A molecular probe carrying anti-tropomyosin 4 for early diagnosis of cerebral ischemia/reperfusion injury
Teng-Fei Yu,Kun Wang,Lu Yin,Wen-Zhe Li,Chuan-Ping Li,Wei Zhang,Jie Tian,,Wen He,
Abstract In vivo imaging of cerebral ischemia/reperfusion injury remains an important challenge.We injected porous Ag/Au@SiO2 bimetallic hollow nanoshells carrying anti-tropomyosin 4 as a molecular probe into mice with cerebral ischemia/reperfusion injury and observed microvascular changes in the brain using photoacoustic imaging with ultrasonography.At each measured time point,the total photoacoustic signal was significantly higher on the affected side than on the healthy side.Twelve hours after reperfusion,cerebral perfusion on the affected side increased,cerebrovascular injury worsened,and anti-tropomyosin 4 expression increased.Twenty-four hours after reperfusion and later,perfusion on the affected side declined slowly and stabilized after 1 week;brain injury was also alleviated.Histopathological and immunohistochemical examinations confirmed the brain injury tissue changes.The nanoshell molecular probe carrying anti-tropomyosin 4 has potential for use in early diagnosis of cerebral ischemia/reperfusion injury and evaluating its progression.
Key Words: cerebral ischemia/reperfusion injury;diagnosis;dynamic monitoring;ischemic stroke;middle cerebral artery occlusion;molecular probe;nanoshells;photoacoustic imaging;tropomyosin 4;ultrasound
From the Contents
Introduction 1321
Methods 1321
Results 1322
Discussion 1323
Introduction
Stroke is classified as either hemorrhagic or ischemic.Ischemic stroke accounts for 85% of all strokes (Saver et al.,2015) and is commonly caused by internal carotid artery stenosis resulting from atherosclerosis.Treatment of acute ischemic stroke is based on reperfusion (Hankey,2017).However,reperfusion after endarterectomy or stenting may be complicated by cerebral ischemia/reperfusion injury (CISI),which is associated with nearly 50%mortality (Hayakawa et al.,2019).Early diagnosis of CISI and identifying the location of the responsible lesion should enable timely intervention (Emberson et al.,2014;Alper et al.,2015).
Computed tomography and magnetic resonance imaging are used to assess CISI (Provost et al.,2019).However,the time required to acquire and process images can delay treatment (Lutsep et al.,1997;Ryu et al.,2002;Ma et al.,2021).Rapid non-invasive high-resolution imaging techniques with high diagnostic sensitivity are needed.Photoacoustic imaging (PAI) is a relatively new radiation-free imaging technique that might meet this need.
PAI overcomes the problems of resolution and scatter that occur when light is transmitted through solid tissue and offers the contrast of optical imaging and the high resolution of ultrasound (US) imaging at depths of up to several cm (Nie et al.,2014;Liu et al.,2016;Wang and Yao,2016;Cai et al.,2019).PAI is also useful for studying morphological damage,functional metabolism,and physiological and pathological properties of tissue.Furthermore,it can be used for extended continuous monitoring and has potential for imaging organelles and organs (Zemp et al.,2008;Taruttis et al.,2010;Lv et al.,2018).Moreover,PAI can be performed rapidly,which enables earlier treatment,and can identify hemorrhage,which is important when deciding treatment in the CISI setting.
In a study of localization patterns of myosin heavy chain isoforms in normal arteries and atherosclerotic lesions of increasing severity,Abouhamed et al.reported that during atherogenesis,tropomyosin 4 (TPM4) expression occurs primarily in smooth muscle cells (SMCs) of the synthetic phenotype in the media and intima;furthermore,upregulated TPM4 mRNA is a marker of vascular SMC dedifferentiation (Abouhamed et al.,2003).Nanostructures with discrete metallic,hollow,and porous construction have shown promise in catalysis and nanomedicine applications because of their large surface area,lightweight nature,and broadly tunable localized surface plasmon resonance(Wu et al.,2012).This study aimed to examine the use of anti-TPM4-coated Ag/Au hollow nanoshells@SiO2nanoparticles as a PAI contrast agent to diagnose and evaluate CISI in a mouse model of IS (Watson et al.,1985).
Methods
Synthesis of Ag/Au hollow nanoshells
According to a standardized protocol,Ag sol was prepared using the sodium citrate reduction method (Wu et al.,2012).Next,1.6 mL of 0.1% HAuCl4and 0.8 mL of 0.02 M NH2OH were added to 40 mL of Ag nanoparticles(approximately 1.5 × 1011particles/mL) in a glass vial and mixed using a magnetic stirrer before 5 mL of water was added.Then,a range of volumes(45–550 μL) of 0.1% HAuCl4and 0.1 mL of 0.02 M NH2OH were added to the vials and mixed using a magnetic stirrer.After heating and refluxing the solution for a few minutes,it was cooled to room temperature before etching the nanoparticles with 0.1 M H2O2(Figure 1).
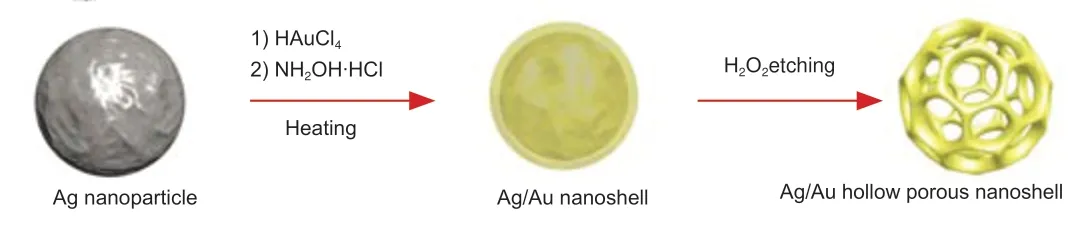
Figure 1|Schematic diagram of the Ag/Au hollow nanoshells.
Synthesis of Ag/Au hollow nanoshells@SiO2 nanoparticles
Ag/Au hollow nanoshells@SiO2nanoparticles were synthesized as described by Li et al.(2017).While vigorously stirring,a suitable amount of 1 mM(3-aminopropyl) trimethoxysilane was added drop by drop to 20 mL of the Ag/Au hollow nanoshells.To ensure complete complexation of amine groups with the gold surface,the mixture was stirred for at least 20 minutes.A sodium silicate solution (0.54 weight percentage) and 0.1 M H2SO4were then added to the solution,which was vigorously stirred then heated at 90°C for 1 hour.After cooling to room temperature,the nanoparticles were centrifuged at 4500 ×gfor 20 minutes and dispersed in 10 mL Milli-Q water (Millipore,Boston,MA,USA).The particles were then transferred to a solution containing 40 mL of ethanol and 1.2 mL of ammonia.After adding 1 mL of 1% tetraethyl orthosilicate,the mixture was stirred at room temperature (25°C) for 24 hours.Subsequently,the nanoparticles were centrifuged and redispersed into 10 mL of isopropanol.Finally,the Ag/Au hollow nanoshells@SiO2nanoparticles were carboxylated by adding 3-(triethoxysilyl) propylsuccinic anhydride and heated at 85°C for 24 hours.
Molecular probe synthesis: Ag/Au hollow nanoshells@SiO2 nanoparticle conjugation with anti-TPM4
1 mL aliquots of nanospheres were first dispersed with US for 15 minutes before 1.5 μL (20 mg/mL) of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide was added.After reacting at room temperature for 15 minutes,100 μL of antibody was desalted in a G25 centrifuge column and added to the activated nanospheres.After 30 minutes at room temperature,centrifugation was performed at 6000 ×gfor 10 minutes,and the precipitate was suspended in phosphate-buffered saline.The precipitate was then suspended in Trisbuffered saline for 10 minutes.
Measurement of the photoacoustic effects of in vitro samples
To assess PAIin vitro,Ag/Au hollow nanoshells@SiO2nanoparticle composite nanosheets of different concentrations (0.185–3.0 mg/mL) were dispersed in aqueous solution.The PA images were acquired using a Vevo LAZR photoacoustic imaging system (Visual Sonics,FUJIFILM Inc.,Toronto,Canada)equipped with an MX-250 probe.Imaging parameters were as follows: region of interest,20 mm;frequency,21 MHz;photoacoustic gain,35 dB;excitation wavelength,700 nm.
Animals
C57BL/6N male mice aged 7–8 weeks (specific-pathogen-free level;weight 23 ± 2 g) were obtained from Charles River (Beijing,China,SCXK (Jing) 2016-0006).Allin vivostudies were conducted according to protocols approved by the Institutional Animal Care and Use Committee at Beijing Tiantan Hospital,Capital Medical University (No.202102002,on March 26,2021).
MCAO stroke model
Mice were placed in the supine position and the neck skin was sterilized.The skin was then incised using scissors and elbow ophthalmic forceps were used to passively separate the neck muscles and nerves to visualize the leftcommon carotid artery.Next,the arterial sheath was carefully isolated to avoid injuring the vagus nerve and the exterior and interior carotid arteries were divided.Then,the common and external carotid arteries were ligated with silk thread.The internal carotid artery was gripped with a clip and a small gap was created using ophthalmic scissors.A 6–0 silicone-coated monofilament was then embedded into the common carotid artery and advanced toward the arterial clamp.The filament was held in place with a distal silk tie to remain in place during the occlusion (Fan et al.,2017).Evaluations were performed using the Zea Longa scale (Longa et al.,1989).Three experimenters scored and recorded the mice in a single-blind manner.Mice with scores of 3 to 4 were selected for follow-up experiments.
CISI model
One hour after arterial occlusion,the 6–0 silicone-coated monofilament was extracted to restore cerebral blood flow on the affected side and cause reperfusion injury.Flow was monitored using the RFLSI III laser-speckle blood flow imaging system (RWD,Shenzhen,China) over 168 hours.The experimental timeline with experimental details is shown inFigure 2.
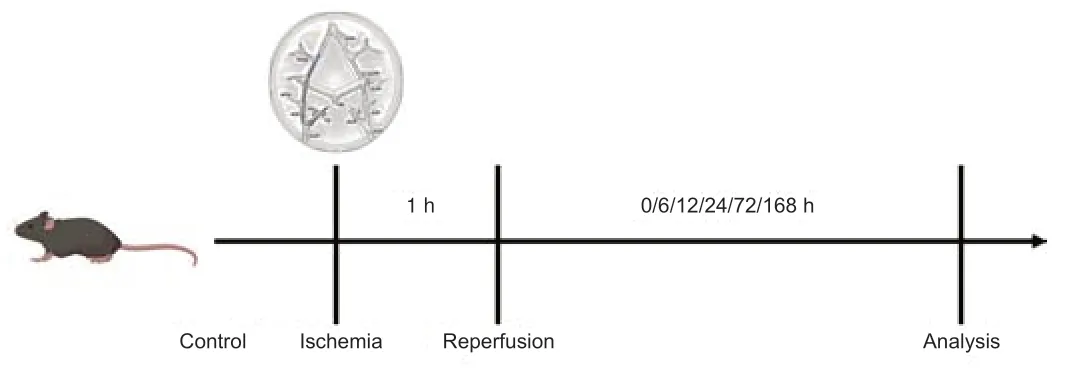
Figure 2|Experimental timeline.
US/PAI detection
A pulse laser integrated with a high-frequency US system (Vevo LAZR-X,VisualSonics,FUJIFILM,Tokyo,Japan) was used to observe microvascular brain changes at the State Key Laboratory of Natural and Biomimetic Drugs,School of Pharmaceutical Sciences,Peking University.Molecular probes were injected into the tail vein immediately and 1,6,12,24,72,and 168 hours (n=3/time point) after the filament was removed.
Histological analysis
After anesthetizing the mice with 1% isoflurane using a R540IP gas anesthesia machine (RWD),they were sacrificed at specific time points via intracardiac injection of 60 mL of 4% paraformaldehyde.Brains were then removed and stored in 4% paraformaldehyde.Next,the injured areas of the right brain were dissected and embedded in paraffin to prepare a series of adjacent horizontal sections (5 μm thickness).Hematoxylin and eosin staining and immunohistochemical staining were performed to detect the degree of braintissue damage.Brain tissue was sectioned,air-dried at room temperature,fixed for 30 seconds,stained with hematoxylin (60°C) for 60 seconds,differentiated with 1% hydrochloric acid ethanol,and stained with eosin for 3 minutes.Then,sections were dehydrated using an ethanol series (70%,80%,95%,and 100% ethanol;5 minutes each),cleaned with xylene three times (5 minutes each),and fixed with balsam.The heart was first infused with 0.9% normal saline and then fixed with 4% paraformaldehyde (Sinopac Chemical Reagents,Shanghai,China).After dehydration in 20% sucrose,the left brain injury area was dissected and embedded in paraffin and 5 μm sections were cut using the S700 slicer (RWD).The primary antibody was incubated at 4°C overnight,and the secondary antibody was washed with phosphate-buffered saline and incubated at room temperature for 1 hour (primary antibody,TPM [EPR13316](1:200,Abcam,Cambridge,UK,Cat# ab181085);secondary antibody,goat anti-rabbit IgG H&L (1:1000,Abcam,Cat# ab6721,RRID: AB_955447)).All sections were scanned using the Pannoramic MIDI scanner (3DHistech,Ltd.,Budapest,Hungary).Five fields were randomly selected from each damaged area and ImageJ software version 1.8.0 (National Institutes of Health,Bethesda,MD,USA) was used to quantify integrated optical density (Schneider et al.,2012).The images were reviewed by experienced physicians who were blinded to grouping.The reports were subsequently group-matched by a researcher.Degree of cerebral cortex tissue injury on the affected side was classified as mild,moderate,and severe (Zhao et al.,2014;Ludwig et al.,2018).
Statistical analysis
Sample size was not statistically predetermined;however,our sample sizes were similar to those reported in a previous publication (Rosenkrans et al.,2022).No animals or data points were excluded from analysis.Quantitative data are presented as means ± standard deviation (SD).Statistical analyses were performed using SPSS software version 21 (IBM Corp.,Armonk,NY,USA).Groups were compared at different time points using one-way repeated measures analysis of variance.P< 0.05 was considered significant.
Results
Characterization of TPM4-targeted Ag/Au hollow nanoshells@SiO2 nanoparticles
The prepared material showed a nanometer particle with a cage structure and diameter of approximately 80 nm (Figure 3).Based on a distribution mapping diagram of the elements O,Au,Si,and Ag,the elements were evenly distributed throughout the nanoparticles.The ultraviolet-visible absorption spectra for Ag/Au and Ag/Au@SiO2showed peaks at 808 nm.
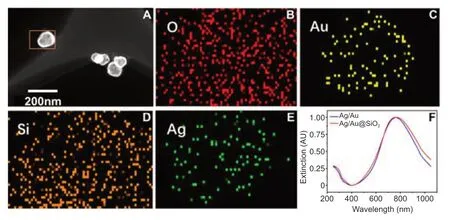
Figure 3|Characterization of TPM4-targeted Ag/Au hollow nanoshells@SiO2 nanoparticles.
Monitoring using laser-speckle blood flow imaging systemand the US/PAI system
During cerebral ischemia,cerebral blood perfusion in the affected (left)side was almost zero on the Zea Longa scale,which indicated successful construction of the ischemia model.After experimental restoration of blood flow,cerebral blood perfusion on the affected side increased over time (Figure 4A–H).Change in perfusion significantly differed between the affected and healthy sides (F=6.127,degrees of freedom 15,P< 0.05).Change in perfusion was significantly higher at 12 hours compared to other periods then decreased gradually (Figure 4I).
Transcranial US examination coupled with the color Doppler flow mode revealed that the blood flow signal of the left middle cerebral artery was apparently weaker than that of the right side.Moreover,the blood flow velocity of the middle cerebral artery was determined by pulsed-wave Doppler and that of the left side was lower than the right one (Figure 4J–L).
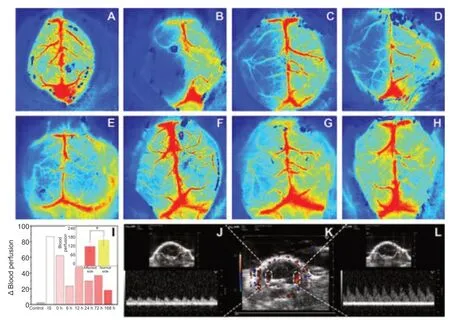
Figure 4|Monitoring using laser-speckle blood flow imaging system and the ultrasound/photoacoustic imaging system.
Evaluation of the probe in the mouse CISI model using the US/PAI system
Molecular probes were injected into the tail vein immediately and 1,6,12,24,72,and 168 hours after removal of the monofilament.Photoacoustic examination was performed immediately after removal and brain reperfusion injury was quantitatively and qualitatively analyzed.As shown inFigure 5,the photoacoustic signal was significantly stronger on the affected (left) side than on the healthy (right) side.At 12 hours,the difference in photoacoustic signal between the two hemispheres increased transiently then became gradually smaller over time.At each time point,the total photoacoustic signal was significantly higher on the affected side than on the healthy side (F=5.045,degrees of freedom=13,P< 0.05).However,the photoacoustic signal on the affected side did not depend on the time after reperfusion.
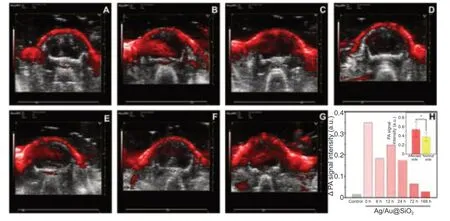
Figure 5|Photoacoustic imaging with the co-registered ultrasound system was used to evaluate the probe in the mouse model of cerebral ischemia/reperfusion injury.
Histological analysis in the mouse CISI model
After photoacoustic examination at each time point,the mice were sacrificed and their brain tissue extracted for histological examination.Brain tissue damage was assessed on hematoxylin and eosin-stained slides (Figure 6).TPM4 immunohistochemical staining showed tissue damage on the affected side was severe in the 1-,24-,and 168-hour groups,mild in the 12-and 72-hour groups,and moderate in the 6-hour group (Figure 7).
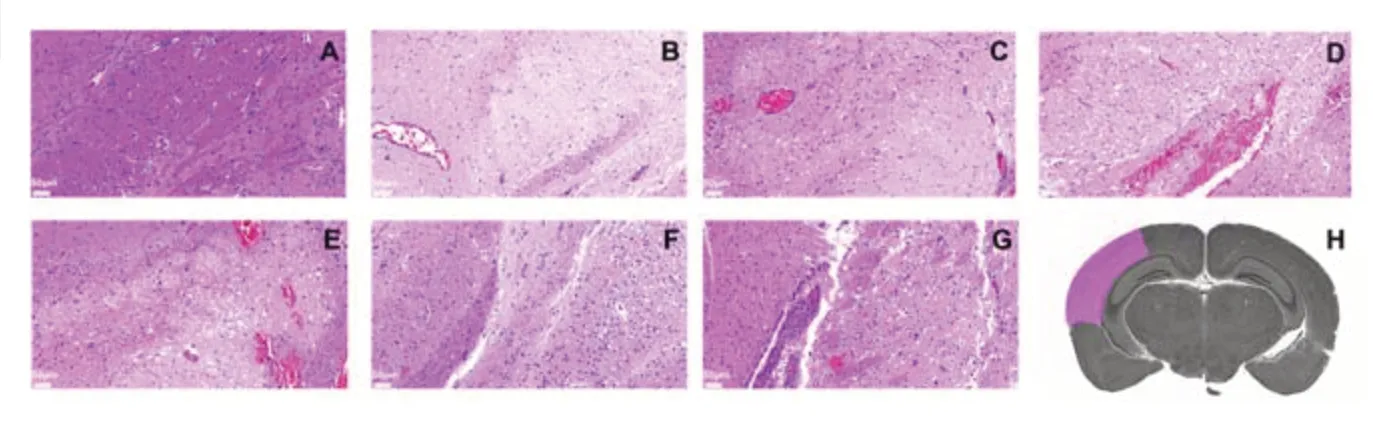
Figure 6|Pathological analysis of cerebral cortex supplied by the left middle cerebral artery (affected side).
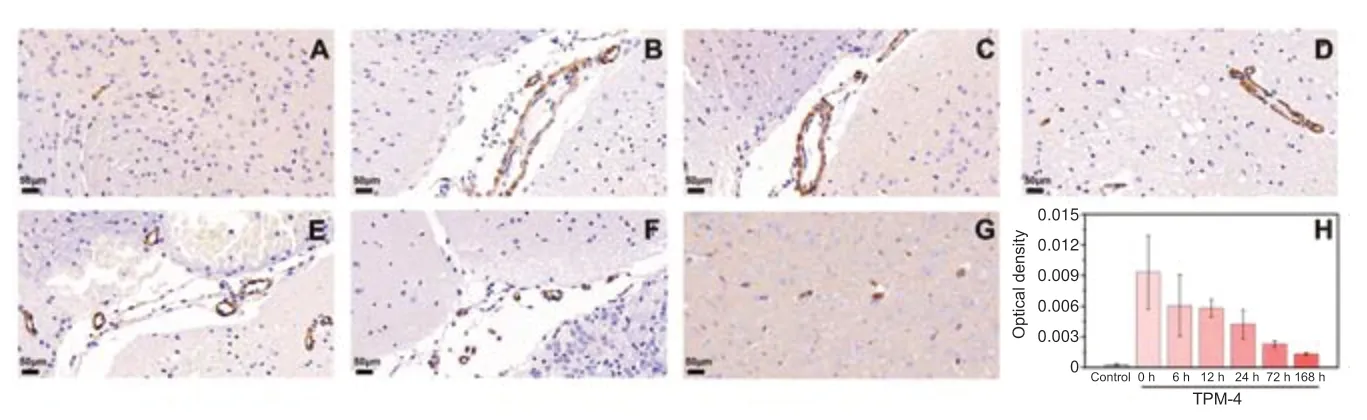
Figure 7|Tropomyosin 4 (TPM4) immunohistochemical analysis of cortex supplied by the left middle cerebral artery(affected side).
Discussion
Previous ischemic stroke research has concentrated on decreasing hypoperfusion time to maintain brain function (Lee et al.,2018).Nevertheless,clinical results after restoration of blood flow to ischemic brain remain suboptimal.Basic and clinical research have not yet revealed the detailed mechanisms underlying CISI (Silvestri et al.,2017).Understanding these mechanisms may provide a solid basis for developing CISI treatments and preventing brain injury (Wu et al.,2018).
Vascular SMC phenotype governs the properties and functions of the vessel wall.In the tunica media,SMCs are responsible for maintaining vessel wall tone.Enhanced TPM4 mRNA expression is a fiducial differentiation marker for synthetic SMCs (Abouhamed et al.,2003).Because damage to cerebrovascular SMCs can promote TPM4 expression,we prepared and injected a molecular probe carrying anti-TPM4 nanocages into the tail veins of male mice.As in other relevant studies,female mice were excluded to reduce experimental errors caused by the mice themselves (Alamri et al.,2021).In our study,a molecular probe was designed to enter the cerebral vasculature,pass through the vascular endothelial cells and target vascular SMCs.US/PAI was then used to predict and diagnose CISI.Furthermore,the nanocages we prepared are capable of carrying drugs,and therefore may have a role in CISI treatment in the future.
US/PAI is a non-ionizing,non-invasive,and real-time imaging technique that has been used in preclinical studies of various diseases (Hu et al.,2021).It has sufficient penetration depth and spatial resolution to detect fine inner organ structures and allow detailed CISI research.Moreover,the molecular probe used in our study is non-toxic.Further developments and improvements upon it should enable human studies in the near future.
Rather than using the conventional molecular probe observation time,we used an observation method that was in line with the contrast-enhanced US procedure: brains were observed immediately after intravenous injection.Because nanomaterials are absorbed by brain tissue itself,we examined blood perfusion volumes and TPM4 expression in both affected (ischemic)and healthy tissue.Early after reperfusion of ischemic brain,the vessel walls and brain tissue were severely damaged and the number of cortical cells was decreased.Some nerve cells in the cortex were necrotic and degenerated with pyknotic and hyperchromatic nuclei.In addition,some nerve fibers were loosely arranged and irregular with sieve-like structures and many vacuoles.Leakage of blood cells was also observed.At the same time,TPM4 expression in the cerebrovascular wall was significantly increased.It was not until 24 hours after reperfusion that self-regulation and self-repair was observed,and the degree of damage gradually decreased.The injured state gradually recovered within a week,although the brain tissue structure remained slightly abnormal: punctate liquefaction necrosis in the cortex and a small degree of cell edema were observed.Nucleoli in a few cells disappeared and more vacuoles were present.The expression of TPM4 in the cerebrovascular wall also decreased gradually.
We successfully constructed a molecular probe of porous Ag/Au@SiO2bimetallic hollow nanoshells carrying anti-TPM4 for the early diagnosis and dynamic monitoring of CISI using US/PAI.These nanoshells successfully served as a contrast agent for PAI and have potential to carry and release drugsin vivobecause of their unique cage structure.This probe shows promise for use in early diagnosis of CISI and assessment of its complications and progression.And then,early intervention of reperfusion injury can reduce the complications and mortality after recanalization.Further studies are warranted,as our study is only preliminary.
Author contributions:Study conceptualization,resources,projectadministration and funding acquisition: WH,JT;methodology: TFY,KW;software: LY;validation,formal analysis: TFY,WH,KW;investigation: TFY,WH,JT;formal analysis,manuscript draft: TFY;data curation,visualization: TFY,LY;manuscript review and editing: TFY,WZL,CPL,WZ,KW,WH;supervision:KW,WH.All authors have read and agreed to the published version of the manuscript.
Conflicts of interest:There are no conflicts to declare.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
- Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update

