The role of crm-1 in ionizing radiation-induced nervous system dysfunction in Caenorhabditis elegans
Hui-Qiang Long,Jin Gao,Shu-Qing He,Jian-Fang Han,Yu Tu,,Na Chen,
Abstract Ionizing radiation can cause changes in nervous system function.However,the underlying mechanism remains unclear.In this study,Caenorhabditis elegans (C.elegans) was irradiated with 75 Gy of 60Co whole-body γ radiation.Behavioral indicators (head thrashes,touch avoidance,and foraging),and the development of dopaminergic neurons related to behavioral function,were evaluated to assess the effects of ionizing radiation on nervous system function in C.elegans.Various behaviors were impaired after whole-body irradiation and degeneration of dopamine neurons was observed.This suggests that 75 Gy of γ radiation is sufficient to induce nervous system dysfunction.The genes nhr-76 and crm-1,which are reported to be related to nervous system function in human and mouse,were screened by transcriptome sequencing and bioinformatics analysis after irradiation or sham irradiation.The expression levels of these two genes were increased after radiation.Next,RNAi technology was used to inhibit the expression of crm-1,a gene whose homologs are associated with motor neuron development in other species.Downregulation of crm-1 expression effectively alleviated the deleterious effects of ionizing radiation on head thrashes and touch avoidance.It was also found that the expression level of crm-1 was regulated by the nuclear receptor gene nhr-76.The results of this study suggest that knocking down the expression level of nhr-76 can reduce the expression level of crm-1,while down-regulating the expression level of crm-1 can alleviate behavioral disorders induced by ionizing radiation.Therefore,inhibition of crm-1 may be of interest as a potential therapeutic target for ionizing radiationinduced neurological dysfunction.
Key Words: behavior;Caenorhabditis elegans;degeneration;disorder;dysfunction;nerve injury;nervous system;neurodevelopment;neuron;radiation
From the Contents
Introduction 1386
Methods 1387
Results 1388
Discussion 1389
Introduction
The practical applications of ionizing radiation are increasingly extensive.More and more people are becoming involved with the development of both nuclear and radiological technologies.Ionizing radiation sources can be natural or artificial in nature.Artificial radiation sources include the production and testing of nuclear weapons,the application of nuclear technology,nuclear accidents,and medical exposure.Ionizing radiation can affect human health in numerous ways owing to its deleterious effects on biological systems.These include reproductive system damage,hematopoietic system damage,and nervous system dysfunction (Ye,2011;Han et al.,2019;Lalkovicova,2022;Wu et al.,2022).Therefore,understanding the pathophysiological processes by which ionizing radiation produces these effects and exploring their mechanisms is of great value in the safe and effective use of ionizing radiation.
Different tissues in the human body have different degrees of sensitivity to ionizing radiation.This is because of differences in the rate of cell division and the degree of cell differentiation within these tissues.The central nervous system is a mildly sensitive tissue.Many radiobiologists consider the brain’s tolerant dose to be between 55 and 65 Gy,while the fractional tolerant dose is approximately 2 Gy (Marazziti et al.,2016).However,in recent years it has been reported that the sensitivity of central nervous system to ionizing radiation may have been underestimated.Despite the relatively high resistance of nerve cells to the effects of ionizing radiation,the functional activity of the nervous system can undergo significant changes,even under the influence of doses that do not cause deterministic effects (Marazziti et al.,2016;Kosiakova et al.,2020).These include changes in electrophysiological activity,cognitive decline,mental disorders,and decreased motor function.It has been reported that the electroencephalograms of adolescents withtinea capitis displayed abnormal beta waves after receiving 1.21–1.39 Gy of X-ray radiation treatment to the scalp (Yaar et al.,1982;Aguiar et al.,2015).Similarly,previous research,in which mice were subject to transcriptome analysis before and after irradiation with 0.1 Gy of137Cs γ-ray whole-body radiation,showed that the expression levels of cognition-related genes were reduced in irradiated mice.Specifically,there was a down-regulation of genes related to synaptic plasticity,such as glutamate receptor,ionotropic,NMDA1(zeta 1) (Grin1),and glutamate receptor,ionotropic,AMPA3(alpha 3) (Gria3)(Lowe et al.,2009).Memory loss and language disorders have occurred in staff who have worked in the interventional radiology department for an extended period of time (Marazziti et al.,2015),while clean-up workers in the Chornobyl exclusion zone had significantly higher rates of schizophrenia compared with the general Ukrainian population (Loganovsky and Loganovskaja,2000).In a study of female workers from 12 nuclear weapon plants in the United States,an increased mortality from mental disorders was found (Sibley et al.,2003;Marazziti et al.,2016).York et al.(2012) used137Cs γ-rays to irradiate mice with 0.5 and 2 Gy of whole-body radiation and found that the locomotor ability of mice was reduced after radiation.Taking these findings together,it can be seen that even a low dose of ionizing radiation can cause changes in nervous system function that are significant enough to result in diverse clinical symptoms: cognitive impairment,mental disorders,and decreased locomotor capacity.That is to say,low doses of ionizing radiation have a negative effect on the health of the recipient.Therefore,examining the nervous system dysfunction induced by ionizing radiation,exploring its pathophysiology,and finding protective biological targets play an important role in the application of nuclear and radiation technology,especially in the realm of occupational exposure.
To date,there have been relatively few reports in whichCaenorhabditis elegans(C.elegans) has been used as a model to study the impairments of ionizing radiation on the nervous system.However,in other fields,C.elegansis a mature model animal for studying neurobiology (Appleby,2012;Liu et al.,2020;Randi and Leifer,2020).C.eleganshas a relatively simple nervous system,but has motor neurons and sensory neurons,as well as many neurotransmitters such as dopamine.These elements make it a useful model in the study of neuroscience (White et al.,1986).Some studies have found that noxious external environmental stimuli (such as arsenic)can lead to nervous system dysfunction inC.elegans.These manifest as disorders of behaviors including head thrash and body bend behaviors,as well as degenerative changes in dopaminergic and serotonergic neurons.The degeneration of dopaminergic neurons is implicated as a major cause of behavioral disorders inC.elegans(Qu and Wang,2020;Zhang et al.,2020;Chen et al.,2021).Therefore,we investigated the effects of ionizing radiation on nervous system function inC.elegans.To accomplish this,we examined behavioral motor function,sensory function,and dopaminergic neuron development.
Cysteine rich motor neuron protein homolog (crm-1) encodes a putative transmembrane protein with multiple cysteine-rich domains that is known to have bone morphogenetic protein (BMPs) binding activity.This protein has been shown to control body size inC.elegans(Fung et al.,2007).There are many studies oncrm-1in mammals.This gene is involved in the regulation of lens development,cell adhesion and migration in human or mouse,and motor neuron development (Zeng et al.,2015;Zhang et al.,2016;Tam et al.,2018;Brązert et al.,2020).crm-1has been poorly studied inC.elegans.This study mainly explores whether crm-1 is involved in the regulation of nervous system function inC.elegans.
Methods
C.elegans culture
C.elegansstrains were cultured on bacterial lawns of either Escherichia coli OP50 (OP50) or Escherichia coli HT115(DE3) (HT115(DE3)) (Shanghai Weidi Biotechnology Co.,Ltd.,Shanghai,China) at 20°C in the dark and~50% humidity,according to standard methods (Brenner,1974).C.eleganswas grown on standard nematode growth medium (3 g NaCl,20 g agar,2.5 g peptone,975 mL H2O,1 mL 1 M CaCl2,1 mL 5 mg/mL cholesterol,1 mL 1 M MgSO4and 25 mL 1 M K3PO4).It fed on OP50 or HT115(DE3) and took approximately 48 hours to develop from the L1 stage to the young adult stage(Byerly et al.,1976).TheC.elegansstrains used included wild-type strain N2,transgenic strain BZ555(dat-1p::GFP),and transgenic strain PHX5872crm-1(syb5872) (SunyBiotech).In BZ555,the sodium-dependent dopamine transporter (dat-1) is co-expressed with GFP.In PHX5872crm-1(syb5872),crm-1and GFP are co-expressed.Wild-type strains N2 and OP50 were obtained from Soochow University,and transgenic strain BZ555 was obtained from Wenzhou Medical University.When examining behavioral indicators,wild-type strains were divided into two groups: a control and a radiation group.To examine behavioral indicators after irradiation and/or inhibition ofcrm-1,wild-type strains were divided into four groups: a control group,a crm-1 RNAi group,a radiation group,and acrm-1RNAi and radiation group.When examining dopaminergic neurons,transgenic strains BZ555 were divided into two groups: a control group and a radiation group.Worms of the transgenic strain PHX5872crm-1(syb5872) were divided into two groups: a control group and a radiation group after irradiation or a control group and anhr-76RNAi group after inhibition ofnhr-76.TheC.elegans-related experiments were reviewed by the Experimental Animal Ethics Committee of Soochow University on March 16,2020.
Irradiation
The60Co γ irradiator in the State Key Laboratory of Radiation Medicine and Protection,School of Radiation Medicine and Protection,Soochow University,was used in this study.In preliminary experiments carried out prior to this study,it was found that 75 Gy could induce abnormal behaviors inC.elegans.Therefore,C.elegansin the L2 stage was exposed to 75 Gy γ-ray radiation.The dose rate was 25 Gy/min and the irradiation time was 3 minutes.Worms were then cultured to the young adult stage for follow-up experiments (Figure 1).
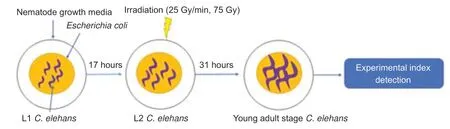
Figure 1|Flow chart showing radiation in Caenorhabditis elegans (C.elegans).
Measurement of behavioral motor and sensory function
InC.elegans,changes of behavioral motor and sensory function are reflected in changes in head thrash,touch avoidance,and foraging behaviors.Measurement of head thrashes was carried out as described previously (Zeng et al.,2017).The young adult stageC.eleganswas transferred to nematode growth media medium without food.M9 buffer (60 μL;5 g NaCl,3 g K2HPO4,15 g Na2HPO4·12H2O,1 mL 1 M MgSO4,H2O to 1000 mL;Sangon Biotech(Shanghai) Co.,Ltd.,Shanghai,China) was added to a singleC.elegansworm,and the number of head thrashes occurring within 60 seconds was recorded.The movement of the head ofC.elegansto one side and then back was recorded as a head thrash.ThirtyC.elegansper group were analyzed.
Touch avoidance was measured as described in previous studies (Kaplan and Horvitz,1993;Hobert et al.,1999;Hart,2006).An eyebrow hair was used to gently touch the area between the posterior bulb and the vulva of young adult stageC.elegans(Figure 2A),and eachC.eleganswas touched 10 times.The number of backwards movements after touching was recorded.ThirtyC.elegansper group were analyzed.
Foraging behavior was studied as described in a previous study (Kohra et al.,2002).Before the test,a petri dish was prepared.One end of the nematode growth medium was set as the starting point.A target circle with a radius of 0.5 cm was coated with OP50.The center of this target circle was 4 cm from the starting point (Figure 2B).Twenty young adult stageC.eleganswere transferred to the starting point.The number ofC.elegansreaching the target circle was observed after 2,4,6,8,and 24 hours,and six parallel samples were set in each group.The foraging level was considered to be the number ofC.elegansreaching the target circle divided by the total number ofC.elegans.
Dopamine neurons analysis
Morphological changes in dopamine neurons of the transgenic strain BZ555 were observed (Nass et al.,2002;Zhang et al.,2020).The young adult stageC.eleganswere transferred to a 3% agarose pads,anesthetized by dropwise addition of 10 μL of 4 mM levamisole hydrochloride (Sangon Biotech (Shanghai) Co.,Ltd.),and covered with a coverslip.Dopamine neurons of wholeC.eleganswere counted under a fluorescent microscope(Guangzhou Micro-shot Technology Co.,Ltd.,Guangzhou,China).The number of discontinuous dendrites and the comparative fluorescent intensity of cell bodies were considered to reflect the extent to which the neuronal development of dopamine neurons was negatively affected.FiftyC.elegansper group were analyzed.
RNA sequencing analysis
Gene expression of irradiated and control nematodes was analyzed using RNA sequencing (RNA-seq).After the total RNA of wholeC.eleganswas extracted,mRNA was enriched and cut into short fragments before being synthesized into complementary DNA (cDNA) and purified.End repair was performed,a poly(A) tail was added,and it was connected with the sequencing adapter.Appropriate fragment size was selected for polymerase chain reaction (PCR)amplification and Illumina HiSeq X Ten was used for sequencing.Proteincoding gene expression was calculated using the fragments per kb per million reads method to compare different samples (Roberts et al.,2011).Differentially expressed genes with a significance level ofP< 0.05 and a fold change greater than 2 were screened for using the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis tools(Viau et al.,2020).Transcriptome sequencing and analysis were conducted by OE Biotech Co.,Ltd.(Shanghai,China).
String online protein-protein interaction networks analysis and literature survey
The string online protein-protein interaction networks (PPI) analysis system(https://string-db.org/) was used to screen out genes that were highly related to cysteine rich motor neuron protein homolog (crm-1) interaction,andC.eleganswas selected as the species.Results were analyzed using the cytoHubba plug-in in cytoscape3.8.2 software (https://github.com/cytoscape/cytoscape/releases/3.8.2/).This assigns values to each gene using the topological network algorithm.Key genes (hub genes) related tocrm-1were subsequently sorted and mined.The following parameters were used:a degree threshold of 2,a node score threshold of 0.2,a k score of 2,and a maximum depth of 100.
Differentially expressed genes were screened out using to the KEGG pathway analysis,GO function analysis and string online PPI analysis systems.Literature searches for each of these genes using PubMed (https://pubmed.ncbi.nlm.nih.gov/) and wormbase (https://wormbase.org//#012-34-5) were carried out to understand the relevant function of these genes.
RNA interference (RNAi)
An 807 bp fragment ofcrm-1was amplified by PCR using cDNA as a template and inserted into theHind III site of vector L4440.The recombinant plasmid was transformed into HT115(DE3) to construct a crm-1 RNAi clone.A 1918 bp fragment of nuclear hormone receptor family (nhr-76) was amplified by PCR using genomic DNA as a template and inserted into theHind III site of vector L4440,and then transformed into HT115 (DE3) to construct an nhr-76 RNAi clone.The primers used were as follows: for thecrm-1RNAi clone,the forward primer 5′-gtc gac ggt atc gat aag ctt GAA ATG CGT TCC ATC CGT GC-3′ and the reverse primer 5′-cag gaa ttc gat atc aag ctt TCA CGA CGA AGT CCA TTG GC-3′ (lowercase letters are homologous end sequences).For the nhr-76 RNAi clone,forward primer 5′-gtc gac ggt atc gat aag ctt ATT CAG GAA GCC GAG GAG GA-3′ and reverse primer 5′-cag gaa ttc gat atc aag ctt CGA CTC GCG TCC GAT AAG TT-3′.crm-1RNAi was performed by feeding synchronized L1 stage with HT115(DE3) carrying thecrm-1RNAi clone.nhr-76RNAi was performed in the same way.HT115(DE3) carrying the empty vector L4440 plasmid was used as a negative control (Fung et al.,2007).The HT115 strain was streaked on Luria-Bertani media (LB) agar (2 g tryptone,1 g yeast,1 g NaCl,3 g agar,H2O to 200 mL,pH 7.5) with 50 μg/mL ampicillin and 10 μg/mL tetracycline.If the LB agar was covered with monoclonal strains,the transformation was considered successful.The irradiation conditions were the same as above,as were the measurements of head thrashes,touch avoidance and foraging.
Quantitative PCR
Young adult stageC.eleganswas collected.The total RNA extracted and then reverse transcribed into cDNA (Zhang et al.,2020).Quantitative PCR(qPCR) was performed using Novozymes ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co.,Ltd.,Nanjing,China) to verify RNA-seq results and RNAi efficiency.The instrument setup conditions were as follows: initial denaturation for 30 seconds at 95°C,followed by 40 cycles of 95°C for 10 seconds and 60°C for 30 seconds.The relative expression level of target genes were calculated using the 2–∆∆CTmethod (Livak and Schmittgen,2001) with the genetba-1being used as the internal control.The primers used for qPCR are listed inTable 1.
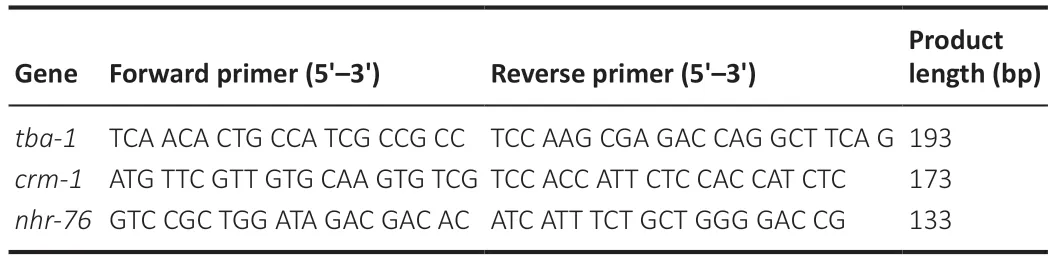
Table 1|qPCR primer sequences
Semi-quantitative analysis of PHX5872 crm-1 (syb5872)
The fluorescence intensity value in the head was scored under a fluorescent microscope after irradiation/sham irradiation ornhr-76RNAi in the transgenic strain PHX5872 crm-1 (syb5872).Thirty young adult stageC.elegansper group were analyzed.
Statistical analysis
All experimental results in this study were statistically analyzed using SPSS 23.0 software (IBM Corp.,Armonk,NY,USA) and GraphPad Prism 8.0.2 software (GraphPad Software,San Diego,CA,USA,www.graphpad.com).The measurement data are expressed as mean ± standard deviation (SD).For statistical analysis between two independent samples,an independent samplet-test was used.For multiple groups of samples,one-way analysis of variance followed by least significant difference (LSD)post hoctest (equal variance) or Dunnett’s T3post hoctest (unequal variances) was used.Each experiment was repeated at least three times.ThirtyC.eleganswere selected per group for this study when examining head thrashes and touch avoidance behaviors.When detecting foraging behavior,six parallel samples were set in each group and 20C.eleganswere selected per parallel sample.FiftyC.eleganswere selected per group when detecting dopamine neurons.ThirtyC.eleganswere selected per group when measuring the fluorescence intensity of PHX5872 crm-1 (syb5872).
Results
The effects of 60Co γ-rays on nervous system function of C.elegans
To evaluate the effects of60Co γ-rays on nervous system function inC.elegans,changes in the head thrash,touch avoidance and foraging behaviors of wild-type strain were examined before and after irradiation.Changes in dopaminergic neurons in the head the transgenic strain BZ555 after radiation were observed to reflect the functional changes of the nervous system ofC.elegans.
Behavioral motor and sensory dysfunction
Worms of the wild-type N2 strains were divided into a control group and a radiation group.After being irradiated with75Gy γ-rays,changes in the behavioral motor and sensory function were detected.The head thrash frequency ofC.elegansdecreased by~8% from 109.83 times to 101.07times per minute (P=0.005;Figure 2C),the proportion of touch avoidance was significantly reduced by~23% from 0.78 to 0.60 (P< 0.001;Figure 2D),and the level of foraging behavior was significantly lessened in the radiation group by~89%,83%,71%,70%,and 47% at 2,4,6,8,and 24 hours compared with the control group,respectively (P=0.003,P=0.001,P=0.001,P=0.001,P=0.003,respectively;Figure 2E).It has been reported that head thrash frequency is dependent on motor neurons such as dorsal A-type and dorsal B-type (White et al.,1976,1986),while foraging is associated with amphid wing neuron A and amphid wing neuron C olfactory neurons,outer labial quadrant and inner labial 1 sensory neurons,and ring motor neuron G motor neurons (Zeng et al.,2017).Anterior ventral microtubule and anterior lateral microtubule sensory neurons ofC.eleganstransmit information to anterior ventral process D and anterior ventral process A interneurons after gentle touch stimulation.These interneurons then innervate Dorsal A-type motor neurons to give rise to backward avoidance responses (de Bono and Maricq,2005).Therefore,this study evaluated the effects of γ-rays on the behavioral motor and sensory function ofC.elegansby examining changes in head thrash,touch avoidance and foraging behaviors.The experimental results show that 75Gy γ radiation can induce nervous system dysfunction in wild-type strains,which manifests as decreased behavioral motor ability and abnormal sensory function.
Degeneration of dopamine neurons
In trangenic strain BZ555,the sodium-dependent dopamine transporter (dat-1) is co-expressed with GFP (Bijwadia et al.,2021).Thedat-1gene encodes a dopamine transporter and so regulates dopamine levels during dopaminergic neurotransmission (Nirenberg et al.,1996).Therefore,fluorescent neurons observed in BZ555 may be considered to be dopaminergic in nature,and the morphology of these neurons can be directly observed using this model.The transgenic strain BZ555 has a total of four pairs of dopaminergic neurons,including one pair of posterior deirid neurons in the tail,two pairs of cephalic sensilla (CEP) neurons and one pair of anterior deirid (ADE)neurons in the head (Figure 3A–C).In this study,the effects of60Co γ-rays on dopamine neurons ofC.eleganswere evaluated by observing the integrity of CEP dendrites and the fluorescence intensity of CEP and ADE cell bodies in transgenic strain BZ555.After 75 Gy γ irradiation,the fluorescence area in the head region ofC.eleganshad shrunk,the fluorescence appeared to be discontinuous and the integrity of CEP dendrites decreased by 3% from 1.00 to 0.97 (P=0.014).After irradiation,the fluorescence area of CEP and ADE cell bodies diminished,and the fluorescence intensity weakened by 10% from 1.00 to 0.90 (P=0.024),indicating that CEP and ADE cell bodies had atrophied (Figure 3D–I).The change in fluorescence level after60Co γ irradiation suggests that ionizing radiation induces degeneration of dopamine neurons inC.elegans.
Genome-wide transcriptional analysis and bioinformatics analysis
To search for key genes that play a regulatory role in ionizing radiationinduced nervous system dysfunction,we performed RNA-seq analysis in wildtype strains,comparing the radiation and control groups.We then screened out differentially expressed genes related to nervous system function through bioinformatics analysis.
Genome-wide transcription analysis and string online protein-proteininteraction networks analysis of wild-type strains after radiation exposure
The screened differentially expressed genes were analyzed using horizontal clustering (Figure 4A) and a volcano plot (Figure 4B) to understand their overall distribution.The genes were then subjected to GO enrichment analysis and KEGG pathway enrichment analysis.On the premise of stable RNA quality,a total of 20,174 genes were sequenced.Compared with the control group,1338 genes were significantly different after radiation.Of these,638 genes were up-regulated and 700 genes were down-regulated(Figure 4C).According to the KEGG analysis,the enriched KEGG pathways included the transforming growth factor-beta signaling pathway,apoptosis,nitrogen metabolism,and tryptophan metabolism.(Figure 4D).According to GO analysis,the GO functions enriched in this study included nervous system development,insulin-like growth factor-activated receptor activity,insulin-like growth factor binding,the extracellular region,serine-type endopeptidase inhibitor activity,and regulation of cell growth (Figure 4E).
According to GO functional enrichment analysis,KEGG pathway analysis,and a literature survey (Kolle et al.,2000;Fung et al.,2007),the differentially expressed genecrm-1is closely related to nervous system development(GO:0007399).The string online PPI analysis system was used to screen out the genes that were highly related tocrm-1interaction inC.elegans.The results were analyzed using the cytoHubba plug-in in cytoscape3.8.2 software,focusing on the hub genes of crm-1.Network modules were mined from the constructed PPI network using the plugin MCODE in cytoscape3.8.2.The parameters were set as follows: a degree threshold of 2,a node score threshold of 0.2,a k score of 2,and a maximum depth of 100.A score greater than 5 indicates a high probability of an interaction between two genes.Therefore,the genes with a score > 5 were selected for analysis (Figure 5).Finally,the differentially expressed genenhr-76(score=6) was screened.It has been reported that nhr-76 is closely related to nervous system function in human or mouse (Li et al.,2020;Yang et al.,2021;Ben-Zvi and Liebner,2022),and is closely related to fatty acid metabolism inC.elegans(Noble et al.,2013).Therefore,it was speculated thatcrm-1andnhr-76could be involved in the regulation of ionizing radiation-induced nervous system dysfunction inC.elegans.
Changes in the expression levels of crm-1 and nhr-76 genes in C.elegans after γ-ray irradiation
Changes in the expression levels ofcrm-1andnhr-76after radiation were assessed by qPCR.It was found that the expression levels ofcrm-1(P=0.028) andnhr-76(P=0.014) induced by 75 Gy γ-rays were increased by approximately 49% (an increase from 1.02 to 1.52) and approximately 50% (an increase of 1.01 to 1.51),respectively (Figure 5C).
The role of nhr-76/crm-1 in ionizing radiation-induced nervous system dysfunction in C.elegans
To confirm hownhr-76andcrm-1interacted and participated in the regulation of ionizing radiation-induced nervous system dysfunction inC.elegans,RNAi technology was used to knock downcrm-1,and expression levels of nhr-76 were measured.Changes in head thrash,touch avoidance and foraging behaviors ofC.eleganswere observed after successful knockdown ofcrm-1.
The interaction between crm-1 and nhr-76
To verify whethercrm-1interacted withnhr-76,RNAi technology was used in this study to knockdowncrm-1andnhr-76in wild-type N2 strains.Their gene expression levels were then measured after successful interference.On knocking down the expression level of thecrm-1gene (P< 0.001),there was no statistical difference in the expression level of thenhr-76gene (P=0.241)inC.elegans.However,knocking down the expression level of thenhr-76gene (P=0.015) produced a change in the expression level of thecrm-1gene(P=0.016) decreased by approximately 59% (a decrease from 1.01 to 0.41)Figure 6).Therefore,nhr-76appears to act upstream ofcrm-1at the gene level.
Fluorescence intensity changes of PHX5872 crm-1 (syb5872)
To demonstrate whether the change incrm-1expression that occurs after irradiation is regulated bynhr-76,and to further investigate whethercrm-1is regulated bynhr-76,the transgenic strain PHX5872 crm-1 (syb5872) was used.The fluorescence intensity of the transgenic strain increased by 35%from 1.00 to 1.35 after irradiation when compared with the control group.This suggests thatcrm-1expression is increased by irradiation.Conversely,the fluorescence intensity weakened by 36% from 1.00 to 0.64 compared with the control group after knocking down the expression level of thenhr-76gene(P< 0.001,P< 0.001;Figure 7).This provides further evidence thatcrm-1is regulated bynhr-76.
The role of crm-1 in ionizing radiation-induced nervous system dysfunction in C.elegans
To verify whethercrm-1is involved in the regulation of ionizing radiationinduced nervous system dysfunction inC.elegans,we used RNAi technology to knockdowncrm-1.Changes in head thrash frequency,touch avoidance,and foraging behaviors ofC.eleganswere observed.Four experimental groups were set up: the control group,thecrm-1RNAi group,the radiation group,and thecrm-1RNAi and radiation group.Comparing the control andcrm-1RNAi group,there were no significant changes in head thrash frequency(P=0.264),touch avoidance (P=1.000),or foraging at 2 (P=0.078),4 (P=0.376),6 (P=0.285),8 (P=0.315),or 24 hours (P=0.659).The head thrash frequency and the proportion of touch avoidance were increased by about 12% from 94.17 times to 105.63 times per minute (P=0.001) and 24%from 0.58 to 0.72 (P=0.007),respectively in thecrm-1RNAi and radiation group when compared with the radiation group.There was no statistical difference in foraging at 2 (P=0.903),4 (P=0.823),6 (P=0.690),8 (P=0.986),or 24 hours (P=0.659) (Figure 8).This suggests that inhibition ofcrm-1can effectively alleviate ionizing radiation-induced head thrash and touch avoidance,but cannot alleviate ionizing radiation-induced foraging.It could be seen that knocking down the expression level of thecrm-1gene effectively improved the behavioral disorders observed inC.elegansthat are induced by ionizing radiation.From previous experimental results,the expression ofcrm-1gene was regulated bynhr-76.Therefore,thenhr-76/crm-1pathway may be involved in the regulation of ionizing radiation-induced nervous system dysfunction.
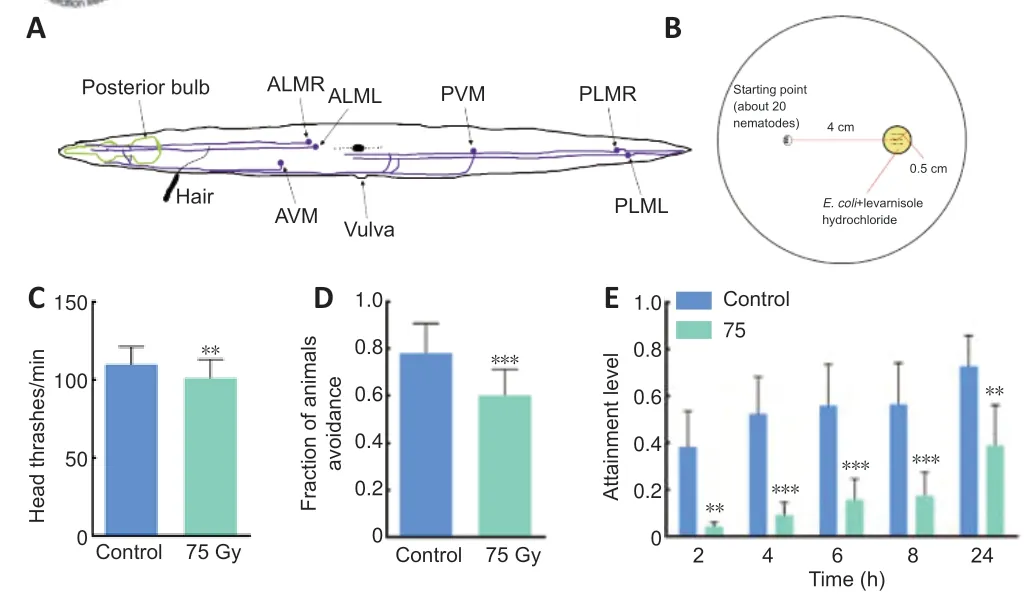
Figure 2|Schematic diagram of behavioral assays and the effects of ionizing radiation on behavior of Caenorhabditis elegans (C.elegans).
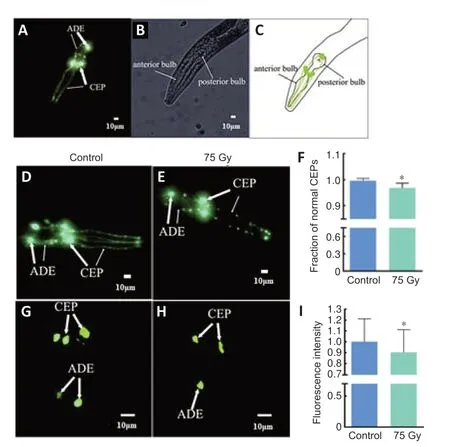
Figure 3|Visualization of dopamine neurons in the head neurons of Caenorhabditis elegans (C.elegans) and the effects of ionizing radiation.
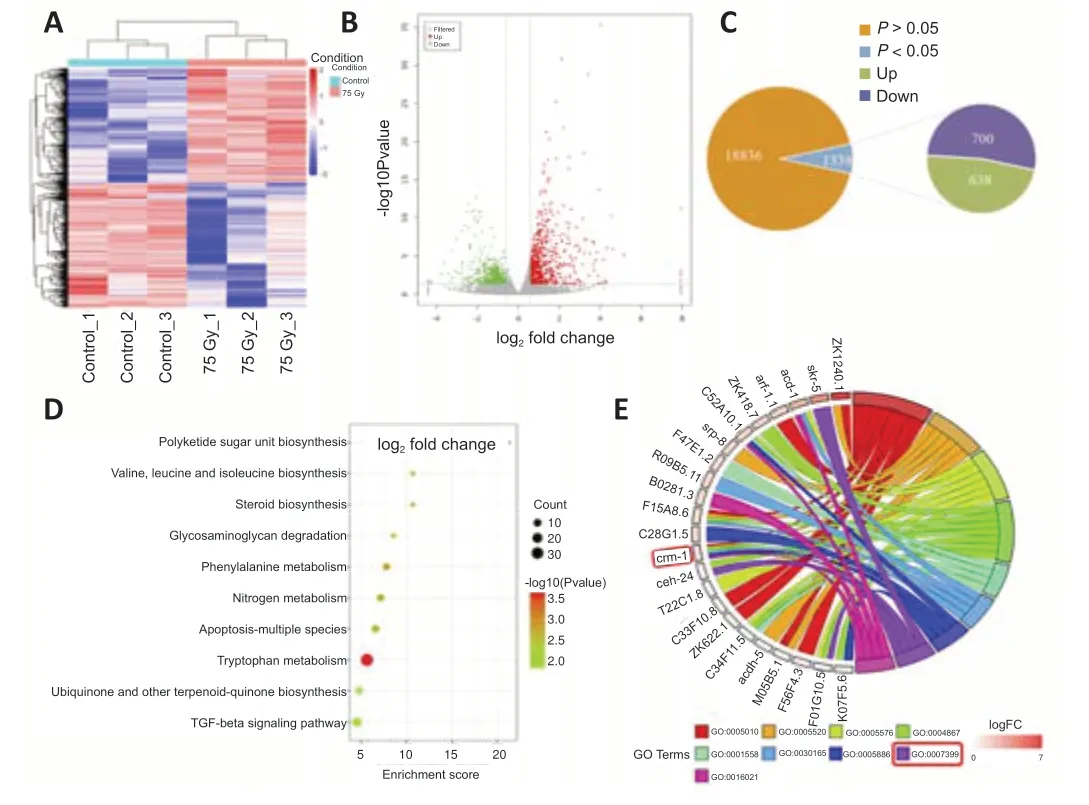
Figure 4|Analysis of differentially expressed genes induced by ionizing radiation in Caenorhabditis elegans (C.elegans).
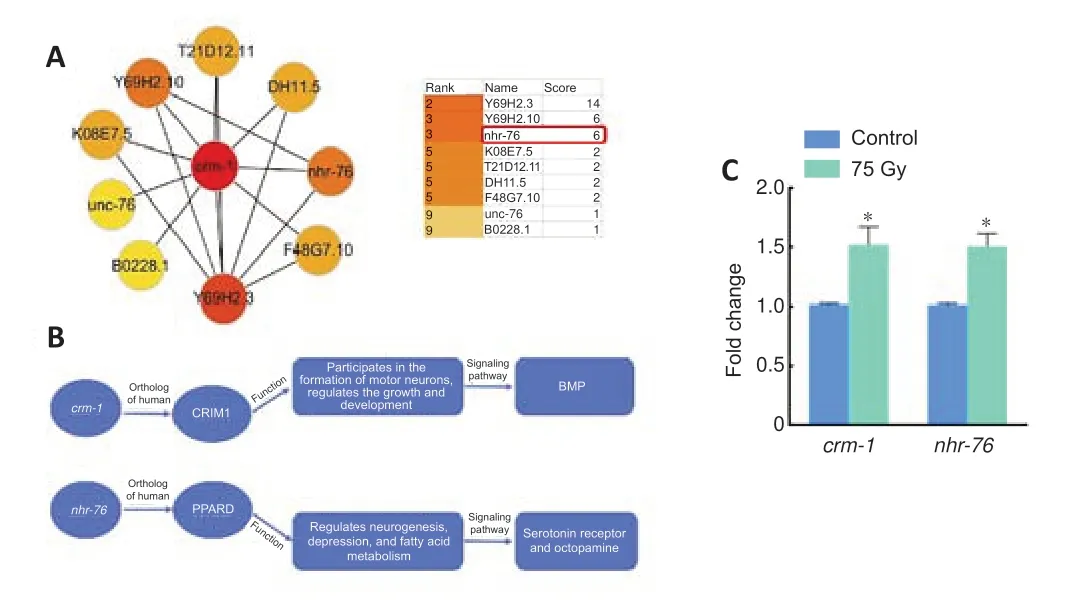
Figure 5|Interaction analysis of crm-1,functions,and expression level changes of crm-1 and nhr-76 in Caenorhabditis elegans (C.elegans) using PPI analysis.
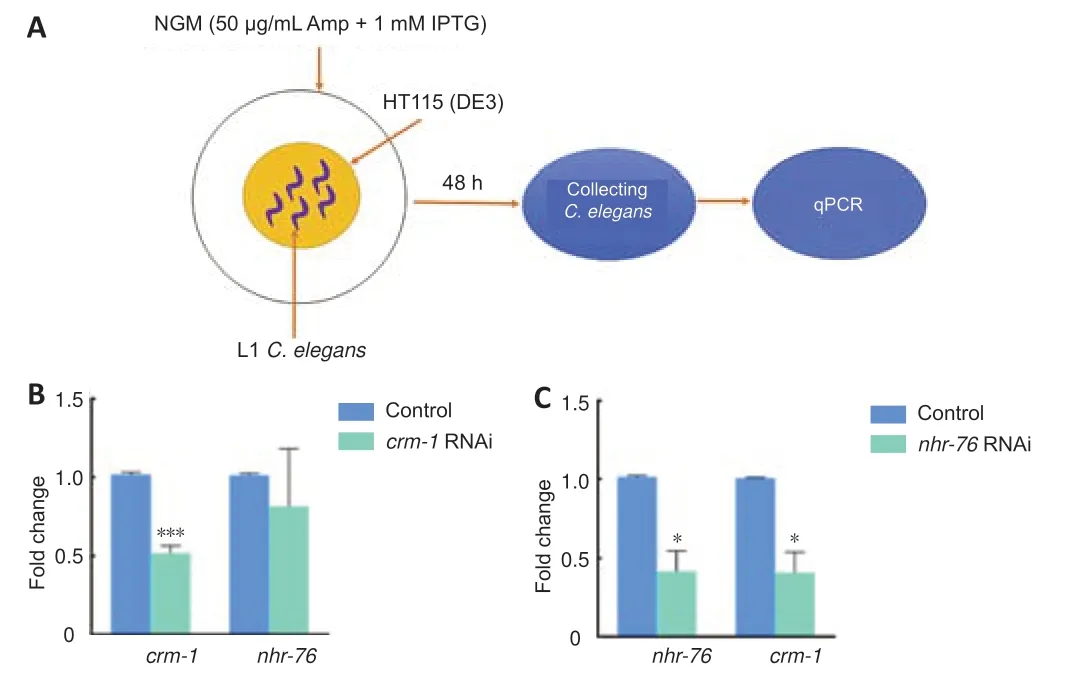
Figure 6|Interaction and RNAi efficiency between crm-1 and nhr-76 in Caenorhabditis elegans (C.elegans).
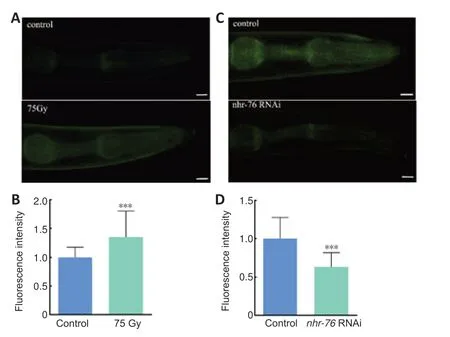
Figure 7|Fluorescence intensity changes after irradiation or knockdown of nhr-76 in transgenic strain PHX5872 crm-1 (syb5872).
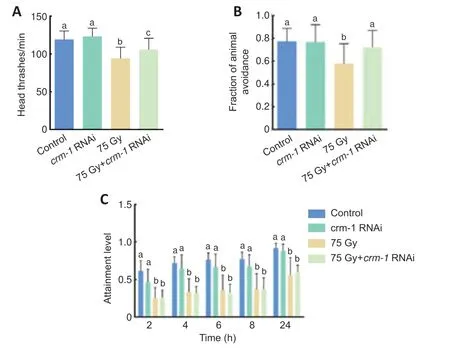
Figure 8|The effects of knockdown of crm-1 gene expression on behavior of Caenorhabditis elegans (C.elegans).
Discussion
The nervous system ofC.eleganshas 302 neurons,which may be classified into motor neurons,sensory neurons,interneurons,and polymodal neurons.It is relatively simple,transmitting information through about 6400 chemical synapses,900 gap junctions and 1500 neuromuscular junctions.Similar to higher animals,the neurons ofC.eleganshave many neurotransmitters,such as dopamine,serotonin and glutamate.The behavior ofC.elegansincludes not only basic behaviors such as locomotion,foraging,feeding and defecation,but also complex behaviors such as the avoidance response and chemotaxis.Many behaviors ofC.elegansare quantifiable.Researchers can therefore use these to evaluate the functional activities of the nervous system (Hart,2006).Many researchers have examined behavioral indicators such as head thrash,body bend,foraging and chemotaxis,as well as morphological changes in dopaminergic neurons and glutamatergic neurons,to evaluate the nervous system function ofC.elegans(Cao et al.,2019;Liu et al.,2020).Therefore,we usedC.elegansas a model animal to study the impairment of nervous system function induced by ionizing radiation in this study.
C.eleganslarvae are still able to develop into adults after being irradiated with 1000 Gy X-rays whole-body radiation (Ishii and Suzuki,1990).Similarly,the mortality ofC.elegansis not changed significantly when they are irradiated with 500 Gy γ-rays whole-body radiation (Weidhaas et al.,2006).Therefore,we consider 75 Gy to be a relatively low dose inC.elegans.Some studies have shown that ionizing radiation can induce nervous system dysfunction inC.elegans,which is reflected in behavioral disorders such as body bend and chemotaxis (Sakashita et al.,2008;Suzuki et al.,2009,2017,2020;Ye,2011).In this study,it was also found that 75 Gy of γ-rays could induce behavioral motor and sensory dysfunction inC.elegans,manifesting as disorders of head thrash,touch avoidance and foraging behaviors.This indicates that ionizing radiation inhibits nervous system functions inC.elegans,producing dysfunction in both motor and sensory functions.Using the transgenic strain BZ555,we also found that γ-rays could induce neuronal degeneration,indicated by an increase in dendrite defects and cell body atrophy in dopaminergic neurons.Dopamine signaling plays a key role in many aspects of nervous system function,and dopamine is able to affect crucial functions including learning and locomotion (Wise,2004;Schultz,2007).Abnormal dopamine function is associated with a variety of diseases,including attention deficit hyperactivity disorder and Parkinson’s disease (Rangel-Barajas et al.,2015;Cooper and Van Raamsdonk,2018;Abrantes Dias et al.,2020;Miyazaki and Asanuma,2020).It has been reported that a reduction in dopamine signaling can lead to apparent behavioral abnormalities in both mammals andC.elegans(Omura et al.,2012;Alghasham and Rasheed,2014;Kim et al.,2014).Previous studies have also found that dopaminergic neuron damage could be the cause of decline in behavioral motor ability (Qu and Wang,2020;Zhang et al.,2020;Chen et al.,2021).Therefore,degeneration in dopamine neurons could be responsible for ionizing radiation-induced behavioral disorders inC.elegans.
The transcriptomes ofC.elegansin the control group and the radiation group were sequenced to explore the molecular mechanisms of ionizing radiationinduced nervous system dysfunction inC.elegans.Through a literature survey,bioinformatics analysis,and the string online PPI analysis system,we screened out the genes related to nervous system function:crm-1andnhr-76.The human homolog of crm-1 is cysteine rich transmembrane bone morphogenetic protein (BMP) regulator 1 (CRIM1),which is involved in the formation of motor neurons and the regulation of growth and development.A previous study found that cysteine rich transmembrane BMP regulator 1 (chordin like) (Crim1) was expressed in the notochord,floor plate,early motor neurons,and interneuron subpopulations within the developing spinal cord.This indicates thatCrim1is closely related to the development of the central nervous system (Kolle et al.,2000).It was found thatcrm-1could affect the growth and development ofC.elegansthrough the BMP pathway and is expressed in both dorsal A-type and dorsal B-type motor neurons of the ventral nerve cord (Fung et al.,2007).The human homolog of nhr-76 is peroxisome proliferator activated receptor delta (PPARD),a nuclear receptor that regulates neurogenesis,homeostasis,and fatty acid metabolism in mammals and inC.elegans(Noble et al.,2013;Ji et al.,2015;Ben-Zvi and Liebner,2022).Previous study has shown that murine peroxisome proliferator activator receptor delta (Ppard) gene is involved in the proliferation and differentiation of neural stem cells in the mouse hippocampus (Ji et al.,2015).Ppard may also be involved in regulating the barrier of mature vascular endothelial cells in mice,thereby regulating tissue homeostasis in the central nervous system (Ben-Zvi and Liebner,2022).C.elegansnhr-76may regulate fatty acid metabolism by regulating serotonin (Noble et al.,2013).Therefore,it is speculated that crm-1 and nhr-76 may be involved in the regulation of ionizing radiation-induced nervous system dysfunction.PPARD is one of the three known PPARs that are part of the nuclear receptor superfamily of transcription factors (Yang et al.,2021).PPARs negatively regulate the BMP pathway (Zhang et al.,2011).Therefore,PPARDis thought to be involved in regulating the BMP pathway,from which it is deduced that thePPARDhomologous gene nhr-76 may be involved in regulating the BMP pathway.At the same time,CRIM1 is involved in the negative regulation of the BMP pathway (Wilkinson et al.,2003): a classic nervous system developmental regulatory pathway (Wilkinson et al.,2003;Yamamoto and Oelgeschläger,2004).Therefore,we speculate that bothcrm-1andnhr-76may regulate nervous system function by regulating the BMP pathway.It is predicted thatcrm-1may interact withnhr-76according to the results of our PPI analysis.
The expression levels of bothcrm-1andnhr-76increased after irradiation.To explore whether these two genes are involved in the regulation of ionizing radiation-induced nervous system dysfunction,we knocked down the expression levels ofcrm-1andnhr-76,respectively,and examined their association.Then behavioral measurement was conducted.In this study,the expression level of nhr-76 inC.eleganswas measured after knocking downcrm-1using RNAi technology.After knocking downnhr-76,the expression level ofcrm-1was assessed in wild-type strain N2 by qPCR,and then in transgenic strain PHX5872crm-1(syb5872) by an assessment of changes in fluorescence intensity.These experiments showed that nhr-76 could positively regulate the expression level ofcrm-1at the gene level.
RNAi technology was used to knock down the expression ofcrm-1.Changes in head thrash,touch avoidance and foraging behaviors were observed to verify whethercrm-1was involved in the regulation of nervous system function.We found that down-regulating the expression level ofcrm-1gene could effectively alleviate the effects of ionizing radiation-induced head thrashes and touch avoidance.Therefore,it may regulate the function of the nervous system.Because the expression level ofcrm-1gene was shown to be regulated bynhr-76in a previous experiment,we conclude that bothnhr-76andcrm-1are involved in the regulation of ionizing radiation-induced nervous system dysfunction inC.elegans.
In the present study,the change in expression level ofnhr-76was not statistically significant after knockdown ofcrm-1.However,the standard deviation is large,which may be because of the interference efficiency ofcrm-1.It may be thatcrm-1andnhr-76have other regulatory roles.In future studies,we aim to knock outcrm-1,and then measure the expression level ofnhr-76.Additionally,we will insert a reporter gene intonhr-76,creating a new transgenic animal to further study the role of this gene.
In conclusion,ionizing radiation exposure can inhibit behaviors such as head thrashes,touch avoidance,and foraging inC.elegans,as well as induce degeneration of dopamine neurons.Ionizing radiation exposure also induced increases in expression of both crm-1 andnhr-76genes.After knocking down the expression ofcrm-1gene,the ionizing radiation-induced changes in head thrash and touch avoidance behaviors were improved.Because the expression level ofcrm-1gene is regulated by the nuclear receptor genenhr-76,we conclude that thenhr-76/crm-1pathway may be involved in the regulation of ionizing radiation-induced nervous system dysfunction inC.elegans.
Acknowledgments:We greatly appreciate Dr.Hui-Min Zhang from Soochow University for the wild-type strain and Dr.Jing-Juan Ju from Wenzhou Medical University for the transgenic strain BZ555.
Author contributions:NC and YT conceived and designed the experiment.HQL,SQH and JFH performed the experiments.JG analyzed the data.HQL wrote the article.HQL,JG,SQH and JFH contributed equally to this work.All authors approved the final version of this paper.
Conflicts of interest:The authors declare that there is no conflict of interests.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Hanin Abdel-Haq,Istituto Superiore Di Sanita,Italy;Peng Li,Virginia Commonwealth University,USA.
Additional file:Open peer review reports 1 and 2.
P-Reviewers: Abdel-Haq H,Li P;C-Editor: Zhao M;S-Editors: Yu J,Li CH;L-Editors: Farmer D,Song LP;T-Editor: Jia Y
- 中国神经再生研究(英文版)的其它文章
- Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
- Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update

