Overexpression of vascular endothelial growth factor enhances the neuroprotective effects of bone marrow mesenchymal stem cell transplantation in ischemic stroke
Cui Liu,Zhi-Xiang Yang,Si-Qi Zhou,Ding Ding,Yu-Ting Hu,Hong-Ning Yang,,Dong Han,,Shu-Qun Hu,,,Xue-Mei Zong,
Abstract Although bone marrow mesenchymal stem cells (BMSCs) might have therapeutic potency in ischemic stroke,the benefits are limited.The current study investigated the effects of BMSCs engineered to overexpress vascular endothelial growth factor (VEGF) on behavioral defects in a rat model of transient cerebral ischemia,which was induced by middle cerebral artery occlusion.VEGF-BMSCs or control grafts were injected into the left striatum of the infarcted hemisphere 24 hours after stroke.We found that compared with the stroke-only group and the vehicle-and BMSCs-control groups,the VEGF-BMSCs treated animals displayed the largest benefits,as evidenced by attenuated behavioral defects and smaller infarct volume 7 days after stroke.Additionally,VEGF-BMSCs greatly inhibited destruction of the blood-brain barrier,increased the regeneration of blood vessels in the region of ischemic penumbra,and reducedneuronal degeneration surrounding the infarct core.Further mechanistic studies showed that among all transplant groups,VEGF-BMSCs transplantation induced the highest level of brain-derived neurotrophic factor.These results suggest that BMSCs transplantation with vascular endothelial growth factor has the potential to treat ischemic stroke with better results than are currently available.
Key Words: bone marrow mesenchymal stem cell;brain-derived neurotrophic factor;CD31;microtubule associated protein 2;middle cerebral artery occlusion;stroke;transplantation;vascular endothelial growth factor
From the Contents
Introduction 1286
Methods 1287
Results 1288
Discussion 1289
Introduction
Acute ischemic stroke is a cerebrovascular disease that causes adult disability and death,and is a worldwide economic problem (Li et al.,2018).During ischemic stroke,a thrombus occludes blood flow,leading to irreversible neurological damage (Moskowitz et al.,2010).Early vascular reperfusion is crucial for good clinical outcomes in patients with ischemic stroke (Saver et al.,2016),and endovascular thrombolytic therapy is the most effective treatment available for patients in the acute phase (Kunadian and Gibson,2011;Goyal et al.,2016;Khelif et al.,2018).However,treatment timing is critical for clinical outcomes,which are limited within narrow therapeutic windows (up to 4.5 hours for thrombolysis,between 6 and 24 hours for mechanical thrombectomy;Campbell and Mitchell,2015;Goyal et al.,2015;Hacke,2018;Huang et al.,2018).Thus,a considerable number of patients can only receive supportive treatment for the resulting symptoms,which can lead to lifelong disability (Nogueira et al.,2018).
Cell transplantation for treatment of ischemic stroke is on the agenda (Boltze et al.,2014).Studies have shown that treatment with bone marrow mesenchymal stem cells (BMSCs) is effective for brain damage in rats with acute ischemic stroke,and the mechanism of action is associated with the promotion of neuronal regeneration and functional recovery (Shen et al.,2008;Komatsu et al.,2010;Chau et al.,2018).Furthermore,BMSCs have low immunogenicity and they regulate immune responses and reduce inflammation via interaction with immune cells in both the innate and adaptive immune systems.Indeed,BMSCs can easily expand after transplantation and attach to injured and inflamed sites to promote tissue repair.A previous study reports that BMSCs can protect the brain after cerebral ischemia (Komatsu et al.,2010).However,local microcirculation markedly changes during tissue ischemia and hypoxia,and the deteriorating microenvironment following stroke adversely affects the survival rate of exogenous transplanted stem cells,resulting in low therapeutic efficacy (Li et al.,2014).
As a pleiotropic growth factor and neurotrophic factor,vascular endothelial growth factor (VEGF) plays a prominent role in brain recovery (Chen et al.,2003).In response to acute ischemic stroke,a rapid increase in VEGF and its receptor levels leads to multiple functional outcomes,including decreased neuronal death (Wang et al.,2005),improved angiogenesis (Quittet et al.,2015;Li et al.,2017b),enhanced post-ischemic blood-brain barrier (BBB)integrity (Zhang et al.,2002),and reduced infarct volume (Zechariah et al.,2013) in the peri-infarct region.However,because of VEGF’s short biological half-life,protecting the ischemic brain via continuous injection of purified VEGF protein is difficult (Harrigan et al.,2002).Recent research using an oxygen and glucose deprivation model suggests that VEGF can improve the survival of BMSCsin vitro(Penna et al.,2013).VEGF might promote the survival of MSCs by reducing MSC culture stress,reducing the expression of cell cycle inhibitors,or reducing the expression of p16INKand P21 in MSCs (Pons et al.,2008).Because BMSCs can differentiate into vascular endothelial cells afterVEGFtreatment (Li et al.,2017a),here we hypothesized that engineering BMSCs to over-express VEGF could potentiate the beneficial effect of BMSCs therapy in ischemic stroke.We used an adenovirus as aVEGFgene-expression vector and designed the study to examine whether administration of grafts containingVEGF-expressing BMSCs improves recovery in ischemic stroke beyond what can be achieved with otherwise.The potential mechanisms underlying this cell-based gene therapy strategy was also explored.
Methods
BMSCs culture and transfection with VEGF-GFP adenoviruses
Cryopreserved BMSCs at the Emergency Laboratory of Xuzhou Medical University (Xuzhou,China) (Shen et al.,2022) were recovered and resuspended in α-DMEM (Dulbecco’s Modified Eagle’s Medium;Hyclone Laboratories,Inc.,Logan,UT,USA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin.Afterward,the cells were cultured at 37°C in a humidified incubator (Jiangsu Weihua Medical Instrument Co.,Ltd.,Taizhou,Jiangsu Province,China) with an atmosphere of 5% CO2.Briefly,tibias and femurs of 4-week-old rats were removed and cut along both ends of the diaphysis.Then the bone marrow was rinsed with 10 mL of cell culture medium and collected in a petri dish.BMSCs were identified as cells that adhered to plastic substrates,and which flow cytometry analysis showed were negative for CD11b,CD34,and CD45,and positive for CD29 and CD90.
According to a previous study (Zong et al.,2017),the efficiency of the green fluorescent protein (GFP) reporter gene transduced into BMSCs withVEGFwas more than 80%,with a multiplicity of infection (MOI) of 100.In that study,the BMSCs were infected with the desired gene at an MOI of 100.Briefly,30 μL of GFP reporter gene (Genechem Co.,Ltd.,Shanghai,China)and 30 μL ofVEGF-GFP adenoviruses (Genechem) were transfected into the BMSCs by incubation in DMEM (Hyclone) without fetal bovine serum for 6 hours.The cells were moved to α-DMEM with PBS and incubated for 24 hours before transplantation.The effects of transducing BMSCs with GFP (Ad-BMSCs)or withVEGF-GFP (Ad-VEGF-BMSCs) were detected using a fluorescence microscope (Zeiss,Oberkochen,Germany) after 24 hours of incubation.Western blot analysis was used to assess VEGF protein expression in the BMSCs 24 hours after transduction.
Experimental groups and the middle cerebral artery occlusion model
Animal experiments were approved by the Animal Care and Use Committee of Xuzhou Medical University (approval No.201801A016) on January 19,2018.Male Sprague-Dawley (SD) rats (specific-pathogen-free [SPF] grade),weighing 250–280 g (4–6 weeks old),were selected and purchased from the Experimental Animal Center of Xuzhou Medical University (license No.SYXK(Su) 2015-0030).Four to five rats were kept per ventilated cage to ensure adequate water and food.The indoor temperature of the feeding house was controlled at 20–26°C,humidity was controlled at 40–70%,the noise was less than 60 dB,and a stable 12-hour day/night cycle was provided.The rats were randomly assigned to the following six experimental groups (n=15 per group): sham,middle cerebral artery occlusion (MCAO),DMEM vehicle (MCAO+DMEM),BMSCs (MCAO+BMSCs),Ad-BMSCs (MCAO+Ad-BMSCs),and Ad-VEGF-BMSCs (MCAO+Ad-VEGF-BMSCs).
The MCAO model was induced as previously reported (Quittet et al.,2015).Rats were anesthetized by intraperitoneal injection of 1% pentobarbital sodium (100 mg/kg,Probos Biotechnology Co.,Ltd.,Beijing,China).The left common carotid was separated,and a small incision was made with ophthalmic scissors.Then,the pre-prepared suture (4-0 surgical nylon suture with a distal cylinder,diameter 0.26 mm) was inserted into the incision until resistance was felt after approximately 18–20 mm.At this time,the head of the suture should have reached the middle cerebral artery,and the suture was ligated and fixed.After 90 minutes,the suture was removed,resulting in ischemia/reperfusion injury of the left hemisphere.During the surgery,the core temperature of the rats was kept at 37°C.Rats in the sham group were treated identically,except that the nylon suture was not inserted.
The experimental flow chart and the number of rats used in each stage are shown inAdditional Figure 1and the experimental timeline is shown inAdditional Figure 2.
BMSCs transplantation
Twenty-four hours after ischemia reperfusion,BMSCs were transplanted into the striatum,as previously described (Fukunaga et al.,1999).Briefly,the rats were anesthetized by intraperitoneal injection of 1% pentobarbital sodium and fixed on a stereotaxic apparatus (Shenzhen Reward Life Technology Co.Ltd.,Shenzhen,Guangdong Province,China).A 1.5-cm longitudinal incision was made in the middle of the scalp to expose the skull.The anterior fontanelle was then exposed by cauterizing the periosteum with H2O2.A borehole (1 mm) was then drilled into the skull and a 26-gauge needle with a Hamilton syringe was slowly inserted into the cerebral parenchyma (1.0 mm anterior;2.0 mm left lateral;4.5 mm ventral relative to Bregma based on the atlas;Paxinos and Watson,2006).BMSCs were transplanted into the BMSCs,Ad-BMSCs,and Ad-VEGF-BMSCs groups with a cell density of 1 × 106cells/mL,suspended in 10 μL DMEM medium at 0.5 μL/min.The same injection protocol was used for the DMEM group,but only DMEM medium was injected.Before slowly withdrawing the needle,it was kept in the brain for 5 minutes to avoid backflow.
Behavioral assessment
Behavioral tests were used to evaluate sensorimotor functions in all rats.All tests were conducted by investigators blinded to the objective of the experiment.Standardized behavioral tests were performed 1 day before inducing the strokes (baseline),and 1 and 7 days after BMSCs transplantation.
The Modified Neurological Severity Scores (Chen et al.,2001) (mNSS) scale was adopted to assess neurological deficits.The mNSS evaluates neurological function,including motor function,sensation,reflex,and balance (normal=0,maximal deficit=18).Higher scores reflect more severe neurological deficits.Only rats that scored 0 before MCAO surgery were used in subsequent experiments.
The adhesive removal test (Schallert et al.,2000) was used to evaluate sensorimotor function.In this test,a small piece of sticky tape (0.35 cm ×0.45 cm) was attached in the same way and with the same strength to the center of the right front paw of each rat.Given the chance,normal rats will try to remove the sticky tape.Thus,the rats were placed into a clean cage without litter,and we recorded the time it took to remove the tape,with a maximum of 2 minutes.Less time needed to remove the tape reflects better sensorimotor function.
The cylinder test was used to evaluate preference for paw use and the influence of BMSC transplantation on the asymmetry of right forelimb use(Ahmed et al.,2016).Normal rats explore new environments,and in the case of cylinders,they use their front paws to touch the walls of the cylinder.In this test,we recorded the pattern with which rats explored a transparent cylinder (diameter: 10 cm;height: 15 cm).We observed them for 2 minutes and counted the number of times each forepaw contacted the side wall.The right forepaw-utilization rate was calculated for each animal (number of right paw touches/total number of paw touches × 100%) and averages for each group were obtained.Because the left brain was damaged,higher utilization rate of the right forelimb reflects better motor function recovery after stroke.
Grip strength and motor coordination were evaluated with the hanging wire test (Vaibhav et al.,2013).A metal wire was straightened and hung 60 cm above the ground,with soft cushions placed underneath to prevent rats from injury if they fell.Rat was guided to grab the wire with their forepaws,without the help of the tester.Scores were assigned as follows: 0 points: the rat fell immediately;1 point: the rat hung on the metal wire with its two forepaws;2 points: achieved level 1 and tried to climb the wire;3 points: achieved level 2 and was able to put one or both hind legs on the wire;4 points: the rat grasped the wire with all four paws or wrapped their tail around it.Each rat performed three suspension-wire grip-strength tests,with 5 minutes between attempts.Subsequent statistical analysis used the highest score of each animal from the three tests.Higher scores indicate better motor coordination.
Quantification of infarct volume
Rats were euthanized 7 days after BMSCs transplantation via overdose anesthesia with an intraperitoneal injection of pentobarbital sodium.The brain was quickly removed,and 2-mm thick coronal sections were made using a brain mold (Rayward Life Technology Co.,Ltd.,Shenzhen,China).The fresh brain slices were placed under a fluorescence microscope to observe the survival of GFP-expressing cells.Then they were immersed in 2%2,3,5-triphenyltetrazolium chloride (TTC) (T8877,Sigma-Aldrich,St.Louis,MO,USA) solution at 37°C for 30 minutes,and the infarcted area of each stained section was measured using ImageJ (1.53 k) analysis software (National Institutes of Health,Bethesda,MD,USA;Schneider et al.,2012).The infarct volumes (V,mm3) were calculated by the following formulaV=t(A1+A2+...+An)– t(A1+An)/2,wheretindicates the slice thickness,Aindicates the area of infarction for a slice,and the numbers 1 tonindicate the slice number).
Detection of BBB integrity
Seven days after BMSCs transplantation,rats were euthanized via overdose anesthesia with an intraperitoneal injection of pentobarbital sodium.Then,Evans blue (E2129,Sigma-Aldrich) was injected into the tail veins and circulatedin vivofor 4 hours.Anesthetized rats were then transcardially perfused with normal saline followed by 4% paraformaldehyde.Brains were removed,post-fixed,and cryoprotected with 30% sucrose.A cryostat(Leica) was used to make 20-μm thick brain sections,which were mounted onto slides.Slides were imaged under a ZEISS LSM700 confocal microscope(Zeiss).Evans blue fluorescence intensity,which represents dye leakage,was analyzed using ImageJ software to evaluate the BBB integrity of the rats.All photographs were taken by researchers who did not understand the purpose of the experiment.
Immunofluorescence
Immunofluorescence staining was performed to analyze the expression of microtubule associated protein 2 (MAP2),brain-derived neurotrophic factor(BDNF),and CD31 (Wang et al.,2018) after rats were euthanized via overdose anesthesia with an intraperitoneal injection of pentobarbital sodium.In brief,rats were perfused,brains were processed,and 20-μm-think brain sections were made as described above.Brain slices were then blocked with 10% bovine serum albumin (BSA) for 1 hour,incubated with primary antibody overnight at 4°C,and then incubated with appropriate secondary antibodies (goat anti-rabbit or anti-mouse IgG) in the dark for 2 hours at room temperature.The following primary antibodies were used: rabbit anti-BDNF(1:200,sc-546,Santa Cruz Biotechnology,Santa Cruz,CA,USA),rabbit anti-MAP2 (1:100,17490-1-AP,Proteintech Group) and mouse anti-CD31 (1:200,ab64543,Abcam).Nuclei visualization was achieved via DAPI (Abcam,London,UK) staining.All fluorescent images were acquired under a spectral confocal LSM 700 microscope (Zeiss) and analyzed using ZEN 2011 (blue edition)software (Zeiss).
Western blotting
After rats were euthanized via overdose anesthesia with an intraperitoneal injection of pentobarbital sodium,BMSCs transfected withVEGF-GFP adenoviruses,or brain tissue within 2 mm of the striatal injection site,were collected and homogenized in ice-cold radio immune precipitation assay(RIPA) buffer (pH 7.4,1% Nonidet p-40,0.1% sodium dodecyl sulfate,0.5%sodium deoxycholate,10 mg/mL PMSF [Phenylmethyl sulfony1 fluoride],and 1 mg/mL aprotinin) for western blotting.The supernatant (lysate protein)was collected after centrifugation at 12,000 ×gfor 5 minutes at 4°C and its concentration was measured with a bicinchoninic acid protein assay kit(Beyotime,Shanghai,China).Protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Beyotime)and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore,Billerica,MA,USA).The membranes with protein were blocked with 5%non-fat dried milk (Difco,Franklin Lake,NJ,USA) and then incubated with primary antibodies overnight and then secondary antibodies for 1 hour at room temperature.The primary antibodies were rabbit anti-BDNF (1:1000,sc-546,Santa Cruz Biotechnology),rabbit anti-MAP2 (1:1000,17490-1-AP,Proteintech Group),and mouse anti-CD31 (1:1000,ab64543,Abcam).The secondary antibodies were goat anti-rabbit IgG antibody and goat anti-mouse IgG.Protein signals were detected with electrogenerated chemiluminescence(ECL) assays (Beyotime).Protein band intensities on the blots were analyzed using ImageJ software.Relative protein expression was normalized to β-actin expression.
Morphometric measurement of vasculature
Seven days after transplant,the endothelial cells within the brain slices were detected using anti-CD31 antibody after rats were euthanized via overdose anesthesia with an intraperitoneal injection of pentobarbital sodium.The images obtained by confocal microscopy were processed with Fijian software(ImageJ) for threshold,noise filtering,and binarization to separate the vascular segments.Vascular density was then analyzed using the Fiji Analyze Particles measurement module.Vascular surface-area analysis was performed using the Imaris (Bitplane,Zurich,Switzerland) measurement module.Five coronal brain sections were selected from each group and 3–5 regions of the ischemic ipsilateral cerebral hemisphere were analyzed.Representative images are presented.
Statistical analysis
No statistical methods were used to predetermine sample sizes;however,our sample sizes are similar to those reported in previous publications (Borlongan et al.,2004a).All data are presented as the mean ± SEM.One-way or two-way analysis of variance followed by Student-Newman-Keulspost hoctest were used to compare mean values among the treatments.When only two groups were compared,a Student’st-test was used.SPSS 22.0 (IBM Corp.,Armonk,NY,USA) software was used for analyses,with statistical significance set at aP< 0.05.
Results
Adenovirus carrying the VEGF gene transfects BMSCs and promotes over-expression of VEGF
Twenty-four hours after transfecting BMSCs cultures withVEGF-GFP adenoviruses,we examined cell morphology in bright-field and fluorescence microscopy.We found strong GFP signaling in the BMSCs (Figure 1A).Western blot analysis was used to detectin vitroprotein levels of the transferredVEGF(Figure 1BandC).Analysis indicated that the level of VEGF expression was significantly higher in the Ad-VEGF-BMSCs group than those in the Ad-BMSCs and BMSCs groups (allP< 0.01).
Ad-VEGF-BMSCs improves behavioral deficits in the rat model of ischemic stroke
At baseline,1 day,and 7 days after BMSCs transplantation,the rats were followed-up with a series of behavioral tests.mNSS score was first used to assess neurological deficits.As shown inFigure 2A,the mNSS scores were significantly higher for all rats with stroke than for the sham group (P< 0.05).Compared with the MCAO and DMEM groups,the Ad-BMSCs group exhibited lower mNSS scores on day 7 (P< 0.05),but not on day 1.In contrast,mNSS scores were significantly lower in the Ad-VEGF-BMSCs group than in all other transplantation groups,both on days 1 and 7 (P< 0.05).An adhesion test was performed to compare the sensory function.As shown inFigure 2B,compared with those at baseline and the sham control groups,animals with stroke needed more time to remove the sticky tape (P< 0.05).However,on days 1 and 7,the time required for tape removal in the Ad-VEGF-BMSCstreated animals was significantly lower,especially on day 7 after BMSCs transplantation (P< 0.05).Analysis showed that contralateral paw use was significantly reduced in all animals with stroke compared with those at baseline and in the sham groups (P< 0.05).However,use of the right paw in the Ad-VEGF-BMSCs group was improved on day 7 compared with the BMSCs and Ad-BMSCs groups (P< 0.05;Figure 2C).These behavioral improvements were further confirmed by improved scores on the wire-hanging grasp test on day 7 after BMSCs transplantation,suggesting that the animals treated with Ad-VEGF-BMSCs showed significant improvements in motor coordination function and forelimb strength compared with those treated with BMSCs or Ad-BMSCs (P< 0.05;Figure 2D).
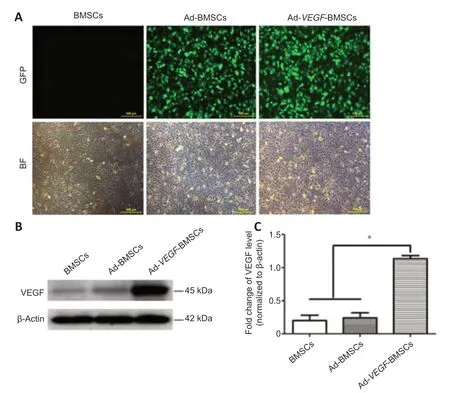
Figure 1|Identification of BMSCs,expression of GFP in BMSCs after transfection,and the western blot results.
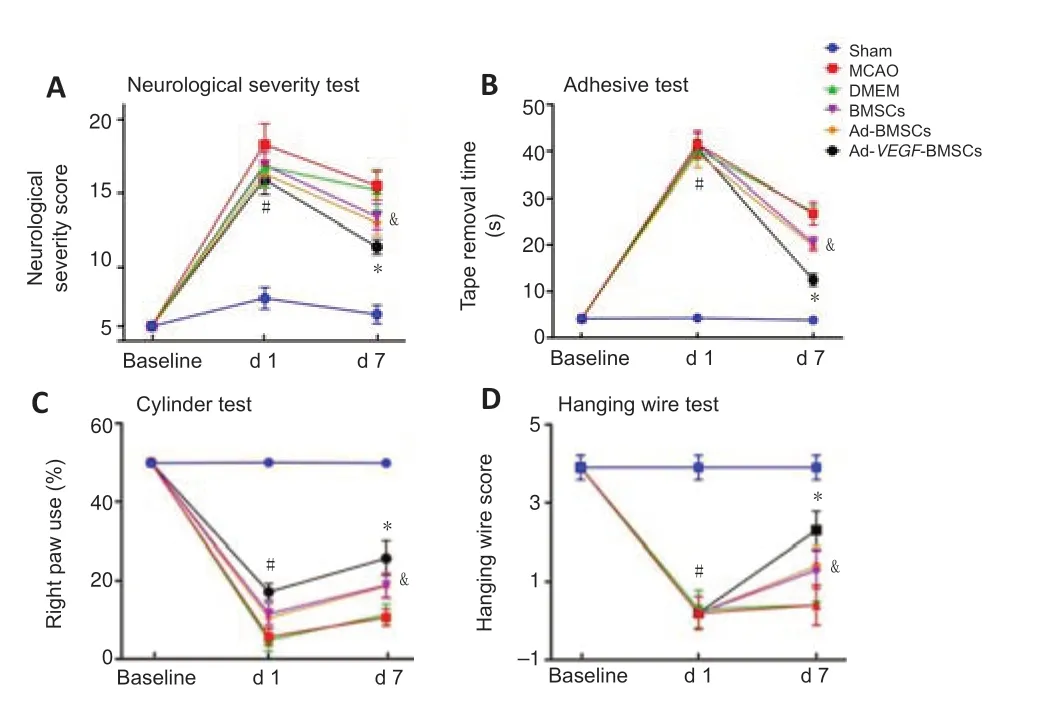
Figure 2|Transplantation of Ad-VEGF-BMSCs improves the behavioral deficits in rats after MCAO.
Ad-VEGF-BMSCs reduces infarct volume in rats
On day 7 after BMSCs transplantation,TTC staining did not reveal any infarcts in sham group.As expected,marked infarcts were observed in the remaining five stroke groups.However,infarct volume was smaller in the BMSCs and Ad-BMSCs groups than in the MCAO and DMEM groups (P< 0.05;Figure 3AandB).Notably,it was even smaller in the Ad-VEGF-BMSCs group than in the BMSCs and Ad-BMSCs groups (P< 0.05).
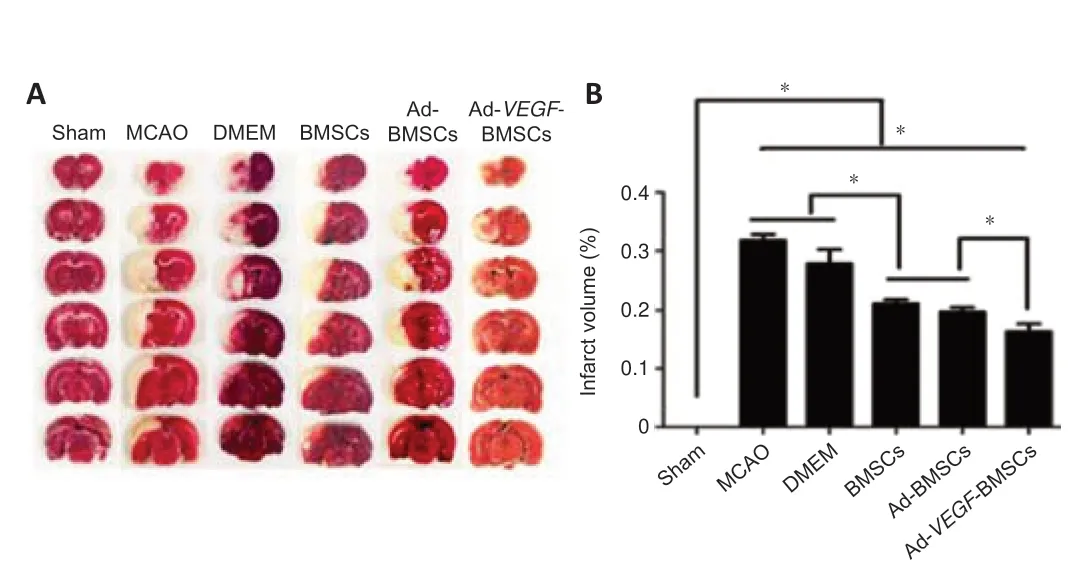
Figure 3|Ad-VEGF-BMSCs transplantation reduces infarct volume in rats after MCAO.
Survival of transplanted Ad-VEGF-BMSCs in vivo
Seven days after BMSCs transplantation,the left striatum and its surrounding area were observed under a fluorescence microscope.The results showed GFP-expressing BMSCs in the left striatum region of the Ad-VEGF-BMSCs group (Figure 4,right panel).In contrast,no GFP signals were detected in the control BMSCs group (Figure 4,left panel).
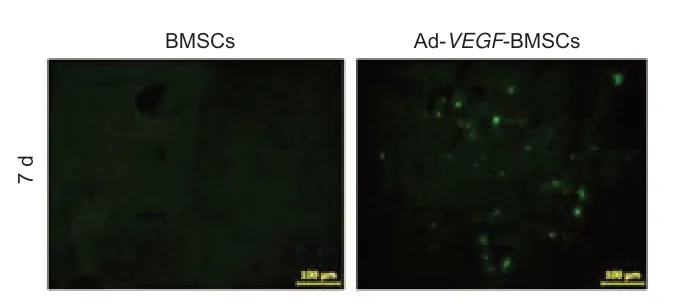
Figure 4|Survival of Ad-VEGF-BMSCs transplanted into the rat brain after middle cerebral artery occlusion.
Ad-VEGF-BMSCs transplantation protects the integrity of the BBB
Stroke is known to induce extensive dysregulation of BBB integrity (Huang et al.,2020;Song et al.,2021).Here,confocal microscopy and fluorescence intensity analyses showed that leakage of Evans blue was significantly lower in the BMSCs and Ad-BMSCs groups than in the MCAO and DMEM control groups (P< 0.05).Intriguingly,Evans blue leakage in the animals injected with Ad-VEGF-BMSCs was significantly less than that in the BMSCs-and Ad-BMSCstreated groups (P< 0.05;Figure 5).
Transplantation with Ad-VEGF-BMSCs preserves vascular morphology
To determine whether Ad-VEGF-BMSCs transplantation promotes angiogenesis,CD31 immunofluorescence staining was used to quantify morphometric changes of the vasculature on day 7 after transplantation.As shown inFigure 6A–C,both vascular surface area and vascular density were significantly higher in the Ad-VEGF-BMSCs-treated group than in the BMSCsand Ad-BMSCs-treated groups (P< 0.05).Vascular morphology was also mildly higher in the BMSCs and Ad-BMSCs groups than in the MCAO and DMEM groups (P< 0.05).Western blot analysis further confirmed the confocal microscopy findings;CD31 was higher in both BMSCs-and Ad-BMSCs-treated groups than in the MCAO and DMEM groups,and even higher in the Ad-VEGF-BMSCs-treated animals (P< 0.05;Figure 6DandE).

Figure 5|Ad-VEGF-BMSCs transplantation protects the integrity of the blood-brain barrier in rats after MCAO.
Transplantation with Ad-VEGF-BMSCs reduces the damage of MAP2
MAP2 is expressed mainly in neuronal dendrites.Immunofluorescence staining showed the degree of MAP2 signaling that remained in the host neurons on day 7 after transplantation (Figure 7A).Compared with the other stroke groups,the Ad-VEGF-BMSCs group exhibited significantly greater MAP2 staining.In addition,this was also true for the Ad-BMSCs and BMSCs groups compared with the DMEM and MCAO groups.Furthermore,we quantified MAP2 expression with western blotting.Analyses confirmed that MAP2 protein levels were significantly higher in the Ad-VEGF-BMSCs group than in the other stroke groups (P< 0.05;Figure 7B).Likewise,compared with those in the MCAO and DMEM groups,MAP2 protein expression levels were significantly greater in the BMSCs and Ad-BMSCs groups (P< 0.05).
Transplantation with Ad-VEGF-BMSCs increases BDNF expression
Immunofluorescence staining and western blot analysis were used to detect the expression levels of BDNF in local brain tissue on day 7 after treatment(Figure 8AandB).BDNF levels in the ischemic hemisphere were highest in the Ad-VEGF-BMSCs-treated group.These levels were significantly higher than those in the Ad-BMSCs or BMSCs groups (P< 0.05),which were moderately and significantly higher than what was observed in the DMEM and MCAO groups (P< 0.05).
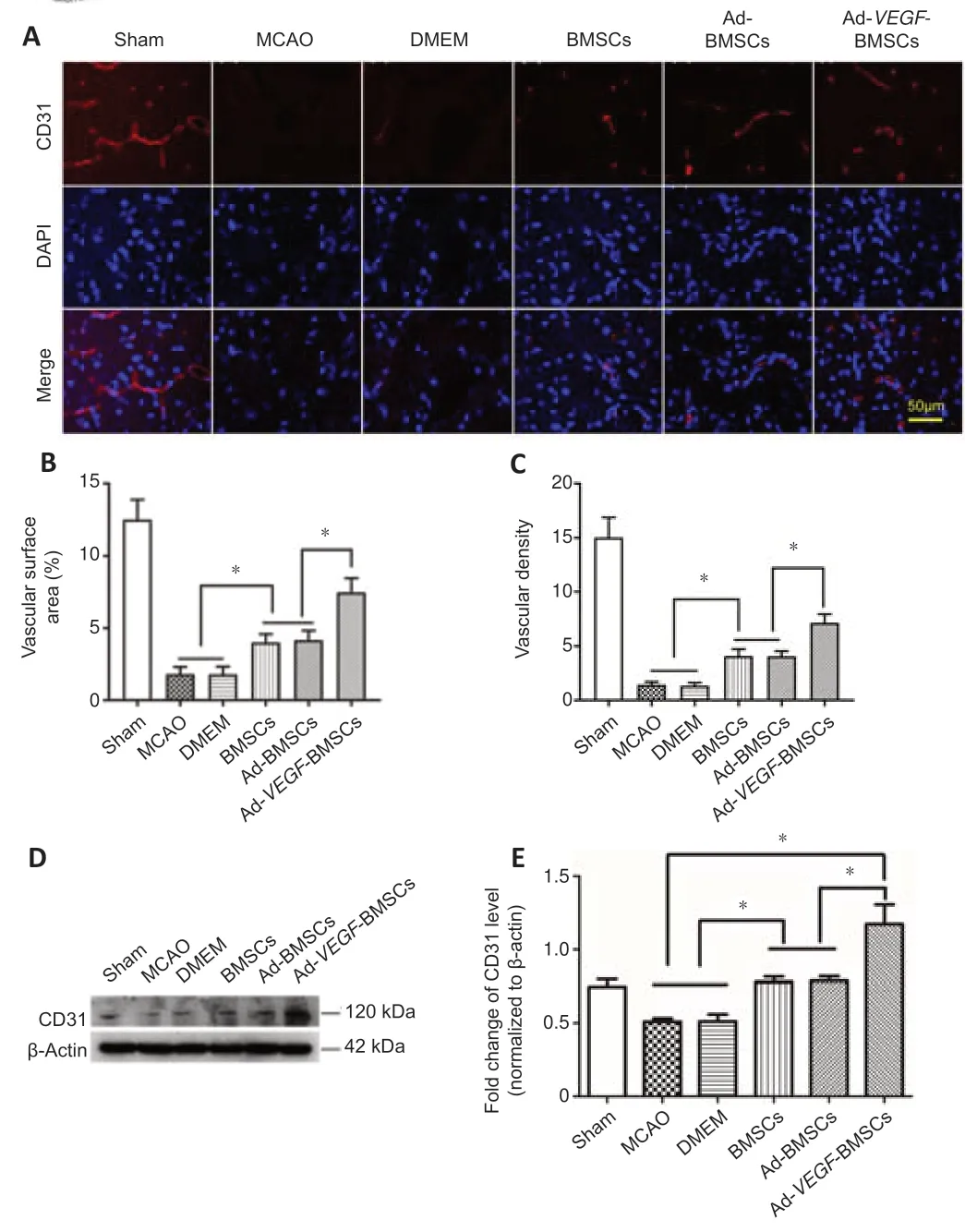
Figure 6|Ad-VEGF-BMSCs transplantation preserves vascular morphology in the brain of rats after MCAO.
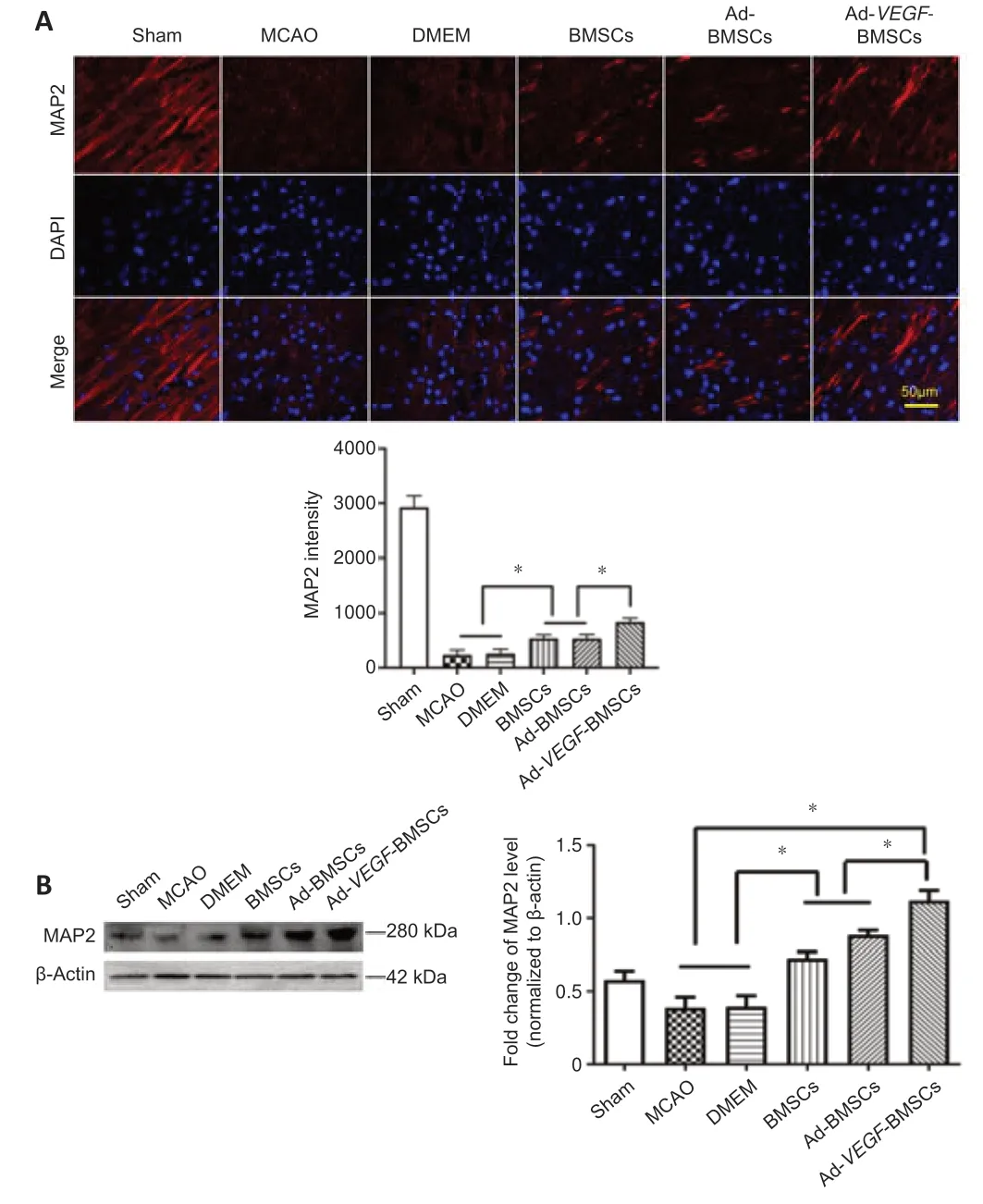
Figure 7|Ad-VEGF-BMSCs transplantation reduces the deficit in MAP2 levels.
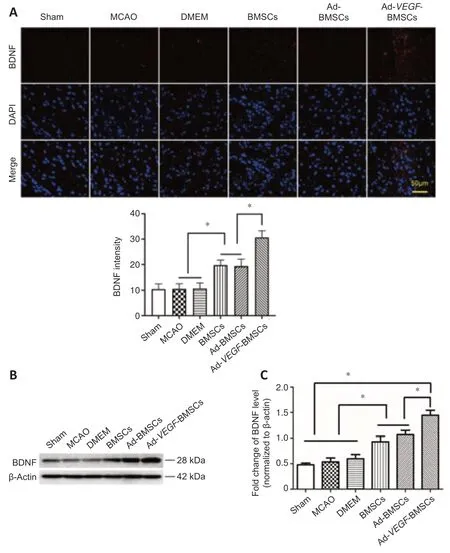
Figure 8|Effect of Ad-VEGF-BMSCs transplantation on BDNF protein expression.
Discussion
In this study,we successfully transfected BMSCs with an adenovirus carryingVEGFto construct Ad-VEGF-BMSCs.By transplanting BMSCs into the brains of rats with ischemic stroke,brain cells can be saved from dying,which improves behavioral recovery.BMSCs are adult stem cells that originate from the mesoderm,and are characterized by self-renewal and multidirectional differentiation (Ahmed et al.,2016).They have the ability to differentiate into neurons,and rejection in allogeneic transplantation is minimal (Chumnanvej and Chumnanvej,2020).Currently,BMSCs are being used in preclinical studies of many neurological conditions,including ischemic stroke (Li et al.,2019).Indeed,there have been many clinical attempts to use BMSCs in stroke treatment (Levy et al.,2019).The routes through which BMSC transplantation are used to treat ischemic stroke include intravascular transplantation (arterial and venous),lateral ventricle transplantation,brain parenchymal directional transplantation,and subarachnoid transplantation (Lalu et al.,2020).Each of these methods has different advantages and disadvantages.For example,intravenous injection of BMSCs seems to be the easiest to perform and is the least pourable,but after the cells pass through the whole body and pulmonary circulatory system,they can arrive at other systems such as the liver,spleen,and lungs,and ultimately few cells can reach the brain (Schmuck et al.,2016).Lateral ventricle transplantation may require more cells and cannot confine the cells to the ischemic penumbra (Maria Ferri et al.,2016).Considering safety and effectiveness,in the current experiment,we injected BMSCs into the striatum to treat rats with ischemic stroke.A large number of animal models and clinical studies in the past have shown that an increase in VEGF after stroke may help ischemic brain tissue to re-establish blood circulation and improve prognoses (Kong et al.,2018;Chan et al.,2020).However,VEGF has a short biological half-life and therapeutic effects cannot be sustained.Considering its role in inducing angiogenesis,VEGF also has the advantage of stimulating neurogenesis and promoting neuron survival(Kanazawa et al.,2019).Therefore,we constructed Ad-VEGF-BMSCsin vitroand transplanted them using a stereotactic method into the penumbra area of the rat brain after acute ischemic stroke.Consistent with previous studies(Penna et al.,2013),BMSCs survival was better afterVEGFtransplantation.We showed that Ad-VEGF-BMSCs transplantation can attenuate poststroke behavioral deficits,limit BBB leakage,preserve vascular morphology(probably partly by promoting angiogenesis),and protect neuronal dendrites.Furthermore,mechanistic study revealed that these effects were due,in part,to the upregulated expression of BDNF.Numerous studies have shown that treatment with BMSCs promotes the recovery of neurological function and reduces infarct volume after acute ischemic stroke (Das and G K,2018;Medeiros et al.,2020).In the current study,treatment with Ad-VEGFBMSCs significantly improved sensory and motor functions beyond what was achieved with BMSCs alone,with similar findings in terms of reduced infarct volume.Thus,transplantation ofVEGF-expressing BMSCs clearly has the highest potential to improve behavioral deficits and limit brain damage after ischemic stroke.
However,the specific mechanism underlying this phenomenon is still unclear.In the current study,we found that transplantingVEGF-expressing BMSCs highly protected the BBB following ischemic stroke.The BBB is composed of pia mater,cerebrovascular,and stellate glial tissue.Under normal circumstances,it can prevent harmful substances from entering the brain(Huang et al.,2020).However,ischemic stroke causes brain damage,changes the morphology of astrocytes around blood vessels in the damaged braintissue,and the tightly connected cells become loose,resulting in damage to the BBB (Song et al.,2021).Since the early 2000s,scholars have proposed that striatal transplantation of BMSCs can restore the damaged BBB.Furthermore,transplantation of BMSCs into the brains of rats with stroke is thought to have a therapeutic effect,likely due to the early repair of the BBB and subsequent blocking of apoptosis,which ultimately allows rescue of the ischemic penumbra (Borlongan et al.,2004a,b).Damage to the BBB after ischemic stroke can lead to cerebral edema and a poor prognosis after stroke(Jiang et al.,2018).In the current study,BMSCs transplantation significantly improved motor behavior and the BBB exhibited limited damage.Therefore,we speculate that the therapeutic effect of BMSCs in rats is related to protection of the BBB,and the subsequent reduction in the amount of toxins that enter the brain,which prevents apoptosis.
In animal models of acute ischemic stroke,increased VEGF expression can promote angiogenesis around the injury (Zechariah et al.,2013).In the current study,we observed neovascularization,as evidenced by the presence of CD31.The increased blood vessel density suggested that BMSCs promote the production of endothelial cells and reconstruction of the blood vessel network.In fact,the two main predisposing factors related to the occurrence of neurological deficits include the reduction of cerebral blood flow and the disruption of the blood-brain barrier in stroke.Therefore,we believe that the protective effect ofVEGF-BMSCs is primarily due to their ability to protect the blood-brain barrier and the promotion of angiogenesis.We think that the benefits of VEGF were likely extended by making the BMSCs self-generate them (avoiding the need for multiple injections),which allowed them to play a long-lasting role in brain protection.
MAP2 is the most abundant microtubule-associated protein,which can regulate cytoskeleton structure and function.It is primarily expressed in dendrites and neuron cell bodies (Doki et al.,2020).After ischemic stroke,the degree of neuronal damage can be detected as an attenuation of MAP2 protein levels (Chen et al.,2020).Therefore,in the current study,we used immunofluorescence and western blotting to detect the expression of MAP2 and thus assess the degree of neuronal damage following ischemic stroke and the various treatments.We found that MAP2 expression was higher in the BMSCs transplantation groups than the MCAO and DMEM groups,and that it was highest in the Ad-VEGF-BMSCs group.Therefore,we speculate that transplantation of BMSCs into the rat brain reduces neuronal damage and promotes the recovery of damaged neurons;VEGF-BMSCs can promote the recovery of blood flow in ischemic brain tissue by producing more VEGF,and thus play a better protective role.BDNF is a growth factor that is closely related to the induction of neuroplasticity (Sasi et al.,2017).For several decades,scientists have believed that BDNF is beneficial to the nervous system (Palasz et al.,2020).A previous study reported that it mediated neurogenesis in the subventricular zone,and its overexpression enhanced subventricular zone neurogenesis after ischemic stroke (Tan et al.,2021).Here,we showed that BDNF expression was higher after BMSCs transplantation,and was even higher when the BMSCs expressed VEGF.On this basis,we can conclude that there are a variety of mechanisms underlying the effects of BMSCs transplantation in treating ischemic stroke,including the secretion of neurotrophic factors that protect neurons,reduce nerve damage,increase neurogenesis,repair the damaged BBB,and promote vascular regeneration.
Furthermore,BrdU injection or co-staining with other cell markers can be used to detect the division and regeneration of other types of cells such as endothelial cells.Then triple-staining with TUNEL could be used to evaluate whether VEGF overexpression increases the survival of transplanted stem cells.Cell culture models can also be used to test whether VEGF overexpression inhibits the inflammatory activation of microglia.
This experiment showed that the transplantation of Ad-VEGF-BMSCs into the brain after ischemic stroke can improve sensory and motor behavior in rats.In terms of the mechanism,although CD31,MAP2,and other indicators partially support the role of BMSCs to promote vascular regeneration and nerve regeneration in the penumbra,the related mechanism through whichVEGFoverexpressing BMSCs further promote brain protection and/or repair need to be studied.For example,how does the timing of transplantation affect the outcome of treatment? (i.e.,brain protection or promotion of nerve and blood vessel regeneration and repair).
In conclusion,our results show that BMSCs transplantation in the treatment of acute ischemic stroke rats can promote the recovery of sensorimotor functions and reduce the area of cerebral infarction,protect BBB integrity,increase the secretion of neurotrophic factors,and preserve brain vasculature.Furthermore,these effects can be enhanced by engineering the BMSCs to overexpressVEGF.Our data suggest that administration of grafts containingVEGF-expressing BMSCs provides better efficacy in facilitating recovery following ischemic stroke than does transplantation with BMSCs alone.
Author contributions:Conceptualization: XMZ;conceptualization,writingreviewing and editing: SQH;writing-original draft preparation,methodology:CL;methodology: ZXY;supervision: DH;data curation: SQZ,DD;formal analysis: YTH;software: HNY.All authors reviewed and approved the final manuscript.
Conflicts of interest:The authors declare that there is no conflict of interest.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Luodan Yang,Louisiana State University in Shreveport,USA;Mohammad Ejaz Ahmed,University of Missouri,USA
Additional files:
Additional file 1:Open peer review reports 1 and 2.
Additional Figure 1:Flowchart of the experiment.
Additional Figure 2:Timeline of the experiment.
- 中国神经再生研究(英文版)的其它文章
- Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
- Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update

