Exosome-transported lncRNA H19 regulates insulin-like growth factor-1 via the H19/let-7a/insulin-like growth factor-1 receptor axis in ischemic stroke
Jue Wang,Bin Cao,Yan Gao,Yu-Hua Chen,Juan Feng,*
Abstract LncRNA (long non-coding RNA) H19 is a transcript of the H19 gene that is expressed during embryogenesis.We previously discovered a role for circular lncRNA H19 in the onset and prognosis of cerebral ischemic stroke.In this study,we used serum from patients with ischemic stroke,and mouse and cell culture models to elucidate the roles of plasma and neuronal exosomes in the regulatory effect of lncRNA H19 on insulin-like growth factor-1 and its mechanism in ischemic stroke,using western blotting,quantitative real-time polymerase chain reaction,and enzyme-linked immunosorbent assays.Plasma exosomal lncRNA H19 was negatively associated with blood levels of insulin-like growth factor-1 in samples from patients with cerebral ischemic stroke.In a mouse model,levels of exosomal lncRNA H19 were positively correlated with plasma and cerebral lncRNA H19.In a cell co-culture model,we confirmed that lncRNA H19 was transported from neurons to astrocytes by exosomes to induce downregulation of insulin-like growth factor-1 through the H19/let-7a/insulin-like growth factor-1 receptor axis.This study provides the first evidence for the transportation of lncRNA H19 by exosomes and the relationship between lncRNA H19 and insulinlike growth factor-1.
Key Words: cerebral ischemia;exosomes;H19;insulin-like growth factor-1;insulin-like growth factor 1 receptor;ischemic stroke;long non-coding RNA
From the Contents
Introduction 1316
Methods 1317
Results 1318
Discussion 1318
Introduction
Ischemic stroke has been shown to be caused by the interaction of environmental and genetic factors,and recent studies have revealed a series of genetic markers for this disorder (Amouyel,2012).TheH19 gene is a highly conserved imprinting gene (Wang and Qi,2021) that is involved in embryonic development and growth control,and can be transcribed into a long noncoding RNA (lncRNA).LncRNAs are RNAs longer than 200 nucleotides that cannot be translated,but which regulate protein expression by acting as mediators of signaling pathways,molecular decoys,molecular guides for transcriptional co-activators,and scaffolds for functional complex formation(Poller et al.,2018).LncRNA H19 is highly expressed during the embryonic period and levels fall rapidly after birth,and it only occurs in the skeletal muscle and heart in healthy adults (Gabory et al.,2010).We previously demonstrated lncRNA H19 expression in the plasma in ischemic stroke patients,and elucidated its gene polymorphism and function in regulating neuronal autophagy,neurogenesis,and microglial polarization,using animal and cellular ischemic stroke models (Wang et al.,2017a,b,2019).
Exosomes are vesicles with a diameter of 30 to 100 nm,which help transport molecules between cells (Dolz et al.,2017).Brain tissue can secret exosomes and plasma exosome levels can be upregulated by cerebral ischemic stroke (Chen et al.,2017) Exosomes play a role in the onset and prognosis of cerebral ischemic stroke by carrying substances,and may thus be a potential interference target for treating ischemic stroke (Ni et al.,2020).The molecules present in exosomes may reflect certain cerebral functions (Werner and Stevens,2015).Non-coding RNAs transported by exosomes (e.g.,miR-9,miR-124,and miR-223) have been related to neurological defects,infarct volume,and prognosis in ischemic stroke patients (Ji et al.,2016).Moreover,exosomes secreted by some stem cells may protect against cerebral ischemic stroke by transporting certain essential substances (e.g.,miR-124 and miR-17-92) (Yang et al.,2017;Ni et al.,2020),making exosomes a promising target for interfering with the substances that they transport.
Insulin-like growth factor-1 (IGF1) is a hormone and paracrine growth factor.Under normal conditions,IGF1 is expressed by cells in the central nervous system and can enter the circulation through the blood-brain barrier (Fernandez and Torres-Aleman,2012;Pharaoh et al.,2020).IGF1 is involved in the development of the nervous system,synaptic plasticity,and the formation of cognitive functions (Frater et al.,2018).IGF1 also performs important regulatory functions during acute brain injury,and the use of IGF1 after cerebral ischemia could decrease the infarct volume (Sun et al.,2012;Baltazar-Lara et al.,2020).A protective role for IGF1 has also been confirmed in several clinical studies of ischemic stroke,which reported an inverse relationship between plasma IGF1 levels and the risk of ischemic stroke,and showed that reduced IGF1 levels were an independent risk factor for ischemic stroke (Aberg et al.,2020).However,the mechanisms responsible for the decrease in IGF1 during cerebral ischemic stroke remain unclear.IGF1 receptor (IGF1R) is a tyrosine kinase receptor that binds to IGF1 and initiates downstream signal transduction pathways,including phosphatidylinositol-3-kinase and mitogen-activated protein kinase pathways,to induce IGF1-mediated functions (Russo et al.,2005).IGF1 can be regulated by IGF1R,and increased IGF1R helps to decrease circulating IGF1 levels and attenuate its entry into the central nervous system.IGF1 production is attenuated in line with decreased access of circulating growth hormone from the peripheral to the central nervous system (Genis et al.,2014).There is also a compensatory mechanism between serum IGF1 and IGF1R levels,whereby decreased IGF1 levels could induce an increase in IGF1R levels,and vice versa.
Let-7 is one of the earliest discovered microRNAs,with functions in many pathophysiological processes including cell differentiation,migration,and apoptosis.LncRNA H19 could regulate several proteins through sponging let-7 to form competing endogenous RNA regulatory patterns (Cao et al.,2019).Therefore,we considered that lncRNA H19 might induce the regulation of IGF1 through interacting with let-7.
We previously found that neuronal lncRNA H19 could be upregulated by oxygen glucose deprivation/reperfusion (OGD/R) (Wang et al.,2017b).It has also been confirmed that IGF1R was expressed in astrocytes (Sun et al.,2012).In this study,we used clinical samples,a middle cerebral artery occlusion and reperfusion (MCAO/R) mouse model,and a neuron,astrocyte,and co-culture cellular OGD/R model to examine the relationship between lncRNA H19 and IGF1.Our previous studies examined lncRNA H19 at the fundamental and clinical levels,and the current study further explored a promising interference pathway for lncRNA H19 in the regulation of IGF1 via its carrier exosomes.This study aimed to verify the role of exosome-transported lncRNA H19 in regulating IGF1 via the H19/let-7a/IGF1R axis in cerebral ischemic stroke.
Methods
Study participants
The study population included 80 patients with acute ischemic stroke and 80 healthy control subjects recruited from Shengjing Hospital of China Medical University between May 1,2019 and May 31,2020.Acute ischemic stroke was diagnosed according to the following eligibility criteria (Rabinstein,2020):clinical symptoms and signs of focal or global cerebral function loss and new cerebral infarction in the clinically relevant cerebral areas identified by magnetic resonance imaging examination.Healthy controls with no chronic diseases were recruited by advertisement.Two neurologists unaware of the patients’ conditions were responsible for the diagnosis of cerebral ischemic stroke.Patients with kidney disease,coronary artery disease,circulation disorders,or autoimmune disease were excluded.The study was approved by the Ethics Committee of Shengjing Hospital and China Medical University on March 5,2018.All the participants provided signed informed consent.
Extraction of exosomes from plasma
Venous blood was collected from the patients and controls at three time points (immediately,and 7 and 30 days after hospitalization).Plasma was centrifuged at 3000 ×gfor 15 minutes at 4°C.ExoQuick Solution (01.SBI.EXOQ5A-1,Millipore Sigma,Burlington,MA,USA) was added to the supernatant in a new tube,followed by incubation at 4°C for 30 minutes,according to the manufacturer’s protocol,and centrifugation at 1500 ×gfor 30 minutes.The exosomes in the pellet were then obtained by centrifuging for an additional 5 minutes,resuspended in phosphate-buffered saline (PBS),and identified by testing for the expression of markers (Wang et al.,2022).
MCAO/R mouse model
All procedures involving animals were approved by the Ethics Committee of Shengjing Hospital and China Medical University on March 5,2018 and were performed according to the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.Male C57BL/6J mice (3 months old,21–23 g,6 per group) were treated with MCAO for 45 minutes followed by reperfusion for 24 hours.A 4-0 monofilament nylon suture (Beijing Sunbio Biotech Co.Ltd;Beijing,China) with a rounded tip was inserted into the internal carotid artery through the external carotid artery stump,and gently advanced to occlude the right middle cerebral artery.After 45 minutes,the suture was removed to restore blood flow.Sham-operated mice were manipulated in the same manner but without MCAO.The exosome blocker GW4869 (50 μM,Invitrogen,Carlsbad,CA,USA) and H19 small interfering RNA (siRNA) (100 μM,GenePharma,Suzhou,Jiangsu Province,China) were used to inhibit the secretion of exosomes and the expression of lncRNA H19,respectively.GW4869,H19 siRNA,and normal control siRNA(GenePharma) were administered via intracerebroventricular injection,as reported previously (Wang et al.,2017b).SiRNA (100 μM) mixed gently with Lipofectamine RNAiMAX Transfection Reagent (Invitrogen) and incubated at room temperature for 20 minutes,or GW4869 (50 μM),were injected into the right cerebral ventricle of mice (x=1.0 mm,y=0.8 mm,z=1.5 mm) prior to MCAO.All animals were housed in a temperature-controlled room at 25± 1°C after recovery from anesthesia using 2.5% sevoflurane (Invitrogen) via an animal ventilator (Kent Scientific,Torrington,CT,USA).Rectal temperature was controlled at 37.0 ± 0.5°C during and after surgery using a temperatureregulated heating pad (CMA 150;Carnegie Medicin,Stockholm,Sweden).
Mice in each group (n=4) were anesthetized by intraperitoneal injection of 3% pentobarbital (30 mg/kg;Invitrogen) 24 hours after reperfusion and then decapitated.Plasma,plasma exosomes,and brain tissue were collected from the ischemic penumbra.The brains were cut into 2-μm-thick coronal sections,stained with 1% 2,3,5,-triphenyltetrazolium chloride solution (Invitrogen) for 30 minutes at 37°C,incubated in 4% paraformaldehyde (Invitrogen) overnight,and then transferred into saline.Six images per group were captured using an MCID computer imaging analysis system (Image ProPlus software v.6.0,Media Cybernetics,Rockville,MD,USA) and observed by a person unaware of the treatment and grouping.
Neuron and astrocyte culture
The neuronal cell line CATH.a and astrocytic cell line C8-D1 were obtained from the American Type Culture Collection (Manassas,VA,USA).Neurons were cultured in RPMI 1640 medium containing 10% fetal bovine serum(HyClone,Logan,UT,USA) and astrocytes were cultured in Dulbecco’s modified Eagle medium (HyClone) supplemented with 10% fetal bovine serum (HyClone).All the cells were cultured in a humidified atmosphere of 5%CO2/95% air at 37°C.
Astrocyte co-cultures with neuronal exosomes
Astrocytes were cultured in a 24-well plate (2.5 × 105cells per well).Neurons(3.0 × 105) were cultured in a culture pool that matched the 24-well plate.After attachment of the astrocytes to the plate,neurons cultured in the pool (challenged with OGD/R) or exosomes collected from neurons (treated with OGD/R) were transferred to the 24-well plate and co-cultured with astrocytes for 24 hours for the following experiments.For separation of neuronal exosomes,the culture medium of neurons treated with OGD/R was collected and centrifuged at 3000 ×gfor 10 minutes.Sequential supernatants were then collected and centrifuged at 2000 ×gfor 10 minutes,10,000 ×gfor 30 minutes,and 10,000 ×gfor 70 minutes,twice.The pellet was finally resuspended in 100 μL of PBS and stored at-80°C until further use.
OGD/R treatment of cells
We investigated the cell type expressing IGF1R using a neuron and astrocyte OGD/R model.CATH.a cells in the OGD/R group were cultured in an ischemiamimetic solution (140 mM NaCl,3.5 mM KCl,0.43 mM KH2PO4,1.25 mM MgSO4,1.7 mM CaCl2,5 mM NaHCO3,20 mM HEPES,pH 7.2–7.4) (HyClone)in a hypoxic incubator chamber (Billups-Rothenberg,Del Mar,CA,USA)filled with 95% N2/5% CO2for 4 hours at 37°C.After OGD/R,the cells were transferred to normal culture medium (RPMI 1640 medium containing 10%fetal bovine serum;HyClone) and kept in an incubator with 5% CO2at 37°C for 24 hours for reperfusion and subsequent treatments.For the isolation of exosomes,culture medium (RPMI 1640 medium containing 10% fetal bovine serum;HyClone) from neurons treated with OGD/R was collected and centrifuged at 3000 ×gfor 10 minutes.Sequential supernatants were then collected and centrifuged at 2000 ×gfor 10 minutes,10,000 ×gfor 30 minutes,and 10,000 ×gfor 70 minutes twice.The pellet was finally resuspended in 100 μL of PBS and stored at-80°C until further use.
Astrocytes were cultured under normal conditions (without OGD/R) or subjected to OGD/R (OGD/R-A group).Cells in the OGD/R-A/exo and OGD/R-A/exo-H19 siRNA groups were co-cultured with neuronal exosomes or exosomes secreted by H19 siRNA-treated neurons,respectively,before OGD/R.To clarify the role of exosomes and lncRNA H19 in regulating the infarct volume induced by ischemic stroke,we inhibited exosome secretion using the exosome inhibitor GW4869,H19 siRNA to inhibit lncRNA H19,and let-7a inhibitor to decrease the level of let-7a,respectively.The neuronal cell line CATH.a was transiently transfected with H19 siRNA (GenePharma) using Lipofectamine RNAi MAX (Invitrogen).Briefly,after culturing in normal medium for 24 hours,the cell culture medium was replaced by normal medium plus H19 siRNA/Lipofectamine RNAi MAX complex at 1.6 μmol/L.Cells were incubated for a further 24 hours in a humidified incubator.
Quantitation of lncRNA H19 and let-7a expression levels
LncRNA H19 levels were measured by quantitative real-time polymerase chain reaction (qRT-PCR).Plasma lncRNA H19 and exosomal lncRNA H19 were examined in blood samples from patients with ischemic stroke and controls and in plasma,plasma exosomes,and brain tissue of MCAO mice.Total RNA was extracted using Trizol reagent (Invitrogen) and cDNA was prepared using a Superscript III reverse transcriptase kit (Invitrogen).PCR was performed using a StepOne sequence detection system (Applied Biosystems,Carlsbad,CA,USA) with SYBR Green qPCR Master Mix (Fermentas,Burlington,Canada).All procedures were carried out according to the manufacturers’ instructions.The qRT-PCR conditions were as follows: 95°C for 2 minutes denaturation,followed by 40 cycles of 95°C for 15 seconds,and 60°C for 30 seconds.The following primers were used: lncRNA H19,5′-CAT TAC TAA ACC CGT AGA TCC GAT-3′ (forward) and 5′-TAT GGT TGT TCT CGT CTC CTT CTC-3′ (reverse);let-7a,5′-GAC ATG GCG TCG ATC GTA CCT AG-3′ (forward) and 5′-ATC GGA TCG TAT AGT GTA CTA GTC-3′ (reverse);β-actin,5′-CTC CCA TTC CTC CAC CTT TG-3′ (forward),and 5′-CCA CCA CCC TGT TGC TGT AG-3′ (reverse).The relative expression ratios of lncRNA H19 and let-7a mRNA were normalized to β-actin gene expression using the ∆Ct method (2–∆∆Ct) (Wang et al.,2017a).
Quantitation of plasma IGF1 levels in patients
Plasma was isolated from stroke patients and healthy control subjects and IGF1 concentrations were determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit (ab108873,Abcam,Cambridge,MA,USA)according to the manufacturer’s protocol.
Western blotting
Total protein was extracted from C8-D1 astrocytes.Cell lysates were subjected to 10% to 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the separated proteins were transferred to a nitrocellulose membrane(Millipore Sigma),blocked with 5% skimmed milk powder in 0.1% Tween 20 (Ding Guo,Bejing,China) in Tris buffer solution for 1 hour at room temperature,and incubated overnight at 4°C with the following antibodies:rabbit monoclonal IGF1R antibody (1:1000,ab 182408;Abcam) and mouse monoclonal β-actin antibody (1:2000,sc81178;Santa Cruz Biotechnology,Santa Cruz,CA,USA),followed by incubation with a horseradish peroxidaseconjugated antibody (goat anti-rabbit IgG,1:2000,ab 150077;Abcam;goat anti-mouse IgG,1:2000,ab 150133;Abcam).Immunoreactive bands were visualized using a chemiluminescence kit (Millipore Sigma).The integrated density value for each band was calculated using a computer-aided image analysis system (Fluor Chen 2.0;Bio-Rad,Hercules,CA,USA).
Statistical analysis
Data are presented as the mean ± standard deviation.Mean differences were evaluated by one-way analysis of variance followed by Tukey’spost hoctest or Student’st-test using SPSS software (version 17.0;SPSS Inc.,Chicago,IL,USA).Correlations were determined using Pearson’s correlation analysis.P< 0.05 was considered statistically significant.
Results
Plasma lncRNA H19 is negatively correlated with IGF1 levels in patients with cerebral ischemic stroke
Basic information on the included participants is listed inTable 1.
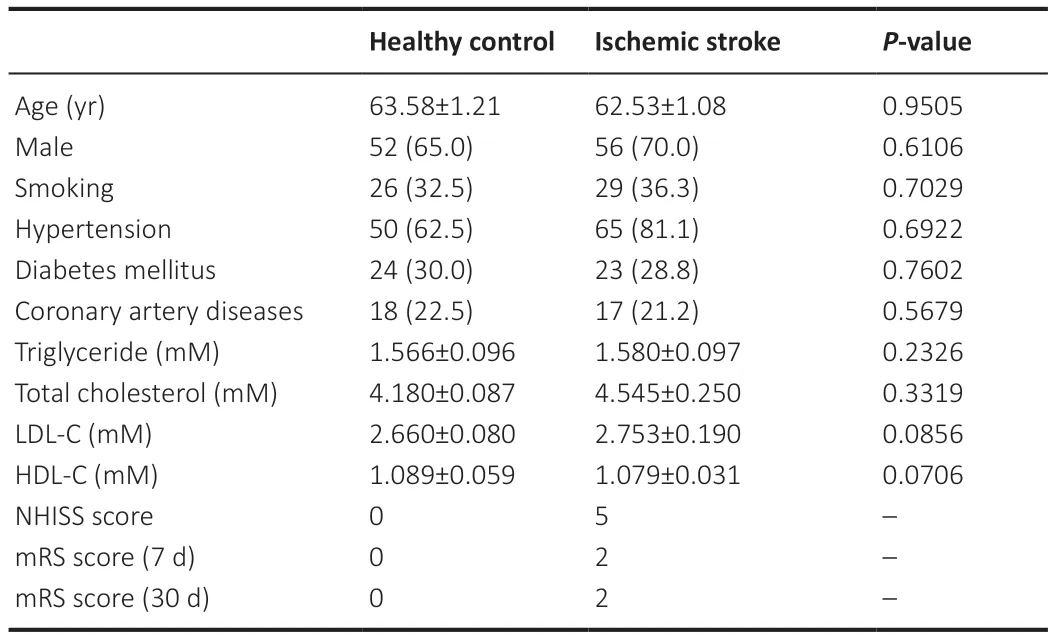
Table 1|Baseline data and mRS score at 7 and 30 days after onset of the included participants
We measured plasma IGF1 concentrations using ELISA.IGF1 levels were significantly downregulated in plasma from ischemic stroke patients compared with healthy controls (P< 0.05;Figure 1A).In addition,plasma lncRNA H19 levels were negatively correlated with IGF1 levels (r=-0.3812,P< 0.0001;Figure 1B).
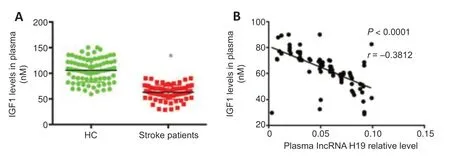
Figure 1|IGF1 expression in plasma of ischemic stroke patients and its relationship with plasma exosomal lncRNA H19 levels at disease onset.
Exosomes may be carriers for transporting lncRNA H19 from brain to plasma
Plasma and plasma exosomal lncRNA H19 levels were both significantly upregulated in ischemic stroke patients compared with the control group(P< 0.05;Figure 2AandB).Plasma exosomal lncRNA H19 was positively correlated with the National Institutes of Health Stroke Scale (NIHSS) and Modified Rankin Scale (mRS) scores 7 and 30 days after disease onset (r=0.5913,0.7494,0.7068,P< 0.0001,respectively;Figure 2C–E).
Plasma exosomal lncRNA H19 levels were positively associated with plasma lncRNA H19 in patients with cerebral ischemic stroke (r=0.7194,P< 0.0001;Figure 2F),indicating that plasma lncRNA H19 might be transported by exosomes.Expression of lncRNA H19 was also upregulated in the penumbra,
plasma,and plasma exosomes of MCAO/R mice compared with the normal control group (Figure 2G–I).To clarify the origin of the circular lncRNA H19,we analyzed the correlation between lncRNA H19 levels in plasma and plasma exosomes and brain tissue from MCAO/R mice.Plasma lncRNA H19 levels were positively correlated with its expression levels in both brain tissue and plasma exosomes in MCAO/R mice (r=0.4606,P=0.0152 inFigure 2J;r=0.6085,P=0.0028 inFigure 2K),indicating that exosomes might act as a carrier for transporting lncRNA H19 from the brain to the blood.
Cerebral inhibition of exosomes or lncRNA H19 reverses the upregulation of plasma lncRNA H19 and IGF1 induced by cerebral ischemic stroke
Intraventricular injection of GW4869 or H19 siRNA significantly reduced the infarction induced by ischemic stroke in a mouse model (Figure 3AandB).GW4869 abolished the upregulation of plasma lncRNA H19 in mice with MCAO/R (Figure 3C),and inhibition of exosomes or lncRNA H19 reversed the attenuation of plasma IGF1 (Figure 3D).
Exosomes transport lncRNA H19 from neurons to astrocytes to decrease let-7a and increase IGF1R
OGD/R induced an increase in IGF1R in astrocytes but not in neurons (Figure4A–D),while lncRNA H19 was upregulated by OGD/R in neurons but not in astrocytes (Figure 4EandF).We then examined the expression of lncRNA H19,let-7a,and IGF1R in astrocytes,OGD/R astrocytes,OGD/R neuronal exosome astrocyte co-culture,and OGD/R neuronal exosome (H19 siRNA)astrocyte co-culture groups.OGD/R treatment had no effect on lncRNA H19 levels in astrocytes,while neuronal exosomes transported lncRNA H19 from neurons to astrocytes,and neuronal lncRNA H19 inhibition decreased the expression of lncRNA H19 in the co-culture system (Figure 4G).Let-7a levels were negatively associated with lncRNA H19 levels (Figure 4H).We also examined the expression of IGF1R in the above groups by western blot.Neuronal exosomal lncRNA H19 induced the upregulation of IGF1R in OGD/R astrocytes,and inhibition of lncRNA H19 abolished the increase of IGF1R(Figure 4IandJ).
Neuronal exosome lncRNA H19 affects astrocyte IGF1R expression by regulating let-7a under OGD/R treatment
The addition of neuronal exosomes to astrocytes promoted the upregulation of IGF1R expression under OGD/R treatment.OGD/R had no further effect on IGF1R levels in the co-culture of astrocytes with exosomes secreted by lncRNA H19-inhibited neurons;however,the production of IGF1R was markedly upregulated when the expression of let-7a and lncRNA H19 were inhibited simultaneously (Figure 5AandB).
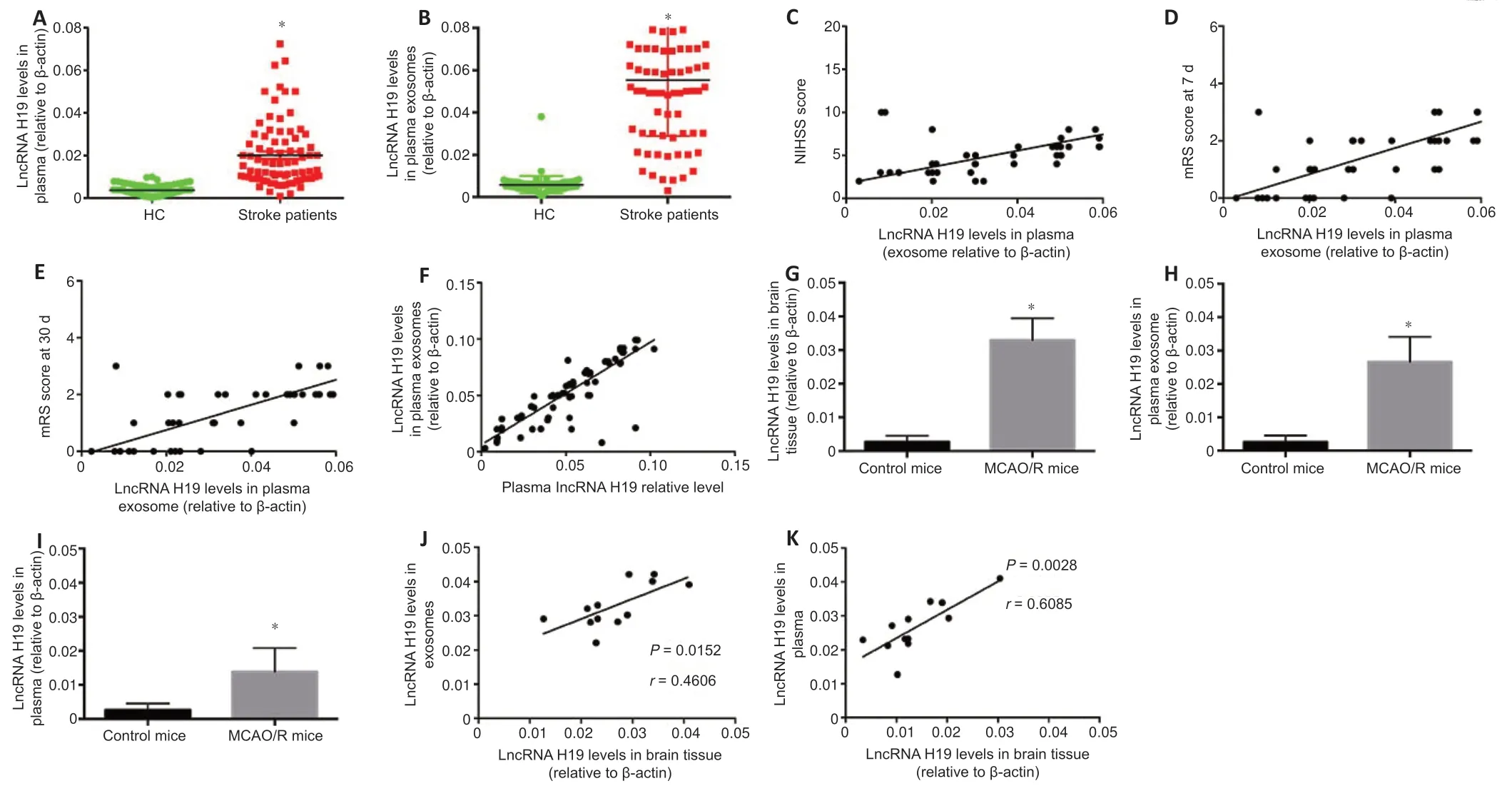
Figure 2|LncRNA H19 expression in plasma and plasma exosomes of patients with cerebral ischemic stroke and in ischemic brain tissue,plasma exosomes,and plasma in mice.
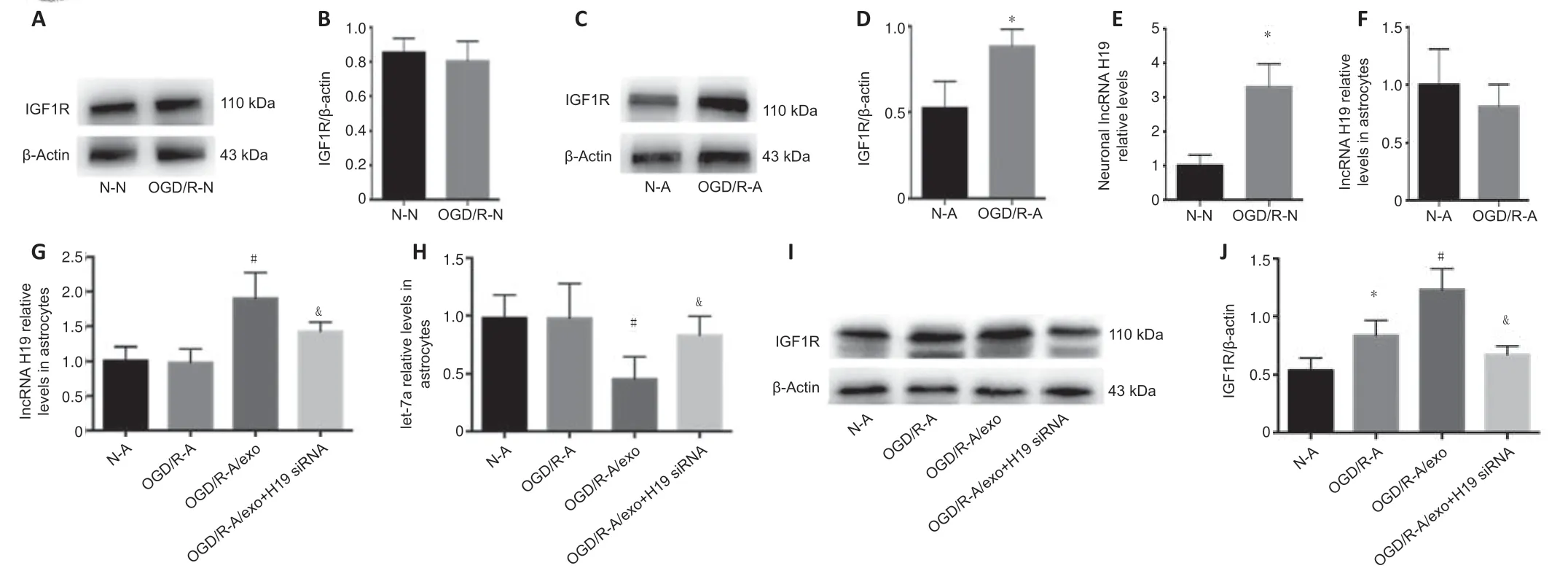
Figure 4|Expression of IGF1R and lncRNA H19 in neurons and astrocytes treated by OGD/R,and effect of neuronal IGF1R and lncRNA H19 inhibition on levels of astrocytic IGF1R and lncRNA H19.
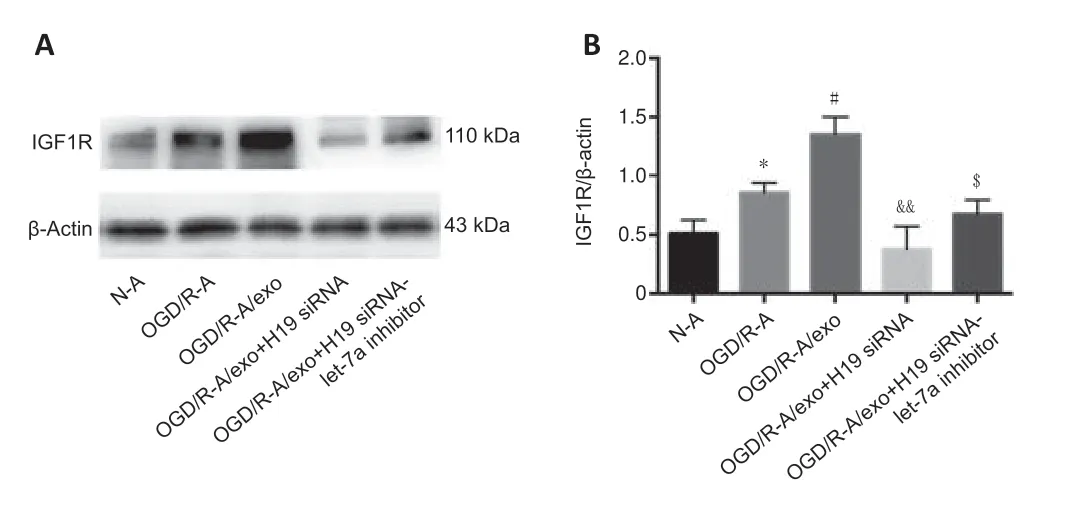
Figure 5|Effects of lncRNA H19 and let-7a inhibition on expression of IGF1R.
Discussion
lncRNA H19 plays a role in hypoxia,hypertension,and atherosclerosis.We confirmed that upregulation of lncRNA H19 in the plasma of patients with cerebral ischemic stroke was positively associated with neurological function defects and disease severity (Wang et al.,2017a),indicating the significant clinical value of lncRNA H19 in cerebral ischemic stroke.However,the origin of plasma lncRNA H19 and the mechanism by which it induces cerebral ischemic stroke remain unclear.In this study,we revealed exosomes as carriers of lncRNA H19 and demonstrated that lncRNA H19 induced its effect via the H19/let-7a/IGF1R axis.
The present study showed that exosomal lncRNA H19 was upregulated in the plasma of ischemic stroke patients and was positively associated with plasma lncRNA H19 levels.We considered that exosomes in the plasma might be released from the brain during ischemic stroke,given that the blood–brain barrier may be damaged during ischemic stroke,resulting in the leakage of substances from the brain into the circulation.We confirmed our hypothesis using the MCAO/R mouse model,and determined that plasma exosomal lncRNA H19 levels were positively associated with lncRNA H19 levels in ischemic brain tissue and plasma,indicating that exosomes might transport lncRNA H19 from the brain to the plasma.
The function of plasma exosomal lncRNA H19 in ischemic stroke patients needs further elucidation.The current results showed that plasma exosomal lncRNA H19 levels were positively related to the patients’ NIHSS scores,suggesting that lncRNA H19 was associated with neurological function deficits.Moreover,lncRNA H19 was positively related to the mRS scores 7 and 30 days after disease onset,indicating that exosomal lncRNA H19 played a role in the prognosis of patients with ischemic stroke and was consistent with the function of plasma lncRNA H19 (Wang et al.,2017b).
IGF1 is regarded as a protective factor against ischemic stroke,and it has been shown to be downregulated in the plasma of ischemic stroke patients.However,the mechanism of IGF1 attenuation in plasma is unknown (Aberg et al.,2020;Hayes et al.,2021).We verified that plasma IGF1 levels were decreased in ischemic stroke patients,and found a negative relationship between plasma IGF1 and lncRNA H19 levels.The mechanism by which lncRNA H19 regulates IGF1 is uncertain.Using a mouse model of cerebral ischemic stroke,we found that an exosome inhibitor prevented the regulatory effect of lncRNA H19 on IGF1R,thus indicating a role for exosomes in the regulatory effect of lncRNA H19 on IGF1R.In addition,lncRNA H19 levels were upregulated in cultured neurons but not astrocytes,IGF1R was upregulated in astrocytes but not neurons,and lncRNA H19 expression was upregulated in astrocytes co-cultured with exosomes secreted from neurons treated with OGD/R.These results confirmed that exosomes transported lncRNA H19 from neurons to astrocytes to induce the upregulation of IGF1R in astrocytes under OGD/R.
LncRNA H19 has recently been reported to act as a molecular sponge to inhibit let-7 bioactivity (Cao et al.,2019).Let-7 targets IGF1R and inhibits its expression by complementary binding to specific sequences,resulting in translational repression and mRNA degradation (Fabian and Sonenberg,2012;Ma et al.,2021).The current study confirmed that lncRNA H19 regulated the expression of IGF1R through let-7a.
This study had some limitations.The number of patients included in the study was small,which could cause selection bias.Furthermore,the mechanism of IGF1 downregulation induced by lncRNA H19 in the plasma of ischemic stroke patients is still uncertain,and further research is needed to confirm our hypothesis.
In conclusion,this study provides the first evidence showing that exosomal lncRNA H19 is upregulated in the plasma of ischemic stroke patients,and that this upregulation is positively related to the severity of neurological function deficits and inversely correlated with plasma IGF1 levels.We also supposed that lncRNA H19 regulated IGF1 through let-7a.These results highlight a possible mechanism by which lncRNA H19 may induce cerebral ischemic stroke,and provides a foundation for the clinical targeting of lncRNA H19 expression by interfering with its exosome carriers.
Author contributions:JW designed the experiment;JW and BC performed the experiment and collected the data;JW and YG wrote the manuscript;YHC and JF supervised the experiment.All authors approved the final manuscript.
Conflicts of interest:The Authors declare that there is no conflict of interest.Editor note: JF is an Editorial Board member of Neural Regeneration Research.She was blinded from reviewing or making decisions on the manuscript.Thearticle was subject to the journal’s standard procedures,with peer review handled independently of this Editorial Board member and their research groups.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative Commons Attribution Non Commercial-Share Alike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
- Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update

