Synaptosome microRNAs: emerging synapse players in aging and Alzheimer’s disease
Subodh Kumar
Synaptosome:Synapses are the most critical portion of neuron connections,necessary for cellular organization of the brain.Synapse integrity is uttermost important for healthy brain functioning.Any perturbation in the synapse structure and/or function initiates neurological disorders.Synapses are the prime targets that are smashed in almost all neurodegenerative diseases.The vital components of synapse are essential for neurotransmission,synaptic plasticity and overall synapse function.Synapse dysfunctions are well studied in Alzheimer’s disease (AD) and other neurodegenerative diseases (Gowda et al.,2021).The best way to study the synapse dysfunctions in neurological diseases is the biochemical analysis of “synaptosome”.Researchers studied the synaptosome to understand the molecular reasons of synapse dysfunction in aging,AD and other neurodegenerative diseases (Kumar et al.,2020;Gowda et al.,2021).Synaptosome maintains the cellular machinery and all vital components necessary for autonomous synapse function.Synaptosome retains mitochondria,synaptic vesicles,lysosomes,endosomes along with the postsynaptic membrane and the postsynaptic density (Lugli et al.,2012;Xu et al.,2013;Li et al.,2015;Kumar et al.,2020).Therefore,synaptosomes hold the molecular machinery necessary for uptake,storage,and release of neurotransmitters,channels,receptors,and local signal transduction.Based on these properties,synaptosomes are called as “Ex vivo” model to study synaptic physiology and pathophysiology.They also pronounced as “halfway house” between neurochemistry and electrophysiology (Lugli et al.,2012;Xu et al.,2013;Li et al.,2015;Kumar et al.,2020).However,there are some technical difficulties while studying the synaptosome.Due to synapse degradation and/or reduced synapse numbers in AD,we need the large amount of braintissue (≥ 50 mg) to isolate the appropriate quantity of synaptosome for electron microscopy,mRNA and protein analysis.Another technical challenge is mitochondria contamination in synaptosome fraction because of the overlapping size of synaptosome (0.6 to 1 μm) and mitochondria(0.5 to 3 μm).Though mitochondria are a part of synaptosome,but sometimes we observed the separate mitochondrial contamination while preparing the synaptosomes.We can avoid the mitochondria contamination by applying the soft and gentle Dounce homogenization for synaptosome extraction (Kumar et al.,2022).
Synaptosome microRNAs:MicroRNAs (miRNAs)are well known for their precise roles in targeted gene regulation.Since,synaptosome contains the mitochondria,synaptic vesicles,and endosomes,therefore presence of miRNAs is not surprising in synaptosome.As we mentioned that synaptosome entity retain the necessary molecular machinery for synapse function.In this scenario,miRNAs could be the critical players to regulate the synaptosome functions locally via modulation of local synaptic proteins.Several studies identified the miRNAs in synaptosomes,endosomes,and even in synaptic vesicles (Lugli et al.,2012;Xu et al.,2013;Li et al.,2015).However,roles of synaptosome enriched miRNAs were not explored in neurological disorders.We assessed the synaptosomal miRNAs and their possible roles in synapse dysfunction in AD and in healthy state(Kumar et al.,2022).
Synaptosome miRNAs in brain aging:Aging is the key factor that impaired the synapse function in several ways such as neuronal shrinking,retracted dendrites,oxidative stress,free radical production and telomere shortening.Aging also increases the numbers of nonfunctional or silent synapses in the human brain.Our study identified several miRNAs expressed more in the synaptosome portion relative to cytosol extracted from unaffected control postmortem brains (Kumar et al.,2022).The Ingenuity Pathway Analysis revealed that some potential miRNAs involved in aging related brain disorders including AD,amyotrophic lateral sclerosis,behavioral variant frontotemporal dementia,epilepsy or neurodevelopmental disorder,schizophrenia,neuronal migration disorders and so on (Figure 1A).The synaptosome miRNAs also take part in various aging related pathways including let-7a-5p: cellular development;miR-103-3p: cell death and survival;miR-24-3p: cellular growth and proliferation;miR-124-3p: nervous system development and function and miR-501-3p:cellular function maintenance and nervous system development (Kumar et al,2022).We also observed the four miRNAs: miR-107,let-7g-5p,let-7f-5p,and let-7c-5p,significantly expressed only in the healthy state synaptosomes,but the not in AD state.As per their aging relevance,Lai et al.(2014)reported the role of miR-107 in aging process.MiR-107 levels were down-regulated from infancy to children,up-regulated in young adulthood,and then showed expression diminishing with aging(Lai et al.,2014).The level of miR-107 was found to be reduced in AD (Dimmeler and Nicotera,2013).Another,miRNA let-7 is also evaluated in aging,which participates in multiple cellular pathways that regulate the aging process,stem cell function,body metabolism,senescence of B cells and various aging-related diseases (Wang et al.,2022).These are just a few examples.Therefore,based on the miRNA’s expression status in healthy synaptosomes,further studies on above mentioned miRNAs are necessary to precisely understand their roles in brain aging and underlying mechanism of aging process.
Synaptosome miRNAs in Alzheimer’s disease:Our recent study identified some potential synaptosomal miRNAs highly expressed in the synaptosomes extracted from the postmortem AD brains (Kumar et al.,2022).We studied the synaptosomal and cytosolic miRNAs in the same samples.Based on the differential distribution of miRNAs,they are classified as synaptosomal miRNAs including miR-124-3p,miR-24-3p,miR-185-5p,miR-151a-5p,miR-103a-3p,let-7d-5p,miR-320a,miR-140-3p,miR-17-5p,miR-138-5p,Let-7a-5p,let-7e-5p,miR-485-5p,miR-502-3p,miR-501-3p,miR-877-5p and cytosolic miRNAs:miR-3656 and miR638.In silico Ingenuity Pathway Analysis revealed that many of these miRNAs are involved in several neurological disorders: AD,mild cognitive impairments,multiple sclerosis,schizophrenia,chronic epilepsy,DiGeorge syndrome,neuropathic pain,thermal hyperplasia,and so on (Figure 1A;Kumar et al.,2022).Similarly,miRNAs gene interaction analysis revealed target genes those were involved in multiple synapse functions and governing synaptic activities.These miRNAs target several ion channels,transporters,peptidases,kinases and phosphatase proteins.As mentioned in previous aging section,AD synaptosome miRNAs are also involved in several biological processes and cellular pathways.However,most of the AD synaptosome miRNAs are implicated in disease-oriented pathways such as Let-7a-5p: AD and sporadic amyotrophic lateral sclerosis;miR-103-3p: schizophrenia;miR-124-3p: depression-related behavior;miR-17-5p:neuropathic pain;miR-138-5p: volume of dendritic spine;miR-502-3p: GABAergic synapse function(Kumar et al.,2022).Many of those miRNAs are well studied in AD,such as miR-103-3p promotes total neurite outgrowth and inhibits cell apoptosis by targeting prostaglandin-endoperoxide synthase 2 in cellular models of AD (Yang et al.,2018).MiR-138 controls the hippocampal interneuron function and short-term memory in mice (Daswani et al.,2022).MiR-124 was identified as potential target for AD via regulation of BACE1 protein (An et al.,2017).Further,elevated expression of miR-17 in microglia abrogates autophagy-mediated Aβ degradation in AD (Estfanous et al.,2021).These evidences showed the importance of these miRNAs in AD pathogenesis and cognitive functions.
However,we also reported some miRNAs those are not explored practically in AD,butin-silicoanalysis revealed their critical roles in synapse function.One of them is miR-502-3p,which showed high expression in AD synaptosomes and positively correlated with disease severity in terms of AD braak stages (Kumar et al.,2022).Bioinformatic analysis showed that high levels of miR-502-3p negatively regulate the GABAergic synapse function.We found that miR-502-3p targets the γ-aminobutyric acid (GABA) receptor alpha 1 (GABRA1) mRNA at multiple sites.Ourinvitroanalysis unveiled that over expression of miR-502-3p reduces GABA receptor protein GABRA1 and GABA current while suppression of miR-502-3p increases the GABA receptor proteins and GABA currents (Figure 1B;unpublished observations).It is well reported that GABAergic neurons are dysfunctional in aging and AD (Limon et al.,2012).Therefore,modulation of GABA functions by miR-502-3p could be a new therapeutic target for AD.
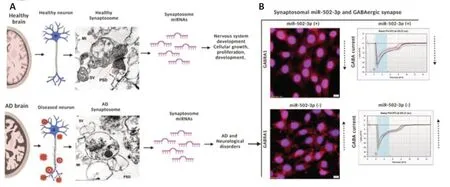
Figure 1|Synaptosome miRNAs analysis in aging and Alzheimer’s disease (AD).
In summary,synapses are the initial target for AD and brain aging.The synaptosome miRNAs,mRNA and synaptic protein establish a dedicated system to ensure accurate,efficient and reliable synaptic transmission in the human brain.MiRNAs alteration at synapse modulates local synaptic proteins and impaired a normal neurotransmission and synaptic plasticity.Our study identified some potential synaptosomal miRNAs deregulated in unaffected control and in AD synapse.Studies are available on a few of them miRNAs and their involvement in aging and AD.However,many of the synaptosomal miRNAs are not explored in AD and aging.Therefore,molecular significance of these synapse centered miRNAs is highly important and needs to be considered in aging and in AD.Further,studies are needed on synapse miRNAs to determine their roles and underlying mechanism in aging process and in AD progression.
Future perspectives:Synapse dysfunction is not only limited to AD and brain aging,precisely it is a common disorder of central nervous system.Synaptic defects are associated with other neurodegenerative diseases and disorders including Parkinson’s disease and Huntington’s disease,autism spectrum disorders,schizophrenia and bipolar disorder.As reported,synaptosome miRNAs regulate GABA function in AD,therefore,synaptosome miRNAs could be crucial players in other neurological diseases.Thus,it is important to determine the roles of synapse-associated miRNAs in other diseases and study their underlying mechanism in synapse function.
The author would like to thank National Institute of Aging (NIA),National Institute of Health (NIH) for the funding the K99/R00 awards-K99AG065645 and R00AG065645 to SK.
The author would like to inform that he filed a patent on “Synaptosomal miRNAs and Synapse Functions in Alzheimer’s Disease” TTU Ref.No.2022-016,U.S.Provisional Pat.App.No.63/332,866 on April 20,2022 related to the contents of this manuscript.
Subodh Kumar*
Center of Emphasis in Neuroscience,Department of Molecular and Translational Medicine;Paul L.Foster School of Medicine,Texas Tech University Health Sciences Center,El Paso,TX,USA
*Correspondence to:Subodh Kumar,PhD,subodh.kumar@ttuhsc.edu.
https://orcid.org/0000-0003-4705-122X(Subodh Kumar)
Date of submission:August 22,2022
Date of decision:September 23,2022
Date of acceptance:October 4,2022
Date of web publication:November 18,2022
https://doi.org/10.4103/1673-5374.360172
How to cite this article:Kumar S (2023)
Synaptosome microRNAs: emerging synapse players in aging and Alzheimer’s disease.Neural Regen Res 18(6):1275-1276.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
- Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update

