Neural differentiation protocols:how to choose the correct approach
Michael Telias
Pluripotent stem cells in neural differentiation:characterization and potential:The establishment and use of pluripotent stem cells (PSCs),including embryonic (ESCs) and induced (iPSCs),constitutes a major scientific breakthrough of the last decades.Human PSCs hold the potential to deliver regenerative therapies in many diseases,including neurological ones.The general approach is to produce functioning human neurons and glial cellsin vitro,to be later implanted in the diseased nervous system,replacing dysfunctional or dead cells.In addition,human and other animal-sourced PSCs make useful and dynamicin vitromodels for neurodevelopmental,neurodegenerative and psychiatric disorders,enabling researchers to continuously produce normal and diseased neurons to investigate basic mechanistic questions,which can eventually lead to new therapeutics.Therefore,the development of efficient protocols to induce neural differentiation in ESCs and iPSCs is currently a major effort in the field (Mertens et al.,2016).
ESCs are derived from the inner cell mass of blastocysts and kept under chemically defined medium that supports pluripotency (Xu et al.,2022),with or without a feeder cell layer.iPSCs are reprogrammed from adult somatic cells by overexpression of pluripotency-promoting genes(Liu et al.,2020),and can also be kept in an undifferentiated state for long periods of time and large number of passages.Both types of PSCs can be induced to differentiate into neural precursors,neurons,and glia cells,including several different cellular types and subtypes (Oh and Jang,2019).Other sources forin vitroneural differentiation include colonies and cultures of multipotent adult stem cells harvested from the fully-developed organism,such as: neural stem cells (NSCs) isolated from brain biopsies (Belenguer et al.,2016);mesenchymal stem cells purified from diverse biological reservoirs including the dermis (Xu et al.,2020),the dental pulp (Rafiee et al.,2020) and fat tissue (Zheng et al.,2017);and from biopsied somatic cells through direct transdifferentiation (Wang et al.,2021).However,as compared to PSCs,somatic or adult stem cells have a more limitedin vitroproliferation and differentiation potential.Therefore,ESCs and iPSCs remain today the best starting material for neural differentiation,as they are the only cells that can be considered truly pluripotent and undifferentiated.In all cases,regardless of the cellular source,in vitroneural differentiation is achieved by manipulating gene expression aimed at suppressing genes that promote non-neuronal fate (pro-endoderm and-mesoderm lineages,negative selection),and activating genes that drive differentiation and maturation of ectodermal derivatives,including neuroblasts and neurons(positive selection).Neural differentiation can be carried out in monolayers of seeded cells (2D cultures) or in multilayers of suspended aggregates(i.e.: 3D cultures or “brain organoids”).
Basic properties of in vitro neurogenesis:Researchers have to decide on the proper neural differentiation protocol that should be chosen for their investigation,according to the underlying scientific hypothesis and goals.Major criteria to be considered include the overall quantitative and qualitative efficiency of the protocol in question.Quantitative efficiency is the ratio between the number of initial undifferentiated cells,to the number of differentiated neurons obtained at the end.The number of neurons required for a specific experiment is affected by the end-goal of the experiment.For example,experiments involving single-cell RNA-sequencing or electrophysiological recordings require a relatively low number of mature neurons,whereasin vivoimplantation of derived neurons or neural precursors in animal models would probably require a much higher number of differentiated cells.Correct assessment of the initial number of PSCs required for each assay is an important tool in planning and executing cost-effective and reproducible experiments.Qualitative efficiency has to do mostly with the molecular,cellular and functional identity of the differentiated cells,during and after the process.For example,if the goal is to model a neurodevelopmental disorder that affects the nervous system at different stages of embryonic life,the protocol needs to be able to recapitulatein vitrothe same developmental stages as they happenin vivo.But if the goal is to transplant and repopulate a defined brain area with a specific cell-type in animal models or neurodegeneration patients,then the protocol to be followed should yield cells with exclusive and identical genotype and phenotype.In this case,recapitulating the stages of embryonic life is pointless while the homogeneity of neuronal identity at the end of the process is of the outmost importance.
To achieve neural induction and neuronal differentiation in PSCs,gene expression can be manipulated directly or indirectly.Direct manipulation of gene expression requires the delivery of a genetic payload into the cell,after which a coding sequence will force the transcriptional activation or inactivation of a target (i.e.: “genetic” differentiation,seeFigure 1).Indirect manipulation employs a variety of pharmacological agents (i.e.: “chemical”differentiation) that exert their effect on receptors,enzymes,and transcription factors;tilting the balance of intracellular signaling towards one favorable of neuronal fate.Both methodologies have pros and cons,and current literature is rich with neural and neuronal differentiation protocols of all kinds (Mertens et al.,2016;Hong and Do,2019;Oh and Jang,2019).
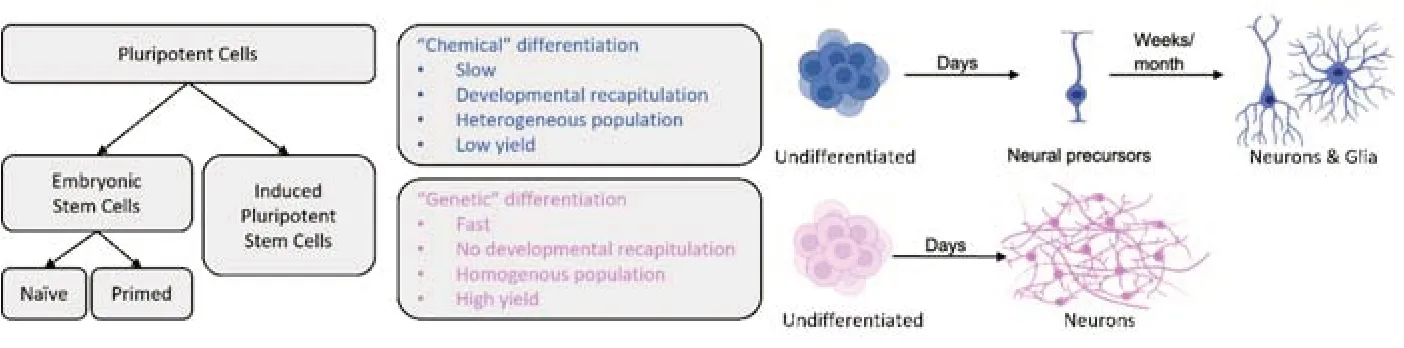
Figure 1|Principal differences between neural differentiation approaches.
Chemically-defined protocols for neural differentiation typically include a mixture of smallmolecule inhibitors and peptides that suppress the activation of molecular pathways leading to non-neuronal fate (i.e.: neural induction),and pro-neural growth factors that promote neuronal differentiation and maturation.The “dual-SMAD inhibition” protocol is a classical example of this (Chambers et al.,2009).SMADs are critical components of the transforming growth factor-β pathway,which promotes non-neuronal lineage development in embryos.In the first induction step,ESCs are deprived of the factors that promote self-renewal and pluripotency,which pushes them towards differentiation.At the same time,they are exposed to the peptide Noggin and the drug SB-431542,which inhibit SMADs and the transforming growth factor-β pathway,repressing differentiation into mesoderm and endoderm derivatives.The only surviving cells will be those differentiating into ectodermal derivatives,including neural crest and neural progenitors.During the second stage,cells will further mature into neuroblasts,and later into neurons,by exposing them to neuronal growth factors including brain-derived neurotrophic factor,nerve-growth factor and glial-derived neurotrophic factor.Effectively,this approach is based on initial negative selection against non-neural lineage,followed by secondary positive selection for neural lineage-precursors.Since proliferation is inversely correlated with differentiation,the more the cells differentiate,the less they proliferate.Therefore,the overall number of cells that can complete the process will be relatively low,creating the need to start the protocol with large numbers of undifferentiated colonies.A valid and useful variant of this approach is to allow spontaneous differentiation into all three germ layers during the first stage,followed by enrichment of the neural fraction with pro-neural growth factors(such as brain-derived neurotrophic factor and nerve-growth factor),and then proceed to sort dissociated cells and re-plate only the desired cell type.
Direct gene manipulation of stem cells with the goal of inducing long-term neuronal differentiation is frequently achieved by introducing cDNA encoding a neuronal gene,and regulatory sequences such as promoters and enhancers.Intracellular delivery of a construct usually involves one of two strategies: (i) plasmid transfection,lipofection or electroporation,or (ii) the use of adeno-associated virus or lentiviral vectors for gene transduction.For example,lentiviral infection of human pluripotent stem cells with a construct overexpressing neurogenin-2 can differentiate pluripotent stem cells into neurons in just two weeks (Zhang et al.,2013),dramatically faster than the natural developmental process or the chemically-induced differentiation protocol.In this case,the process is so fast and aggressive that most initial cells in the culture become the final induced neuronal cells,with little proliferation or cell loss.Besides neurogenin-2,other neuronal transcription factors have been used to induce direct convertion of of PSCs and even somatic cells into neurons,such as NeuroD1,or even a combination of several.In all cases,the selected transcription factors are always overexpressed,presumably greatly surpassing any natural expression in developing neurons.
Important considerations for protocol adoption and optimization:When comparing neural differentiation protocols based on direct gene expression to those based on indirect pharmacological manipulation (Figure 1),several observations can be drawn.It can be argued that direct differentiation through enhanced expression of pro-neural genes in PSCs is faster and more consistent,delivering a high percentage of differentiated cells (reported by some as nearly 100%),characterized as a highly homogenous population,all sharing a defined and testable neuronal identity.The limiting-rate step is the initial introduction of the genetic material into the cells,which will determine the portion of cells that have been effectively transfected or transduced,and the relative change in gene expression in those cells.As most expression cassettes include antibiotic-resistance genes,stable expression and high homogeneity are achieved by positively selecting transfected cells and killing off the rest.Therefore,if the initial gene transduction of undifferentiated cells is highly effective,the number of cells finalizing the process will be high,and the number of non-transduced cells will be low and further reduced by lack of antibiotic resistance.
In comparison,chemically defined neural differentiation protocols are slower and direct genetic differentiation,as they tend to mimic the natural timeline of neurogenesis,and are expected to produce more heterogeneous populations.Positive and negative selection of cells is the result of exposure to complex combinations of drugs and growth factors at different phases of the process,which requires freshly prepared medium and drug cocktails,which in turn can significantly vary between cultures and cell lines,introducing confounding factors that can influence differentiation efficiency and neuronal identity.As there is no “active” negative and positive selection through antibiotic resistance,there is always the possibility that even after months of differentiation,the cultures could be contaminated with non-neural cells from endodermal or mesodermal origin.This is especially important if cells are not sorted or purified ahead of DNA,RNA or protein extraction for downstream applications,and most important if they are intended forin-vivotransplantation.
In general,direct differentiation results in robust overexpression of the differentiation factor (e.g.neurogenin-2,NeuroD1,etc.).This artificial overexpression can be persistent or transient,reversible or irreversible.Persistent gene manipulation is achieved through methods that ensure integration of the expression cassette into the cell’s genome or as a stable nuclear episome;while transient manipulation of gene expressionin vitrocan be achieved through cell transfection via several different methods.Persistent gene expression can be irreversible if using a constitutively active promoter,or reversible if the expression cassette contains a regulatory On/Off switch,such as the Tet-On expression system.The latter entails maintaining cell cultures under relatively high concentrations of two antibiotics,one for transfection selection and the second for temporal regulation of gene expression,which can lead to reduced proliferation and neuronal yield.If gene manipulation is carried out using viral vectors,then the molecular and genetic characteristics of each vector should be considered in each protocol,including serotype,tropism,maximal payload size,infectivity rate,and more.In addition,and regardless of the delivery method,any synthetic DNA construct that is expected to integrate into the cell’s genome can potentially create unexpected mutations and chromosomal abnormalities,introducing the risk of unknown confounding factors.Direct genetic manipulation of PSCs can be made transient if using genetic material with limited physical duration,such as mRNA or siRNAs;but achieving long-term and robust neuronal differentiation using transiently expressed RNAs is not really feasible,unless multiple or continuous dosage can be delivered with high homogeneity and reproducibility.On the other hand,chemically-defined protocols can be fully reversible and transient,by simply changing the cell medium with any desired cocktail of reagents.And because most drugs have target affinities of nM to mM,the large dynamic range of effective concentrations for each specific reagent provides the potential for accurate optimization,while also providing a tool to dissect the molecular mechanisms behind neurogenesis.Since each potential drug or reagent used in the process has its own pharmacodynamics,it is hard to determine whether at every single point during the process all cells are homogenously exposed to the optimal concentrations of every component in the cocktail.Fine-tuning this process can be arduous and ultimately irrelevant,as commercially available reagents,drugs,and peptides often vary from batch to batch and from preparation to preparation,introducing unavoidable heterogeneity between and within experiments.
In summary,planning experiments involving neural or neuronal differentiation of stem cells is no simple task,and careful design is paramount.The plethora of different protocols available in the literature can provide an advanced starting point,but it does not preclude the need for detailed calibration,optimization and customization of each protocol and each experiment.
All types of pluripotent stem cells,including naïve and primed embryonic stem cells,as well as genetically reprogrammed somatic cells can be differentiated into neural precursors,neurons and glia using chemical or genetic methods.“Chemical”or indirect differentiation refers to treating cells with several defined molecules,including small-molecule inhibitors,peptides and other bioreagents,to encourage neural differentiation and discourage non-neuronal differentiation.It can take many weeks to months to obtain mature and functional neurons,depending on the protocol,and it usually ends in the development of both neurons and glia cells.“Genetic” or direct differentiation refers to inducing the overexpression of one or more genes that force the undifferentiated cells to convert to neurons.It can take only a few days (usually between 15 and 30) to produce homogeneous cultures of functional neurons,with a high degree of cell identity homogeneity.
The present work was supported by an unrestricted grant to the University of Rochester’s Department of Ophthalmology from the Research to Prevent Blindness Foundation.
Michael Telias*
Flaum Eye Institute,University of Rochester,Rochester,NY,USA
*Correspondence to:Michael Telias,MSc,PhD,Michael_Telias@urmc.rochester.edu.
https://orcid.org/0000-0002-7632-6942(Michael Telias)
Date of submission:March 3,2022
Date of decision:September 23,2022
Date of acceptance:October 1,2022
Date of web publication:November 18,2022
https://doi.org/10.4103/1673-5374.360171
How to cite this article:Telias M (2023) Neural differentiation protocols: how to choose the correct approach.Neural Regen Res 18(6):1273-1274.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
- Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update

