Ginsenoside Rb1 improves energy metabolism after spinal cord injury
Shan Wen,Zhi-Ru Zou,Shuai Cheng,Hui Guo,Heng-Shuo Hu,Fan-Zhuo Zeng,Xi-Fan Mei,
Abstract Mitochondrial damage caused by oxidative stress and energy deficiency induced by focal ischemia and hypoxia are important factors that aggravate diseases.Studies have shown that ginsenoside Rb1 has neurotrophic and neuroprotective effects.However,whether it influences energy metabolism after spinal cord injury remains unclear.In this study,we treated mouse and cell models of spinal cord injury with ginsenoside Rb1.We found that ginsenoside Rb1 remarkably inhibited neuronal oxidative stress,protected mitochondria,promoted neuronal metabolic reprogramming,increased glycolytic activity and ATP production,and promoted the survival of motor neurons in the anterior horn and the recovery of motor function in the hind limb.Because sirtuin 3 regulates glycolysis and oxidative stress,mouse and cell models of spinal cord injury were treated with the sirtuin 3 inhibitor 3-TYP.When Sirt3 expression was suppressed,we found that the therapeutic effects of ginsenoside Rb1 on spinal cord injury were remarkably inhibited.Therefore,ginsenoside Rb1 is considered a potential drug for the treatment of spinal cord injury,and its therapeutic effects are closely related to sirtuin 3.
Key Words: axon growth;ginsenoside Rb1;glycolysis;metabolic reprogramming;mitochondrion;neuroprotection;oxidative stress;oxygen and glucose deprivation;Sirt3;spinal cord injury
From the Contents
Introduction 1332
Methods 1333
Results 1334
Discussion 1335
Introduction
Spinal cord injury (SCI) is a central nervous system disease with a high incidence and disability rate (Wen et al.,2021).It not only causes patients to lose their working ability but also poses a significant burden to their families and society (Hu et al.,2021).SCI is mainly divided into primary and secondary injuries.Vascular damage and progressive edema caused by the primary injury continuously aggravate ischemia and hypoxia at the injury site,resulting in insufficient neuronal energy production and apoptosis (Jeong et al.,2021;Masterman and Ahmed,2021;Shao et al.,2021;Wahyudi et al.,2022).Secondary injury is the continuation of the primary injury.Its pathological process mainly includes oxygen-free radical formation,local ischemia,and inflammatory reactions,which can last for weeks or months,resulting in the gradual expansion of the lesion area and further aggravation of nerve injury(Orr and Gensel,2018;Jia et al.,2019;Zhou et al.,2020;Huang et al.,2021b).In particular,ischemia,hypoxia,and oxidative stress are considered key to the prognosis of SCI.
Panax ginseng is a commonly used Chinese medicinal herb,the root or stem of which has been used for the treatment of cardiovascular disease in several Asian countries (Lou et al.,2021).Pharmacological studies have shown that Panax ginseng and its extracts exert multiple pharmacological activities,such as antioxidative,platelet aggregation-inhibiting,and neuronal apoptosis-suppressing effects (Kiefer and Pantuso,2003;Mancuso and Santangelo,2017;Xie et al.,2018;Lou et al.,2021).Ginsenoside Rb1 (G-Rb1)is a tetracyclic triterpenoid mainly obtained from the roots or stems of Panax notoginseng and ginseng by extraction and purification (Lou et al.,2021).It is one of the main bioactive compounds of ginseng with minimal toxicity and neurotrophic and neuroprotective effects on brain injury (Chen et al.,2019;Zhang et al.,2021a,b).G-Rb1 promotes hippocampal nerve regeneration,enhances learning and memory,and has anti-aging and anti-fatigue effects(Cheng et al.,2005;Li et al.,2015;Zhao et al.,2018;Lin et al.,2019;Yang et al.,2020).In addition,it activates antioxidants,stimulates the immune system,has anti-apoptotic activity,and maintains cell adenosine triphosphate(ATP) levels (Fan et al.,2020).Moreover,some studies have reported that GRb1 potentially inhibits oxidative stress-induced apoptosis after SCI (Liu et al.,2018;Ye et al.,2019).However,there are limited studies related to the effects of GRb1 on energy metabolism in SCI.
As one of the most important deacetylases,sirtuin 3 (Sirt3) is essential for eukaryotic life and is closely related to metabolism in several organs (Zhang et al.,2020).Moreover,Sirt3 is involved in almost all aspects of mitochondrial metabolism and homeostasisin vivo,protecting mitochondria from various types of damage (Hirschey,2011;Wang et al.,2019).However,its role in SCI has been less reported.
In this study,we aimed to identify an approach for treating SCI that may provide ideas for clinical treatment.To this end,we established a mouse model of blunt SCI and evaluated the therapeutic effect of G-Rb1 using behavioral assays.Then,the role of G-Rb1 in oxidative stress was assessed with mitochondrial probes and oxidative stress-related kits.The role of G-Rb1 in energy metabolism was evaluated by measuring the mitochondrial membrane potential,activity of key enzymes involved in glucose metabolism,and ATP content.Finally,a Sirt3 inhibitor was used to explore the molecular mechanism of G-Rb1 action.
Methods
Animals and surgical procedures
C57BL/6J mice (male and female,25–30 g,6–8 weeks old) used in this study were purchased from Liaoning Changsheng Biotechnology Co.,Ltd.(Benxi,China;license No.SCXK (Liao) 2020-0001).These mice were reared in the Experimental Animal Center of Jinzhou Medical University in a specificpathogen-free environment at 22 ± 2°C and a 12-hour light-dark cycle (six mice per cage).All operations were approved by the Animal Care Committee of Jinzhou University (approval No.SYXK 2019-0007) in January 2021.All experiments were designed and reported according to the Animal Research:Reporting ofIn VivoExperiments (ARRIVE) guidelines (Percie du Sert et al.,2020).Mice were divided into the Sham (n=30),SCI (n=57),G-Rb1 (SCI+30 mg/kg G-Rb1,n=57),and Sirt3–(SCI+Sirt3 inhibitor+30 mg/kg G-Rb1,n=30) groups.
According to previous reports (Liu et al.,2020;Huang et al.,2021a;Lin et al.,2021),after mice were anesthetized with uratan (30 mg/kg,intraperitoneal injection,Sinopharm Chemical Reagent Co.,Ltd.Shanghai,China),the back hair was removed,and the back was disinfected with povidone-iodine(LIRCON,Dezhou,Shandong,China).T9/T10 laminectomy was performed to expose the thoracic spinal cord.Next,a self-made impactor (12.5 g) was used to impact the exposed dorsal surface of the spinal cord from a height of 2 cm (Hu et al.,2021).The sham group was only stripped of the lamina but did not receive spinal cord blows.One hour after surgery,mice were injected intraperitoneally with G-Rb1 (30 mg/kg) dissolved in normal saline or saline(0.1 mL) for 7 days (Ye et al.,2019;Jiang et al.,2021;Zhang et al.,2021a).The Sirt3 inhibitor 3-(1H-1,2,3-triazol-4-yl) pyridine (3-TYP) was intraperitoneally injected at a dose of 50 mg/kg every 2 days for three doses 1 week before the surgery.On postoperative day 7,tissues were obtained (Figure 1).
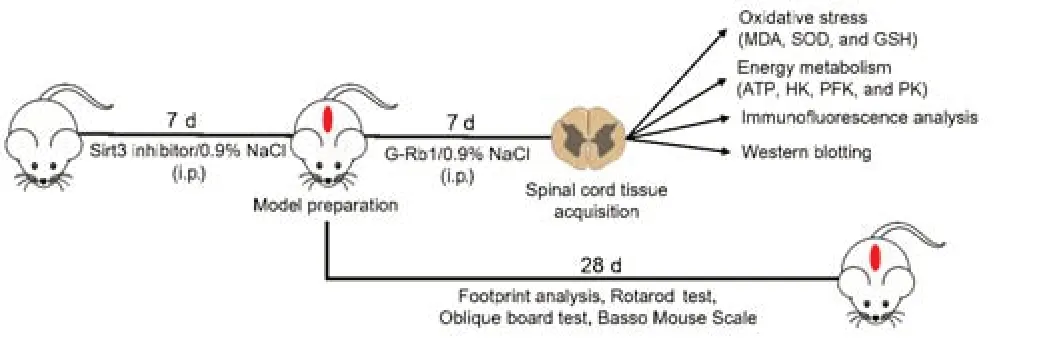
Figure 1|Animal experiment flow chart.
Cell culture and model construction
PC12 cells (Cat# ab279978,RRID: CVCL_0481),as a commonly used neuronal cell line,were obtained from Abcam.Before the experiment,immunofluorescence was performed to confirm that cells were NeuN positive and that their morphology was consistent with PC12 cell characteristics.All cells were cultured at 37°C in a humidified atmosphere of 5% CO2and digested with 0.25% trypsin (Bioind,Shanghai,China) during cell subculture.The medium was Dulbecco’s modified Eagle medium (Gibco,Grand Island,NY,USA) containing fetal bovine serum (10%),penicillin (100 U/mL),and streptomycin (100 μg/mL;Gibco).
For oxygen and glucose deprivation (OGD) (Wang et al.,2022),PC12 cells of a suitable density were selected,and their medium was changed to phosphatebuffered saline (PBS,pH 7.4).The hypoxia incubator was then filled with nitrogen gas (20 L/minute,5 minutes) to maintain the oxygen concentration below 1%.After 2 hours of hypoxia and glucose deprivation,cells were removed from the hypoxic incubator and cultured in normal growth medium for 1 day (Jia et al.,2022).The control group was incubated normally.Cells were incubated with 100 μM G-Rb1 (Aladdin Chemical Reagent Co.,Shanghai,China) for 1 day after OGD treatment.Cells were treated with the SIRT3 inhibitor 3-TYP (50 μM;MedChemExpress,Monmouth Junction,NJ,USA,Cat# HY-108331) for 3 hours before OGD.The concentrations of G-Rb1in vitrowere determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays (Additional Figure 1).
Western blotting
After mice were subjected to different treatments for 7 days,they were anesthetized by an overdose of uratan.The spinal cord tissue (1 cm around the injury site) was collected,and proteins were extracted using radioimmunoprecipitation assay lysis buffer.Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis after adding equal amounts of samples from different groups to a sodium dodecyl sulfatepolyacrylamide gel.Then,the proteins were transferred to polyvinylidene fluoride membranes.Subsequently,polyvinylidene fluoride membranes were blocked with 5% nonfat dry milk for 2 hours and incubated with primary antibodies overnight at 4°C.To detect apoptosis and axon regeneration,the primary antibodies and dilutions were as follows: anti-Bcl-2 (rabbit,1:1000,Abcam,Cambridge,UK,Cat# 1017-1,RRID: AB_289591),anti-Bax (rabbit,1:1000,Abcam,Cat# 1063-1,RRID: AB_351500),anti-cleaved caspase-3(rabbit,1:1000,Abcam,Cat# ab52293,RRID: AB_873720),anti-β-actin(mouse,1:2000,Proteintech,Rosemont,IL,USA,Cat# 66009-1-Ig,RRID:AB_2687938),anti-growth associated protein-43 (GAP43;rabbit,1:1000,Abcam,Cat# 3857-1,RRID: AB_10900622),and anti-neurofilament 70/200 kDa (NF200;mouse,1:1000,Thermo Fisher Scientific,Waltham,MA,USA,Cat# MA5-15234,RRID: AB_10982518).The next day,the secondary antibody(horseradish peroxidase [HRP]-conjugated AffiniPure Goat Anti-Mouse IgG,Proteintech,Cat# SA00012-7,RRID:AB_2 890970;HRP-conjugated AffiniPure Goat Anti-Rabbit IgG,Proteintech,Cat# SA00001-2,RRID:AB_2722564,both 1:1000) was incubated at 37°C for 2 hours.Finally,the optical densities were measured using a Tanon 5500 gel imaging system (Tanon,Shanghai,China).ImageJ V1.8.0 software (National Institutes of Health,Bethesda,MD,USA)(Schneider et al.,2012) was used to analyze signal intensities.
Immunofluorescence analysis
Mice were anesthetized by uratan and fully perfused with saline and 4%paraformaldehyde.Spinal cord tissue (1 cm around the injury site) was collected and placed in sucralose,and frozen sections were made.Frozen sections of spinal cords from different groups were washed with PBS until use.After permeabilizing with 0.3% Triton X-100 for 15 minutes,sections were blocked with goat serum (ZSGB-BIO,Beijing,China) for 2 hours.Subsequently,primary antibodies (anti-NeuN,mouse,1:1000,Abcam,Cambridge,UK,Cat# ab77315,RRID: AB_1566475 and anti-cleaved caspase-3,1:300) were incubated with sections overnight at 4°C.The next day,the corresponding secondary antibody (Alexa Fluor 488 goat anti-mouse IgG,Thermo Fisher Scientific,Waltham,MA,USA,Cat# A32728,RRID: AB_2 633277;Alexa Fluor 568 goat anti-rabbit IgG,Thermo Fisher Scientific,Cat# A-11011,RRID: AB_143157,both 1:1000) was added and incubated at 37°C for 2 hours.Finally,sections were stained for 15 minutes using 4′,6-diamidino-2-phenylindole (DAPI) staining solution (Beyotime Biotechnology Co.,Shanghai,China,Cat# C1002) containing an anti-quencher reagent and photographed with an FV10I confocal microscope.The fluorescence intensity in each group was analyzed with ImageJ software.
Cell samples were processed similarly to tissues.Briefly,cells subjected to different treatments were fixed with 4% polyfluoroalkyl for 30 minutes and then incubated with permeabilizing solution (0.1% Triton X-10) for 15 minutes,blocking solution (goat serum) for 2 hours,primary antibody (anti-β-tubulin: mouse,1:300,Proteintech,Cat# 10094-1-AP,RRID: AB_2 210695;anti-cleaved caspase-3: 1:100;anti-Sirt3: rabbit,1:500,Abcam,Cat# 27-579,RRID: AB_10943254) for 18 hours at 4°C,secondary antibody (Alexa Fluor 488 goat anti-mouse IgG and Alexa Fluor 568 goat anti-rabbit IgG,both 1:1000) for 2 hours at 25°C,and nuclear staining solution (DAPI) for 30 minutes at 25°C.Finally,the fluorescence intensity was observed with a confocal laser scanning microscope (CLSM,BioTek Instruments,Winooski,VT,USA).
Determination of energy metabolism
After C57BL/6J mice were treated for 7 days,the spinal cord tissue (1 cm around the injury site) was collected.Cells were isolated 1 day after the completion of the corresponding treatment.
ATP content
ATP in spinal cord tissues/PC12 cells of different groups was extracted using an ATP detection kit,and then 100 μL of ATP detection working solution(Beyotime Biotechnology Co.,Cat# S0027) was added to each well.After 5 minutes of incubation at room temperature,20 μL of the sample or standard were added.Then,relative light unit values were determined using a microplate reader (Versa Max,Molecular Devices,Sunnyvale,CA,USA).Finally,the relative light unit values of ATP standards with different concentrations were detected,the standard curve was generated,and the ATP content in the sample was calculated.
Hexokinase activity
Hexokinase (HK) was extracted from spinal cord tissue/PC12 cells using a hexokinase assay kit (Beyotime,BC0745).After homogenizing samples in an ice bath,they were centrifuged at 8000 ×gfor 10 minutes at 4°C,and the supernatant was collected.Next,180 μL of the reaction system,10 μL of the reaction substrate,and 10 μL of the sample were added to a 96-well plate and mixed.The absorbance value A1 at 340 nm was immediately recorded for 20 seconds.After 5 minutes,the absorbance value A2 at 340 nm was recorded.Finally,HK activity was calculated following the kit’s instructions.
Phosphofructokinase activity
Phosphofructokinase (PFK) was extracted from spinal cord tissue/PC12 cells using a PFK assay kit (Beyotime,BC0535).After homogenizing samples in an ice bath,they were centrifuged at 8000 ×gfor 10 minutes at 4°C,and the supernatant was collected.Next,170 μL of the reaction system,10 μL of the reaction substrate,10 μL of iron supplement,and 10 μL of the sample were added to a 96-well plate and mixed.The absorbance value A1 at 340 nm was immediately recorded for 20 seconds.After 10 minutes,the absorbance value A2 at 340 nm was recorded.Finally,PFK activity was calculated following the kit’s instructions.
Pyruvate kinase activity
Pyruvate kinase (PK) was extracted from spinal cord tissue/PC12 cells using a PK assay kit (Beyotime,BC0745).After homogenizing samples in an ice bath,they were centrifuged at 8000 ×gfor 10 minutes at 4°C,and the supernatant was collected.Then,180 μL of the reaction system,10 μL of the reaction substrate,and 10 μL of the sample were added to a 96-well plate and mixed.The absorbance value A1 at 340 nm was immediately recorded for 20 seconds.After 2 minutes,the absorbance value A2 at 340 nm was recorded.Finally,PK activity was calculated following the kit’s instructions.
Oxidative stress experiments
PC12 cells were seeded in 24-well plates and allowed to adhere.When the groups finished processing for 1 day,the reactive oxygen species (ROS)fluorescent probe 2,7-dichlorodihydrofluorescein diacetate (Beijing Solarbio Science &Technology Co.,Beijing,China,Cat# CA1410) was added to different groups of cells and incubated in the incubator for 30 minutes.Finally,cells were observed and photographed using a fluorescence microscope (Leica,Wetzlar,Germany).
Superoxide dismutase (SOD;Beijing Solarbio Science &Technology Co.,Cat#BC0175),plasma glutathione peroxidase (GSH-Px),and malondialdehyde(MDA;Beijing Solarbio Science &Technology Co.,Cat# BC0025) detection kits were used to detect SOD activity,GSH-Px levels,and MDA contents in accordance with the manufacturer’s instructions.
Measurement of mitochondrial membrane potential
PC12 cells were seeded in confocal plates and allowed to adhere.Then,the mitochondrial membrane potential probe JC-1 (Beyotime Biotechnology Co.,Cat# C2003S) was added to different groups of cells following the manufacturer’s instructions (Solarbio,Beijing,China).Finally,cells were observed and photographed using a fluorescence microscope.
Behavioral assessments
Footprint analysis
To detect the hind limb mobility of mice,a footprint analysis was performed.After 28 days of different treatments,the forelimbs were painted with black dye,and the hind limbs were painted with red dye.Mice were placed on absorbent paper surrounded by wooden boards and encouraged to walk in a straight line.The step length and step width were measured (Hu et al.,2021).
Rotarod test
The rotarod test was selected to assess the overall motor ability of mice.After 28 days of treatment,the mice were trained to stay on the rolling device (Ugo Basile,47650,Gemonio,Italy) and slowly rotate a rod with a diameter of 2 cm at a speed of 5 revolutions/minute and an acceleration of 1 revolution/minute.The time spent on the stick until each mouse fell was recorded (Gong et al.,2022).
Oblique board test
At 1,3,5,7,14,21,and 28 days after the operation,mice were placed on an inclined board (self-made),and the inclination angle was continuously increased at a speed of 0.5 °/s until the mice fell off.When the mouse slid down,the tilt angle of the slanted plate was recorded (Shen et al.,2019).
Basso Mouse Scale
Behavior was assessed using the Basso Mouse Scale (BMS).Mice were evaluated in a double-blind manner by three examiners at 1,3,5,7,10,14,and 28 days after surgery.The BMS score ranges between 0 and 9,with 0 indicating no ankle activity and 9 indicating completely normal activity (Ge et al.,2021).
Statistical analysis
All animal and cell experiments were biologically repeated three times.The evaluators were blinded to the assignments.Statistical analysis was performed using GraphPad Prism (version 8.0.2,GraphPad Software,San Diego,CA,USA,www.graphpad.com),and all data were reported as the mean± standard deviation.For analyzing the differences in BMS scores between the groups over time,a two-way repeated measures analysis of variance with Bonferroni’spost hoccorrection was performed.Other data were analyzed using a one-way analysis of variance with Tukey’s multiple comparison test.All tests were two sided,and the level of significance was set at 0.05.
Results
G-Rb1 improves the motor function of mice
To detect the effect of G-Rb1 on the motor function of mice with SCI,we used footprint analysis,rotarod system tests,oblique board tests,and BMS scores to evaluate the motor function of mouse hind limbs.The footprint analysis showed that compared with footprints in the SCI group,footprints in the G-Rb1 group were improved,the step length was longer,and the step width was shorter (Figure 2A–C).The rotarod test found that the exercise time of the G-Rb1 group on the rolling axis was longer than that of the SCI group (Figure2D).The oblique board test showed that the inclination angle of the inclined plate in the G-Rb1 group was greater than that in the SCI group (Figure 2E).Finally,the activity of the hind limbs of mice in each group was evaluated using the BMS score.The results showed that the movement of hind limbs in the G-Rb1 group was improved (Figure 2F).The above experiments comprehensively demonstrated the improvement in motor function of mice.Furthermore,immunofluorescence results showed that after treatment,anterior horn motoneuronal survival was higher in the G-Rb1 group than in the SCI group,and motoneurons were more morphologically intact (Figure 2GandH).
G-Rb1 inhibits oxidative stress and protects mitochondria after SCI
After SCI,mitochondria are severely injured,and their energy production is decreased,with increased production of ROS (Slater et al.,2022).G-Rb1 has antioxidant activity in myocardial ischemia (Fan et al.,2020),but whether it protects mitochondria through its antioxidant effects after SCI is unclear.First,a mitochondrial probe was used to detect the number of mitochondria in PC12 cells.The results showed that the number of mitochondria in neurons was increased after treatment (Figure 3AandB).To measure the oxidative stress in each group,ROS,MDA,SOD,and GSH-Px kits were used to detect the content of key substances involved in oxidative stress.The results showed that the level of ROS in neurons in the G-Rb1 group was lower than that in the OGD group (Figure 3CandD).MDA reflects the status of lipid peroxidation (Qi et al.,2014),and its content in neurons was also decreased after treatment(Figure 3E).The content of antioxidant SOD and activity of GSH-Px were increased after treatment (Figure 3FandG).To further verify the antioxidative stress activity of G-Rb1in vivo,ROS,MDA,SOD,and GSH-Px kits were used to detect oxidative stress at the injury site (Figure 3H–J).Consistent with thein vitrofindings,the G-Rb1 group showed decreased contents of ROS and MDA compared with the SCI group.Moreover,the G-Rb1 group exhibited increased SOD contents and GSH-Px activity compared with the SCI group.Therefore,G-Rb1 protected the mitochondria of neurons by inhibiting oxidative stress.
G-Rb1 improves energy deficiency and neuronal apoptosis after SCI
G-Rb1 has been reported to have therapeutic effects on ischemic diseases,such as myocardial ischemia and cerebral ischemia (Slater et al.,2022).Therefore,we considered whether its efficacy is related to the improvement in cell metabolism.First,we collected samples from damaged spinal cordtissue.After G-Rb1 treatment,ATP production in the injured spinal cord was increased (Figure 4A).ATP and lactic acid produced by glycolysis play an important role in neuronal apoptosis and axonal regeneration (Schurr and Passarella,2022).Therefore,we next detected the activities of key glycolytic enzymes.The results showed that the activities of HK and PFK but not PK were increased by G-Rb1 (Figure 4B–D).Because neurons mediate spinal cord signaling (Ding et al.,2022),PC12 cells were used in all cell experiments.The results were similar to those in animal experiments (Figure 4E–H).Furthermore,using an optical microscope,we found that the axon length of neurons was increased after treatment.Moreover,the cell morphology was improved,and the cell density was increased (Figure 4I).To examine axon regeneration,the protein levels of GAP43 and NF200 were detected,and the western blot results showed that the levels of GAP3 and NF200 in the G-Rb1 group were higher than those in the SCI group (Figure 4K–M).Owing to the improvement in cell morphology and cell density after treatment,we speculate that G-Rb1 is related to the inhibition of apoptosis.Therefore,immunofluorescence staining was performed to detect apoptosisrelated proteins,and the results showed that the fluorescence intensity of cleaved caspase-3 in the G-Rb1 group was lower than that in the SCI group in spinal cord tissues.In conclusion,G-Rb1 may reduce neuronal apoptosis by improving energy deficiency (Figure 4N).
Sirt3 is a key factor in the G-Rb1-mediated treatment of SCI
Because Sirt3 regulates glycolysis and oxidative stress (He et al.,2017;Zheng et al.,2018),we questioned whether G-Rb1 works through Sirt3.First,immunofluorescence was used to detect the expression of Sirt3.The results showed that Sirt3 was expressed in neurons,and the fluorescence intensity in the G-Rb1 group was higher than that in the OGD group (Figure 5AandB).The results ofin vivoexperiments showed that the protein expression of Sirt3 in the G-Rb1 group was also higher than that in the SCI group (Figure 5CandD).Next,the Sirt3 inhibitor and G-Rb1 were used together.The rotarod test showed that when the Sirt3 inhibitor was used with G-Rb1,the duration of the Sirt3–group on the rotarod was shorter than that of the G-Rb1 group(Figure 5E).Footprint analysis also showed that the step length of the Sirt3-group was shorter,and the step width was longer compared with those in the G-Rb1 group (Figure 5F–H).Additionally,the results of the oblique board test showed that the grasping ability of the Sirt3-group was weaker than that of the G-Rb1 group (Figure 5I).The BMS score revealed that the hind limb motor ability of the Sirt3-group was lower than that of the G-Rb1 group (Figure 5J).Therefore,G-Rb1 promotes the recovery of motor ability in mice,which is related to Sirt3.
Sirt3 is key for G-Rb1 to inhibit oxidative stress in the treatment of SCI
The above experiments demonstrated that G-Rb1 inhibits neuronal oxidative stress and protects neuronal mitochondria.We next questioned whether it works through Sirt3.First,the functional status of mitochondria was detected by JC-1.Compared with the G-Rb1 group,the Sirt3–group showed decreased red/green fluorescence intensity in PC12 cells,suggesting the aggravation of mitochondrial damage (Figure 6AandB).Moreover,the key substances of oxidative stress,such as MDA,SOD,and GSH-Px,in neurons of each group were detected.The results showed that after Sirt3 was inhibited,the MDA content was higher than that in the G-Rb1 group (Figure 6C).In addition,the SOD content and GSH-Px activity were decreased (Figure 6DandE).To confirm these resultsin vivo,we collected the injured tissues of mice in each group.The results also showed that when Sirt3 was inhibited,the MDA content was increased,and the SOD content and GSH-Px activity were decreased (Figure 6F–H).Therefore,Sirt3 is key for G-Rb1 to inhibit oxidative stress and protect mitochondria.
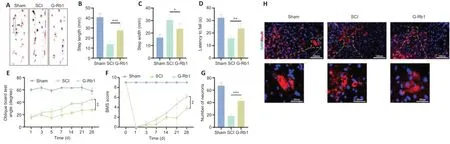
Figure 2|G-Rb1 improves the motor function of mice.
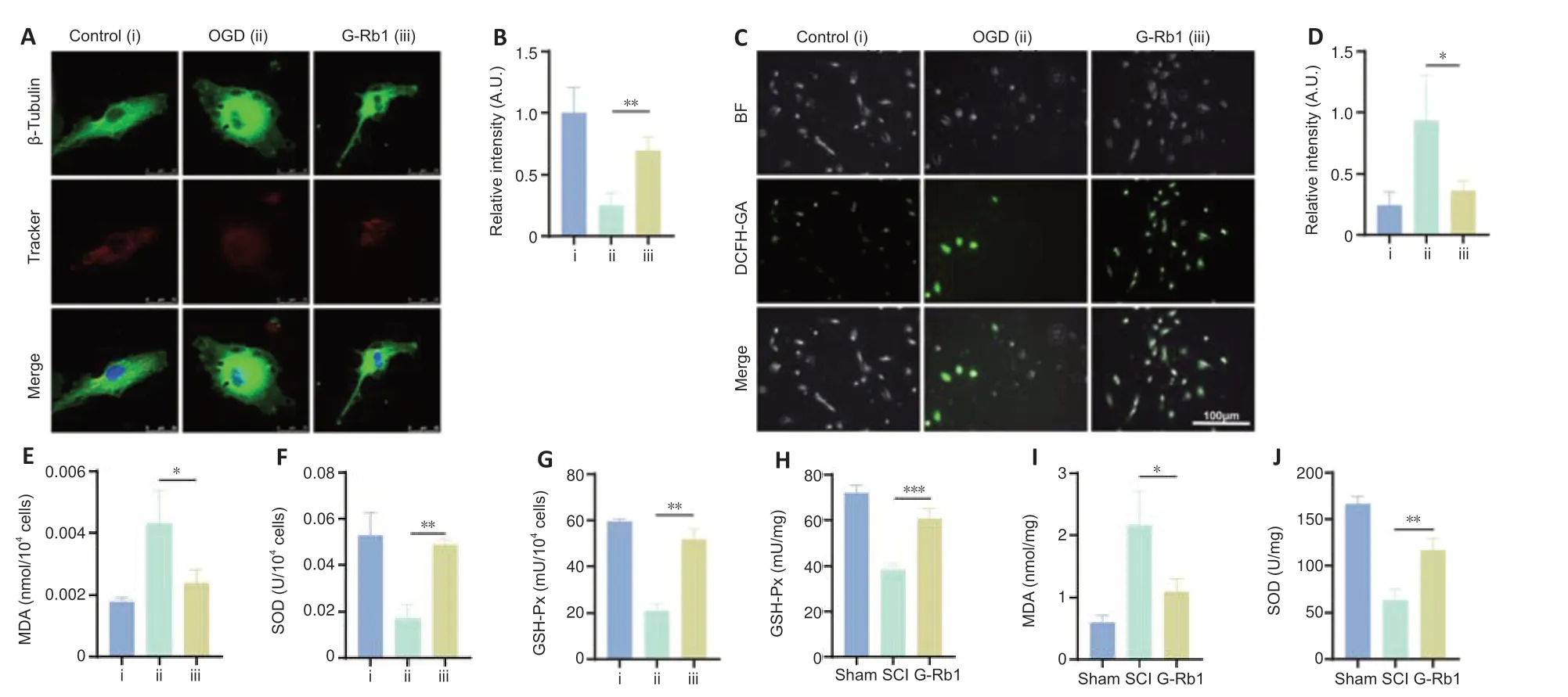
Figure 3|G-Rb1 inhibits oxidative stress and protects mitochondria after SCI.
Sirt3 is involved in the regulation of glycolysis by G-Rb1 in the treatment of SCI
Because Sirt3 is involved in the regulation of SCI recovery by G-Rb1,we next determined which regulatory processes Sirt3 plays a role in.First,Sirt3-inhibited SCI mice were treated with G-Rb1.ATP detection showed that the intracellular ATP content in the Sirt3-group was lower than that in the G-Rb1 group (Figure 7A).The results of enzyme activity experiments showed that compared with those in the G-Rb1 group,the activities of HK and PFK in the Sirt3-group were increased,but there was no difference in PK activity (Figure7B–D).To clarify the effect of Sirt3 inhibition on neurons,PC12 cells were used to detect energy metabolism.The results showed that ATP production,HK activity,and PFK activity were decreased after Sirt3 inhibition (Figure 7E–H).To determine whether G-Rb1 inhibits neuronal apoptosis through metabolic reprogramming related to Sirt3,apoptosis-related proteins were detected.The immunofluorescence results showed that after Sirt3 inhibition,the fluorescence intensity of cleaved caspase-3 was higher than that in the G-Rb1 group (Figure 7I–K).In addition,the expression of Bax and cleaved caspase-3 was higher than that in the G-Rb1 group,and the expression of Bcl-2 was lower than that in the G-Rb1 group (Figure 7L–O).In conclusion,Sirt3 inhibition reverses the neuronal metabolic reprogramming and apoptosis caused by G-Rb1.
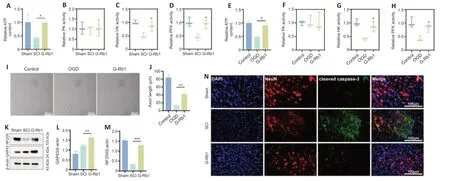
Figure 4|G-Rb1 improves energy deficiency and neuronal apoptosis.
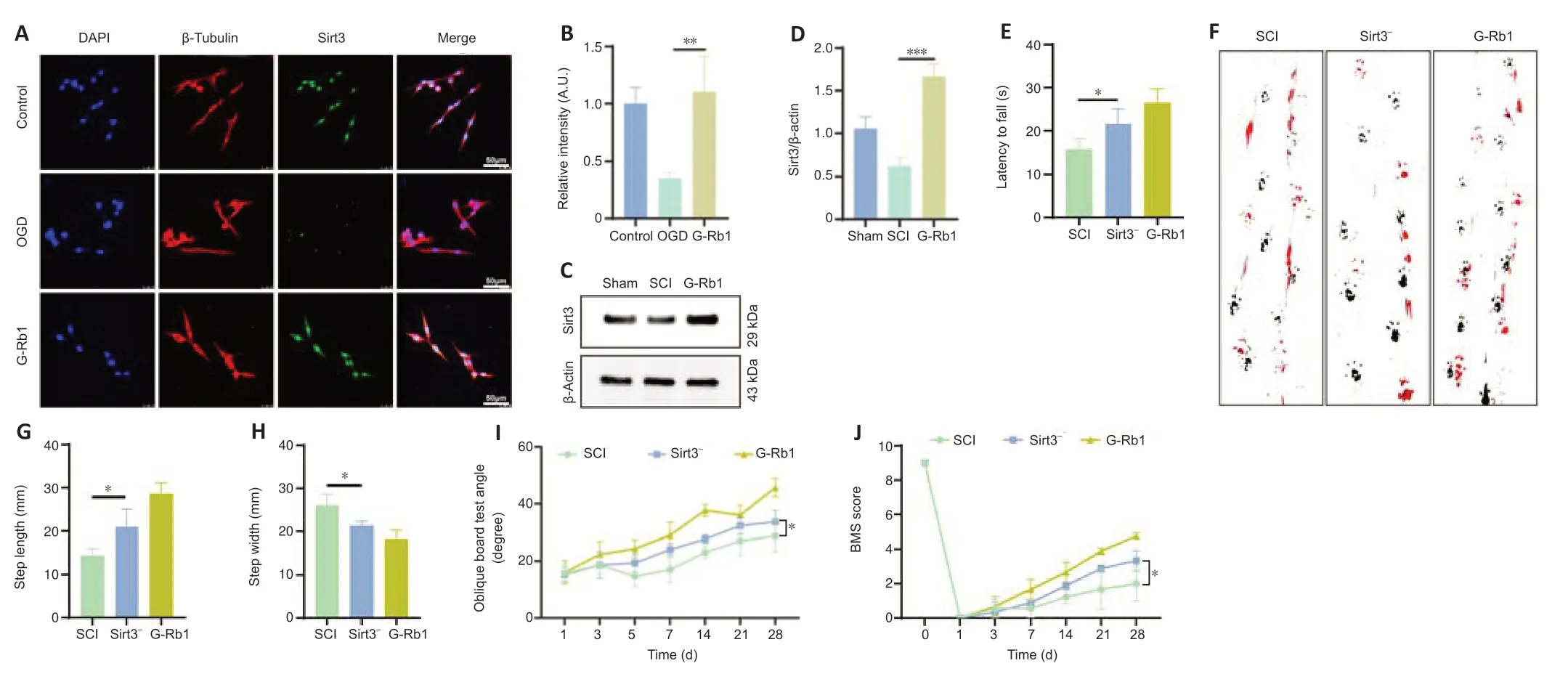
Figure 5|Sirt3 is a key factor in the G-Rb1-mediated treatment of SCI.
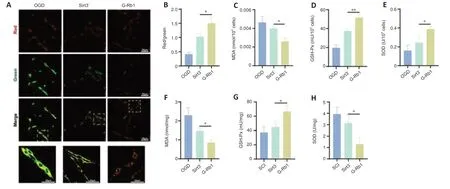
Figure 6|Sirt3 is key for G-Rb1 to inhibit oxidative stress in the treatment of SCI.
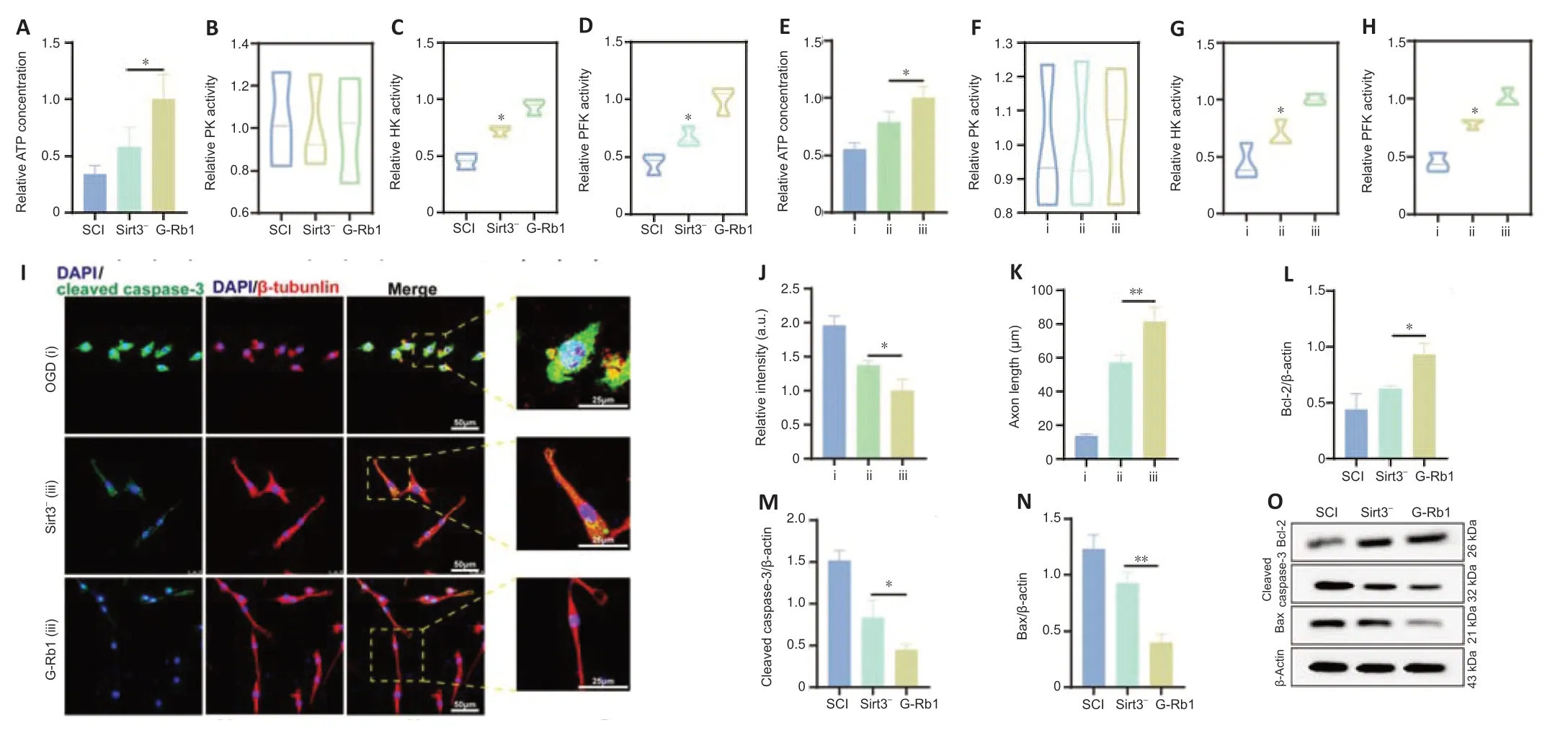
Figure 7|Sirt3 is involved in the regulation of glycolysis by G-Rb1 in the treatment of SCI.
Discussion
SCI triggers oxidative stress,inhibits mitochondrial and glycolytic functions,induces apoptosis,and leads to secondary injury to the spinal cord (Ge et al.,2021).In this study,G-Rb1 improved the motor function of the hind limbs of SCI mice by inhibiting oxidative stress to protect mitochondria and increasing the activity of the key glycolysis enzymes HK and PFK to improve energy deficiency in the ischemic and hypoxic environment of lesions.Using a Sirt3 inhibitor,we determined that Sirt3 is a key factor in the efficacy of G-Rb1.
As the main active ingredient of ginseng,G-Rb1 has low toxicity and neurotrophic and neuroprotective effects on brain injury (Zhang et al.,2021a).This study found that G-Rb1 inhibited oxidative stress after SCI and promoted energy metabolism,thereby improving the motor function of the hind limbs of mice.Although G-Rb1 was less studied in SCI,it has been reported in various diseases,such as stroke,intestinal ischemia,and reperfusion.In a variety of stroke mice,G-Rb1 reduced neuronal death by scavenging free radicals (Xie et al.,2021).Furthermore,in an ischemic microenvironment,G-Rb1 reduces the concentration of glutamic acid and Ca2+and inhibits the apoptosis of hippocampal pyramidal neurons (Wang et al.,2017;Guo et al.,2018).G-Rb1 also had a significant effect in other organ ischemia-reperfusion diseases.Sun et al.reported that after intestinal ischemia and reperfusion in rats,the MDA content was increased,and SOD levels were decreased.G-Rb1 reduced oxidative substances and increased antioxidants,thereby reducing oxidative stress damage (Sun et al.,2013).In this study,G-Rb1 improved the hind limb function score in mice with SCI,reduced the MDA content,increased the SOD content and GSH-Px activity,protected damaged mitochondria,increased the activity of key glycolysis enzymes,and increased the ATP content at the site of injury.Its antioxidant stress mechanisms may include scavenging free radicals,increasing antioxidant enzyme activity,and blocking lipid peroxidation.Neuronal mechanisms to protect against ischemia and hypoxia at the site of injury include increased glycolysis and improvements in mitochondrial function after the inhibition of oxidative stress.Therefore,we speculate that the mechanism by which G-Rb1 improves the physiological function of spinal cord tissue is associated with the inhibition of oxidative stress and improvement in local energy supply deficiencies.
Sirt3 plays an important role in physiological processes,especially energy homeostasis and antioxidant stress (Wu et al.,2014).This study found that Sirt3 inhibition decreased the motor function of SCI mice treated with G-Rb1,increased the oxidative stress capacity,inhibited glycolysis,and decreased ATP production.Therefore,we speculate that Sirt3 is a key factor in the treatment of SCI with G-Rb1.First,Sirt3 has been demonstrated to inhibit oxidative stress in the central nervous system (Hou et al.,2022).As reported by Lee et al.(2021),the overexpression of Sirt3 mitigates oxidative stressinduced cell death and mitochondrial dysfunction in dopaminergic neurons and astrocytes.In Parkinson’s disease,Sirt3-mediated inhibition of oxidative stress has been widely reported (Salvatori et al.,2017;Shen et al.,2020).Sirt3 modulates the activities and biological functions of a variety of proteins involved in various mitochondrial functions.Increasing numbers of studies have suggested that the upregulation of Sirt3 confers a beneficial effect on neuroprotection in various Parkinson’s disease models (Salvatori et al.,2017).First,the antioxidant stress effects of Sirt3 protect mitochondria,thereby improving cellular energy metabolism (Zhai et al.,2017).In addition,Sirt3 regulates glycolysis (Li et al.,2020).After SCI,the injury is locally caused by severe ischemia and hypoxia,and mitochondrial damage and oxygen deficiency increase the importance of anaerobic respiration.This experiment found that Sirt3 increases the activities of key glycolysis enzymes and promotes the production of ATP.Recent studies have also reported a decrease in glycolytic activity and an increase in mitochondrial oxygen consumption in Sirt3-deficient endothelial cells (He et al.,2017;Zeng and Chen,2019).In addition,Sirt3 deficiency inhibits the formation of HKII-VDAC-ANT complexes,resulting in decreased glycolytic enzyme activity,reduced glucose uptake,and increased conversion of metabolic substrates from glucose to fatty acids(Lantier et al.,2015).These reports suggest that Sirt3 not only has antioxidant stress effects but also improves resistance to ischemia and hypoxia damage through metabolic reprogramming that protects mitochondria and glycolysis.
In summary,G-Rb1 inhibits neural oxidative stress and increases energy production through Sirt3,thereby promoting recovery from SCI.These results provide guidance for the application of G-Rb1 in the clinical treatment of SCI.However,there are some shortcomings in this study.The energy metabolism system is large and complex.This study did not investigate the entire process of energy metabolism,and it is difficult to comprehensively explain the regulation of energy metabolism by G-Rb1.In future experiments,we will use a seahorse cell energy metabolism analyzer and metabolome sequencing technology to comprehensively detect the changes in energy metabolism.
Author contributions:Study design and manuscript draft: SW;experiment implementation: SW,ZRZ,SC,HG;data analysis: ZRZ;technical assistance:HSH,FZZ;financial support and study guide: XFM.All authors have approved the final version of the manuscript.
Conflicts of interest:The authors declare that there are no competing financial interests.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional Figure 1:Determination of G-Rb1 concentration in vitro using MTT.
- 中国神经再生研究(英文版)的其它文章
- Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
- Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update

