starts in the periphery and progresses in an arcuate pattern across the visual hemifield.Interestingly,this arcuate pattern,when mapped to the retina,follows the pathway of not only the RGC axon bundles themselves but also the major blood vessels in the superficial layer of the retina (Elze et al.,2015).Patterns of glaucomatous visual field deficits have been categorized into 17 subtypes,but the commonality between them is that vision loss occurs sectorially,following the retinal axon bundles and vascular structures (Elze et al.,2015).What is the reason for this distinctive sectorial RGC dropout? It is feasible that small,undetected changes in retinal vascular function may initialize pro-degenerative events that spread through the neurovascular network from one retinotopic sector to the next.There are several possible mechanisms that explain how the vasculature might impact sectorial RGC loss in glaucoma: 1) RGC degeneration leads to the breakdown of local neurovascular coupling,2) dysfunctional neurovascular function precedes degeneration of RGCs and acts as a prodegenerative trigger,or 3) other pro-degenerative signaling cascades cause dysfunction in both the vasculature and RGCs simultaneously.
Dysfunctional cGMP signaling in retinal neurodegeneration:NO-GC-cGMP signaling is a wide-ranging signal transduction pathway with well-established roles in smooth muscle relaxation,platelet function and visual transduction (Fesenko et al.,1985;Carvajal et al.,2000;Walter and Gambaryan,2009).The NO-GC-cGMP signaling pathway begins with the catalytic conversion of arginine and molecular oxygen into NO and citrulline by nitric oxide synthase.NO is lipophilic and freely diffuses between cells where it binds to its main receptor,GC (predominantly GC1 but also the less abundant GC2;formerly GCα1β1 and sGCα2β1,respectively).Upon NO binding,GC activity increases 200-fold catalyzing the formation of the second messenger cGMP.cGMP can then bind to a variety of effectors,with the most predominant being protein kinase G and cyclic nucleotide-gated ion channels.Retinal expression of GC is widespread,with levels of GC protein detected in RGCs,amacrine cells,astrocytes,bipolar cells,and photoreceptors (Wareham et al.,2018a).Besides its well-characterized role in rod and cone phototransduction,the widespread retinal expression of GC suggests that it may exert a number of other physiological roles in the retina.The first evidence linking dysfunctional GCcGMP signaling to retinal dysfunction in humans was discovered in a candidate gene association study in the GLAUGEN cohort (Buys et al.,2013).The study identified a variant in the GUCY1A3/GUCY1B3 gene locus in primary open-angle glaucoma patients that develop early paracentral vision loss (Buys et al.,2013).In separate studies of glaucoma patients,NO and cGMP levels were reduced in aqueous humor compared to controls(Wareham and Calkins,2020) .The link between dysfunctional GC-cGMP signaling and RGC degeneration has also been observed in mice.Mice lacking the alpha catalytic subunit of GC1(GC1–/–mice,formerly known as sGCα1–/–) develop RGC degeneration with age,with only modest increases in intraocular pressure (Buys et al.,2013).This work provided a normotensive mouse model of glaucoma and further evidence that systemic dysfunctional cGMP signaling results in RGC degeneration.
As a secondary messenger,cGMP levels in the body are tightly controlled through the action of phosphodiesterase enzymes (PDEs) which catalyze the breakdown of cGMP.Breakdown of cGMP by PDEs prevents unnecessary activation of downstream signaling cascades.PDE inhibitors prevent this breakdown,increasing the bioavailability of cGMP and thus activation of downstream effectors such as protein kinase G.By administering an FDA-approved PDE5-specific inhibitor tadalafil at low doses in mouse chow,the degeneration of RGCs was prevented in two glaucoma models: the GC1–/–mouse and microbead model of ocular hypertension(Wareham et al.,2019).Tadalafil treatment was successful in increasing systemic cGMP levels relative to baseline and preventing outright RGC soma dropout as well as axon degeneration in both murine models of glaucoma.Importantly,the protective effects of tadalafil occurred without any effect of cGMP on level of intraocular pressure,suggesting a mechanism of neuroprotection that was independent of intraocular pressure-related RGC stress at the optic nerve head.Further investigation into the neuroprotective effects of cGMP was carried outex vivousing whole mouse retinal explants andin vitrousing primary isolated mouse RGCs (Wareham et al.,2019).In these studies,cGMP had a direct effect on apoptotic signaling pathways;supplementation with a cellpermeable cGMP analog (8-Br-cGMP) resulted in a marked reduction in the expression of apoptotic cell markers,such as cleaved caspase-3.These studies highlight a potential neuroprotective application of FDA-approved PDE inhibitors for retinal neurodegeneration,however,further exploration of the mechanism of neuroprotection of RGCs is warranted.Furthermore,it is important to note that dysregulation of cGMP levels in the other extreme,i.e.,very high biological cGMP levels,can result in retinal photoreceptor degeneration in the disease retinitis pigmentosa(Newton and Megaw,2020).Like the majority of biological compounds,there are optimalin vivoconcentrations of cGMP and efforts to identify the levels of cGMP required to provide neuroprotection will need to be determined.
cGMP signaling in the retinal neurovascular unit:cGMP signaling is integral to systemic vascular function,including in the retina.GC1–/–mice exhibit dysfunctional retinal vascular responses to systemic application of NO-donor sodium nitroprusside (Buys et al.,2013).In the systemic vasculature,a drop in blood pressure due to sodium nitroprusside elicits a vasoconstriction of blood vessels,including the vessels in the retina.In wild-type (WT) mice,this was apparent,however,GC1–/–mice exhibited a reduced vasoconstriction response in both systemic and retinal vessels(Buys et al.,2013).Whether retinal vascular disruption preceded or was a consequence of RGC degeneration in GC1–/–mice was not clear as measurements were carried out in animals that were already exhibiting retinal degeneration.
In our recent work,we examined the effect of dysfunctional cGMP signaling on the retinal vasculature in both young and aged GC1–/–and WT mice (Holden et al.,2022).The goal of our research is to understand how early neurovascular events in glaucoma pathophysiology contribute to RGC degeneration.In the CNS,vascular function is primarily controlled by cells of the neurovascular unit which include glial cells (e.g.,astrocytes),vascular cells (e.g.,endothelial cells and pericytes),and neurons.Among the most important players in this process are astrocytes,the predominant glial cell type in the CNS.Astrocytes extend fine processes throughout the parenchyma to contact blood vessels with structures known as end feet.These contacts serve as sites of communication where vasoactive compounds,such as cGMP,can be released from astrocytes and act directly on both endothelial and contractile mural cells(Boal et al.,2021).The neurovascular unit acts as an interconnected network with widespread physical association and functional communication between diverse cell types.This serves to maintain neurovascular homeostasis;dysfunction at any point throughout the system could have farreaching effects on neuronal function and viability.
In our study of the retinal vasculature,we observed gross changes in blood vessel morphology in GC1–/–mice that was associated with the breakdown of the blood-retinal barrier and these changes were not observed in WT mice (Holden et al.,2022).We also found aberrant astrocyte morphology and increased expression of glial fibrillary acidic protein (GFAP) in GC1–/–mice when compared with WT animals at an age that preceded outright RGC degeneration.The interconnected networks that astrocytes form across the retina relies on the expression of connexin proteins that physiologically connect adjacent cells (Boal et al.,2021).In astrocytes,the most highly expressed connexin is connexin-43 (Cx43) (Boal et al.,2021).In both WT and GC1–/–mice,Cx43 expression was reduced with increasing age (Holden et al.,2022).Furthermore,GC1–/–mice had considerably less Cx43 expression with increasing age than WT mice (Holden et al.,2022).Dysfunctional astrocyte connexin expression is associated with increased susceptibility to intraocular pressure-dependent degeneration of RGCs (Cooper et al.,2020).The astrocytic changes that we observed in GC1–/–animals preceded the age at which outright RGC degeneration occurred.Our results therefore suggest that dysfunctional cGMP signaling alters astrocyte physiology and vascular morphology,likely rendering RGCs more susceptible to agerelated neurodegeneration.
cGMP signaling– a multifaceted retinal neuroprotectant?The role of retinal vascular dysfunction in glaucoma pathophysiology is gaining traction and could represent a new therapeutic area for the disease.However,our understanding of how and when retinal vascular dysfunction occurs in glaucoma is limited.While it is well known that the primary role of cGMP signaling is to mediate proper vascular function,our research suggests that cGMP signaling may have a direct neuroprotective role on RGCs and may play a key role in astrocyte physiology(Figure 1).Elsewhere in the CNS,PDE5 inhibitors have shown potential as neuroprotective compounds.For example,in models of brain injury,increased levels of cGMP promoted the expression of anti-inflammatory pathways and decreased the expression of pro-apoptotic signaling (Zinni et al.,2021).Our findings raise the question: could dysfunctional cGMP signaling lead to the breakdown of the neurovascular unit,underpinning sectorial neurodegeneration in glaucoma? Further investigation into the role(s)of cGMP in retinal degeneration are needed,but initial studies are proving very promising for the future development of cGMP-based therapeutics for neurodegeneration.
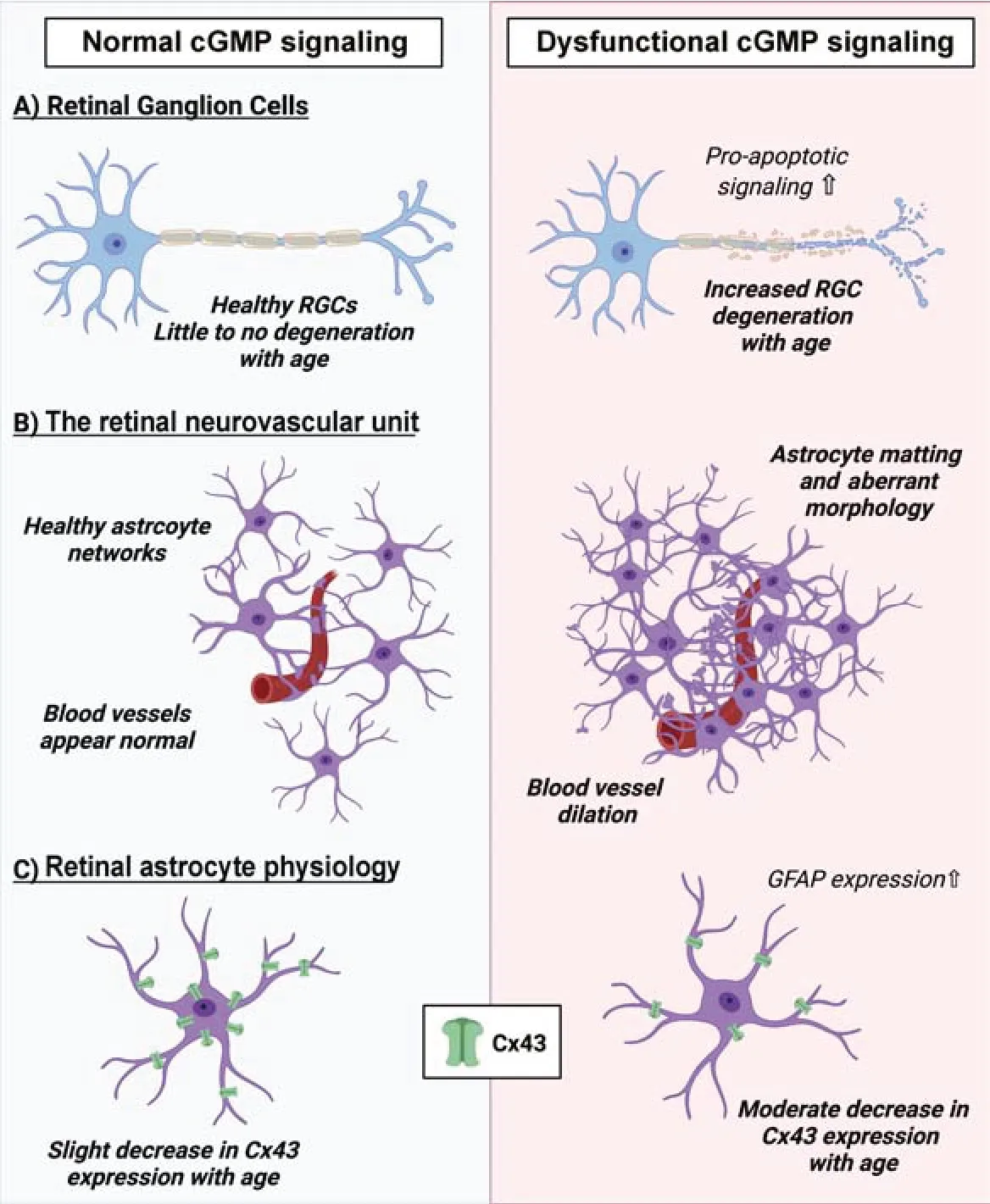
Figure 1|The impact of cGMP signaling on RGC function and the retinal neurovascular unit.
Joseph M.Holden,Lauren K.Wareham*
Vanderbilt Eye Institute,Vanderbilt University Medical Center,Nashville,TN,USA
*Correspondence to:Lauren K.Wareham,PhD,lauren.wareham@vumc.org.https://orcid.org/0000-0003-4512-6355(Lauren K.Wareham)
Date of submission:August 15,2022
Date of decision:September 23,2022
Date of acceptance:October 17,2022
Date of web publication:November 18,2022
https://doi.org/10.4103/1673-5374.360169
How to cite this article:Holden JM,Wareham LK(2023) cGMP signaling: a potential therapeutic target for neurodegeneration in glaucoma? Neural Regen Res 18(6):1267-1268.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
- Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update

