Ginger polysaccharide UGP1 suppressed human colon cancer growth via p53,Bax/Bcl-2,caspase-3 pathways and immunomodulation
Yanfang Qian,Chenying Shi,Chen Cheng,Dengwei Liao,Junping Liu,Guitang Chen*
College of Engineering/National R&D Center for Chinese Herbal Medicine Processing,China Pharmaceutical University,Nanjing 211198,China
Keywords:Ginger polysaccharide Apoptosis p53 Bcl-2 Immunomodulation
ABSTRACT I n previous study,we got a purified ginger polysaccharide UGP1 and verified its significant antitumor activities on colon cancer HCT116 cells.In this article,we aimed to illustrate the underlying mechanism of UGP1 exerted antitumor activities on colon cancer by using in vitro cell models and in vivo animal models.The results demonstrated that UGP1 could induce S-phase cell cycle arrest,up-regulate the expression of Bax and p53,down-regulate the expression of Bcl-2,and activate the downstream protein caspase-9 and caspase-3,which was related to intrinsic apoptosis pathway on HCT116 cells.Moreover,UGP1 signif icantly stimulated RAW264.7 cell proliferation and secretion activity.Similarly,UGP1 inhibited tumor proliferation on tumor-bearing mice,increased the expression of p53 and the ratio of Bax/Bcl-2,enhanced the secretion of pro-inflammatory cytokines TNF-α,IL-2,IL-6 and decreased the secretion of pro-tumor cytokines TGF-β and bFGF in serum.In conclusion,it indicated that the UGP 1 could sup press human colon cancer growth by inducing apoptosis via the regulation of p53,caspase-3,and Bax/Bcl-2 ratio-dependent pathway and regulating immune system activity.Thi s investigation provided basic theoretical mechanism of ginger polysaccharideexerted antitumor activities,and contributed to develop a possible functional food or adjuvant agent for prevention or treatment of colon cancer.
1.Introduction
Col on cancer,one of the most common malignancies,accounts for over 30% of all gastrointestinal cancers,which is the third leading cause of cancer-related mortality throughout the world [1].A comprehensive program including surgery,chemotherapy and immunotherapy is used for treatment for middle and advanced colon cancer [2].While the treatment of colon cancer still faces great challenges due to poor prognosis,there is need for more therapies with low toxicity and high efficiency for colon cancer in clinics.
In the past few decades,natural polysaccharides have attracted many attentions for various of bioactivities,including antioxidant [3],immune-modulatory [4],anti-tumor [2]and many other activities.Compared with chemotherapy drugs,natural polysaccharides show low or no side effects on normal cells [5].Polysaccharide as a biosafety reagent with efficient antitumor activities has been reported widely.Hawthorn polysaccharide has been proved anti-cancer effects on colon cancer cell via cell cycle arrest and apoptosis [6].Dendrobium officinale polysaccharide has been validated to be an anti-colon cancer agent through a combination of energy metabolism and autophagy [7].
Ginger plant is a perennial,tuberous root or rhizome that belongs to family of Zingiberaceae.Evidences fromin vitro,animal,and epidemiological studies suggest that ginger and its active constituents suppress the growth and induce apoptosis of variety of cancer types [8].Zhang et al.[9]reported that curcumin reverses doxorubicin(DOX) resistance in colon cancer cells by regulating metabolic change.Zhang et al.[10]described a nanovector made from edible ginger-derived lipids that could serve as a delivery platform for the therapeutic agent DOX to enhance the chemotherapeutic inhibition of tumor growth in colon cancer treatment.Natural products derived from ginger appear to show a direct or synergistic anticancer effect on colon cancer.Wang et al.[11]reported ginger polysaccharides induce cell cycle arrest and apoptosis in HepG2 cells.Polysaccharide as an important component of the ginger has been reported to possess various activities.However,it is not clear whether ginger polysaccharide has therapeutic effect on colon cancer.What’s more,it is also worth exploring whether ginger polysaccharide has the potential to replace DOX as a new,effective and safe drug for colon cancer treatment.
Many previous studies showed that polysaccharide exerts antitumor bioactivities indirectly via host’s immune system [12,13].Polysaccharide might activate effector cells to express cytokines,such as TNF-α,IFN-γ,and IL-6 [13,14].While there are some researches demonstrated polysaccharide exerts antitumor activities directly on tumor cells [15-17],including arresting cell cycle,inducing the apoptosis of tumor cells and inhibiting the expression of cellular oncogenes.Apoptosis is characterized by mitochondrial membrane depolarization,the release of death-inducing factors and the activation of caspase [18].Activated caspase-3,-6,-7 will cleave a set of vital proteins and thus,initiate and execute the apoptotic degradation phase including DNA degradation and the appearance of typical apoptotic morphological features [16].
In previous study [19],we have isolated a ginger polysaccharide(UGP1) with an average molecular weight of 769.19 kDa by ultrasonic assisted extraction.The chemical compositions of UGP1 were (93.58 ± 0.08)% carbohydrate,(0.73 ± 0.10)% protein and(3.14 ± 0.16)% sulfate.The monosaccharide compositions of UGP1 were 28.0% mannose,59.2% glucose,9.6% galactose and 3.2% arabinose.UGP1 contained the glycosidic linkage of →6)-β-DGalp-(1→ and possessed the triple-helix conformation.It has been verified that UGP1 inhibit HCT116 proliferation significantly,while the specific mechanism of its tumor suppression was not clear.The study of its mechanism will contribute to the development of anticolon cancer drugs.Here,we studied the cell cycle arrest,apoptosis pathway and immune activitiesin vitroandin vivoto verify the antitumor activities of UGP1,which would help to promote the application of ginger polysaccharide in industries of food and pharmacy.
2.Materials and methods
2.1 Materials
UGP1 was extracted by ultrasonic assisted extraction and purified by DEAE cellulose-52 and Sephadex G-200 size-exclusion chromatography according to a previously published protocol [19].3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide(MTT),RPMI-1640 medium,Hoechst 33342,Annexin V-FITC/PI,BCA assay kit,capase-3,-8,-9 assay kits were from KeyGen Biotechnology Co.,Ltd.(Nanjing,China).DMEM medium,Trysin-EDTA and fetal bovine serum (FBS) were obtained from Invitrogen-Gibco (Grand Island,NY,USA).Dimethylsulfoxide (DMSO) and lipopolysaccharide (LPS) were obtained from Sigma Chemical Co.(St.Louis,MO,USA).The antibodies against Bcl-2,Bax,p53,β-actin were obtained from Cell Signaling Technology,Inc.(USA).Cytokine(TNF-α,TGF-β,bFGF) ELISA kits were from CUSABIO Biotech Co.,Ltd.(Wuhan,China).Cytokine (IL-2,IL-6) ELISA kits were from eBioscience,Ltd.(Shanghai,China).All other chemicals used in this study were of analytical grade.
2.2 Cell culture and animals
HCT116 cells and RAW264.7 cells were obtained from Cell and Molecular Biology Experiment Platform of China Pharmaceutical University.HCT116 cells were cultured in RPMI-1640 medium and RAW264.7 cells were cultured in DMEM medium,both supplemented with 10% fetal bovine serum (FBS,Gibco,USA),100 U/mL penicillin and 100 μg/mL streptomycin.The cells were cultured at 37 °C in a humidified atmosphere with 5% CO2.
Male nude mice (BALB/c,18-22 g) aged 6-8 weeks (NO: SCXK-2016-0010) were provided by Nanjing Qing Longshan Biotechnology Company (Nanjing,China).The animals were maintained under sterile conditions ((24 ± 2) °C,humidity of (50 ± 10)% with a 12 h light/dark cycle,and had free access to standard rodent chow and water.Animal procedures in this investigation conform to Guide for Care and Use of Laboratory Animals (NRC 2011),and all experimental protocols were in accordance with Laboratory Animal Care Committee at China Pharmaceutical University (Permit number SYXK-2018-0019).
2.3 Cell proliferation assay
HCT116 cells were seeded in 96-well microtiter plates at a density of 8 × 104-1 × 105cells/mL (100 μL) and incubated for 24 h.UGP1 were added with final concentrations of 25,50,100,150,200 μg/mL.The RAW264.7 cells were seeded and cultured as above.After 48 h incubation,each well was added with 20 μL MTT and incubated for another 4 h.Removal of mixtures,150 μL of dimethyl sulfoxide (DMSO) was added to each well and plates were shaken for 15 min with the condition of avoiding lights.The optical density (OD)values were measured at 490 nm by using a micro-plate reader (Bio-Tek,EXl800,USA).Inhibition rate was calculated as following formula:
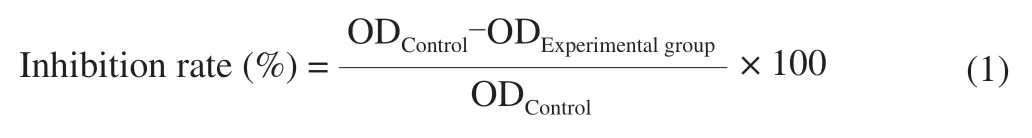
Where ODControland ODExperimentalgroupstand for the absorbance of the control cells and treated cells,respectively.
2.4 Morphological analysis
In order to observe the morphological changes of the cells treated with UGP1,the cells were stained with Hoechst 33342 [20].HCT116 cells were seeded in 6-well plates at a density of 3 × 104cells/mL incubating overnight at 37 °C with 5% CO2and then exposed to 0,25,50,150 μg/mL UGP1 for 48 h.After removal of medium,the cells were washed in cold PBS and fixed in 1 mL ethanol-acetic acid (3:1,V/V) for 5 min.Washed with PBS for 3 times,the cells were stained with Hoechst 33342 for 10 min at room temperature in dark,then analyzed with a fluorescence microscope (DMI6000 B,Leica,Germany).
2.5 Cell cycle analysis
HCT116 cells were seeded in a 6-well plate at 3 × 104cells per well and were incubated with various doses of UGP1 (0,25,50,150 μg/mL) for 48 h.HCT116 cells were harvested following trysinization and centrifugation.The cells were washed twice with PBS and resuspended in 70% ethanol (4 °C) for fixation purpose.Ethanol-suspended cells were centrifuged for 5 min at 2 000 r/min to decant ethanol thoroughly.Cells were washed by PBS and resuspended in 500 uL propidium iodide and RNase A with a final concentration of 250 μg/mL.The mixture was incubated for 30 min 37 °C away from light and analyzed for cell cycle by a flow cytometry(AccuriTM,BD,USA).
2.6 Measurement of apoptosis
Cells apoptosis were analyzed by Annexin V-FITC/PI staining [2].HCT116 cells were dealt with the same method as section 2.5.The cells were collected by trypsinization and centrifugation and washed twice with PBS.The cell density was adjusted to 1 × 105cells/mL.The cell-mixture was centrifuged for 5 min at 1 500 r/min and supernatant was discarded.5 μL of Annexin V-FITC and 10 μL of propidium iodide (PI) were added and incubated in the dark for 30 min,the cells were immediately analyzed by a flow cytometry.
2.7 Caspase activity assay
HCT116 cells were treated with UGP1 (0,25,50,150 μg/mL) for 48 h and harvested to extract the total protein using RIPA reagent.Then,according to the manufacturer’s protocols,the activity of caspase-3,8 and 9 was determined by a commercial kit (KeyGen Biotechnology,Nanjing,China) and measured at 405 nm with an ELISA reader (ELX800,Promega,USA).
2.8 Western blot assay in vitro
After treatment,the cultured HCT116 cells were collected and washed with PBS,then resuspended in an ice-cold RIPA Buffer supplemented with a protease inhibitor.The protein concentration was determined using the BCA protein assay based on the albumin standard.Equal amounts of protein were separated by 12% Trisglycine gels and then transferred electrophoretically onto a nitrocellulose membrane.The membranes were blocked with 5% non-fat milk for 2 h.After washing three times with TBST,the nitrocellulose membranes were separately incubated with primary antibodies overnight at 4 °C,washed with TBST for three times and then incubated with enzyme-linked secondary antibody.The protein bands were visualized using the Enhanced ECL Western blotting kit(KeyGen Biotechnology,Nanjing,China).The protein levels were quantified by using Image J software and expressed relative to the levels ofβ-actin,used as an internal control.
2.9 Cytokine assay in vitro
The RAW264.7 cells were plated in 96-well plates (1 × 105cells/mL)for 24 h.The culture medium was then removed,and the cells were incubated with various concentrations of UGP1 (0,25,50,100,150,200 μg/mL) for 24 h.DMEM and LPS were used as negative and positive controls.After centrifugation at 1 000 r/min for 20 min,the supernatants were collected.The productions of TNF-α in the supernatants were determined by commercial ELISA kits(Shanghai Enzyme-linked Biotechnology Co.,Ltd.) according to the manufacturer’s instructions.The absorbance was measured at 450 nm and the concentrations of TNF-α were calculated by comparing the absorbance with a standard curve.
2.10 Animal experiments
The animal model was established by inoculating 0.1 mL of HCT116 (1 × 106cells/mL) into the right armpit of each mouse.After two weeks,the size of tumor approaching to 30-40 mm3indicated the model was successful.Then the mice were divided into 5 groups(8 mice in each group) at random.The three groups of UGP1-treated mice received different dosages (20,80,200 mg/kg body weight),respectively.The positive control group was treated with adriamycin(DOX,10 mg/kg),and the model control group was administered 0.9% saline solution.UGP1 and saline solution was administered by using intragastric perfusion,and DOX was administered by intraperitoneal injection once daily.On the 27thday,the mice were weighed and sacrificed by cervical dislocation.The spleen,kidney and liver were immediately excised and weighed.Kidney,liver and spleen indexes were calculated as the weight of thymus and spleen relative to body weight.To determine the inhibitory effect of UPG1 on tumors,the inhibition rate was expressed as follows:

WhereWTreatedandWControlare the average tumor weight of the treated and control mice,respectively.
The fresh tumor tissues were collected to perform a western blot assay and the subsequent steps were following section 2.8.
2.11 Cytokine assay in serum
The blood samples of mice in each group were centrifuged at 3 500 r/min for 10 min,and the serum was collected to detect the levels of TNF-α,IL-2,IL-6,TGF-β and bFGF using commercial ELISA kits.Each procedure was performed according to the Manufacturer’s protocols.
2.12 Haematoxylin and eosin (H&E) staining
Tumor tissue samples were fixed in 4% formalin,embedded in paraffin,sectioned and stained with H&E for histopathological assay.The image was captured at 200 ×,400 × by an optical microscope(Nikon Eclipse E100,Nikon DS-U3).
2.13 Statistical analysis
Data presented as mean ± SD andPvalue were determined using an unpaired Student’st-test from SPSS version 19.0 software.P<0.05 was considered as statically significant.
3.Results and discussion
3.1 Effect of UGP1 on proliferation of HCT116
HCT116 cells were cultivated with various concentrations of UGP1 for 48 h.As shown in Fig.1,UGP1 inhibited cell proliferation in a dose-dependent manner at concentrations of 25-200 μg/mL.UGP1 at 150 μg/mL exhibited strongest cytotoxicity with 56.84% inhibitory rate,and there was no significant difference between the 200 and 150 μg/mL treatment groups.Therefore,25,50 and 150 μg/mL were selected as the treatment concentrations for subsequent experiments.
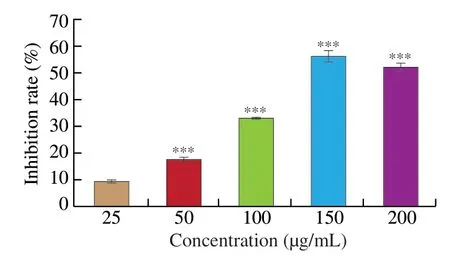
Fig.1 Inhibition rate of UGP1 on HCT116 tumor cells.Values were significantly different compared with the control group using the t-test: ***P <0.001.
3.2 Induction of apoptosis by UGP1
In order to understand whether the reduced cell proliferative activity by UGP1 treatment was due to apoptosis,the morphological change of cells treated with UGP1 was estimated using Hoechst 33342 dye in Fig.2.From the control group,HCT116 cells demonstrated normal spindle-shaped with uniform fluorescence staining and no symptoms of apoptosis.Nevertheless,the HCT116 treated with UGP1 showed the nuclear fragmentation and chromatin condensation,which are characteristic of apoptosis [16].The symptoms were gradually obvious with a dose-manner.From the Hoechst 33342 morphological observation,we concluded UGP1 induced the apoptosis of the HCT116.Besides,Annexin-V/propidium iodide staining in flow cytometry (FCM) was carried out in Fig.3 for further study.Cells sorted into quadrants Q1,Q2,Q3 and Q4 denoted necrotic,late apoptotic,early apoptotic,and viable (live) populations,respectively.Compared to control that unobvious apoptosis occurred,the proportion of apoptotic cells increased to 7.87% (25 μg/mL),24.16% (50 μg/mL) and 53.30% (150 μg/mL),respectively.The late apoptotic cells upregulated significantly,from 7.19% to 40.50%,indicating UGP1 induced apoptosis,especially late apoptosis in HCT116 cells.
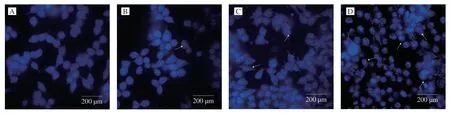
Fig.2 Fluorescence pattern of HCT116 cells treated with different concentrations of UGP1.(A,B,C,and D is the final concentrations of 0,25,50,150 μg/mL,respectively.)
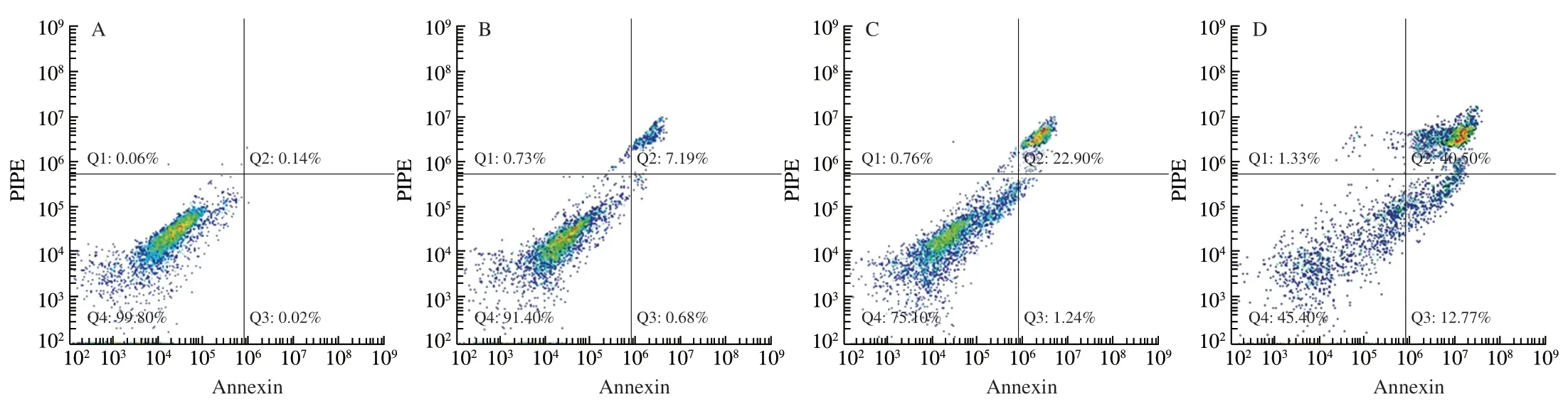
Fig.3 Apoptosis distribution of HCT116 cells treated with different concentrations of UGP1.(A,B,C,and D is the final concentrations of 0,25,50,150 μg/mL,respectively.)
3.3 Induction of cell cycle arrest by UGP1
Growth inhibition is often accompanied by cell cycle arrest [17].To further investigate the growth inhibitory effects from UGP1 in HCT116,cells in different cell cycle phases were counted and percentages of cells in each phase were calculated by comparing to the total cell number.As shown in Fig.4,UGP1 treatments demonstrated different effects in different cell cycle phases.The percentage of cells in S phase increased,while the percentage of G0/G1 and G2/M phase reduced by UGP1.Percentage of cells at G0/G1 and G2/M phase in the three groups of UGP1-treated (25,50,150 μg/mL)samples were 70.73%,50.86%,40.70% and 17.46%,14.31%,8.59%,respectively,while that of control group was 73.86% and 21.58% .A highest percentage of cells in S phase (50.71%) were shown in HCT116 cells treated with 150 μg/mL UGP1,compared to that of the other groups,indicating cell cycle arrest of HCT116 was most significant at 150 μg/mL.Hence,the results suggested that UGP1 exerted its anticancer activity by regulating cell proliferation,blocking cell cycle at S phase and preventing cell mitosis in HCT116 cells.
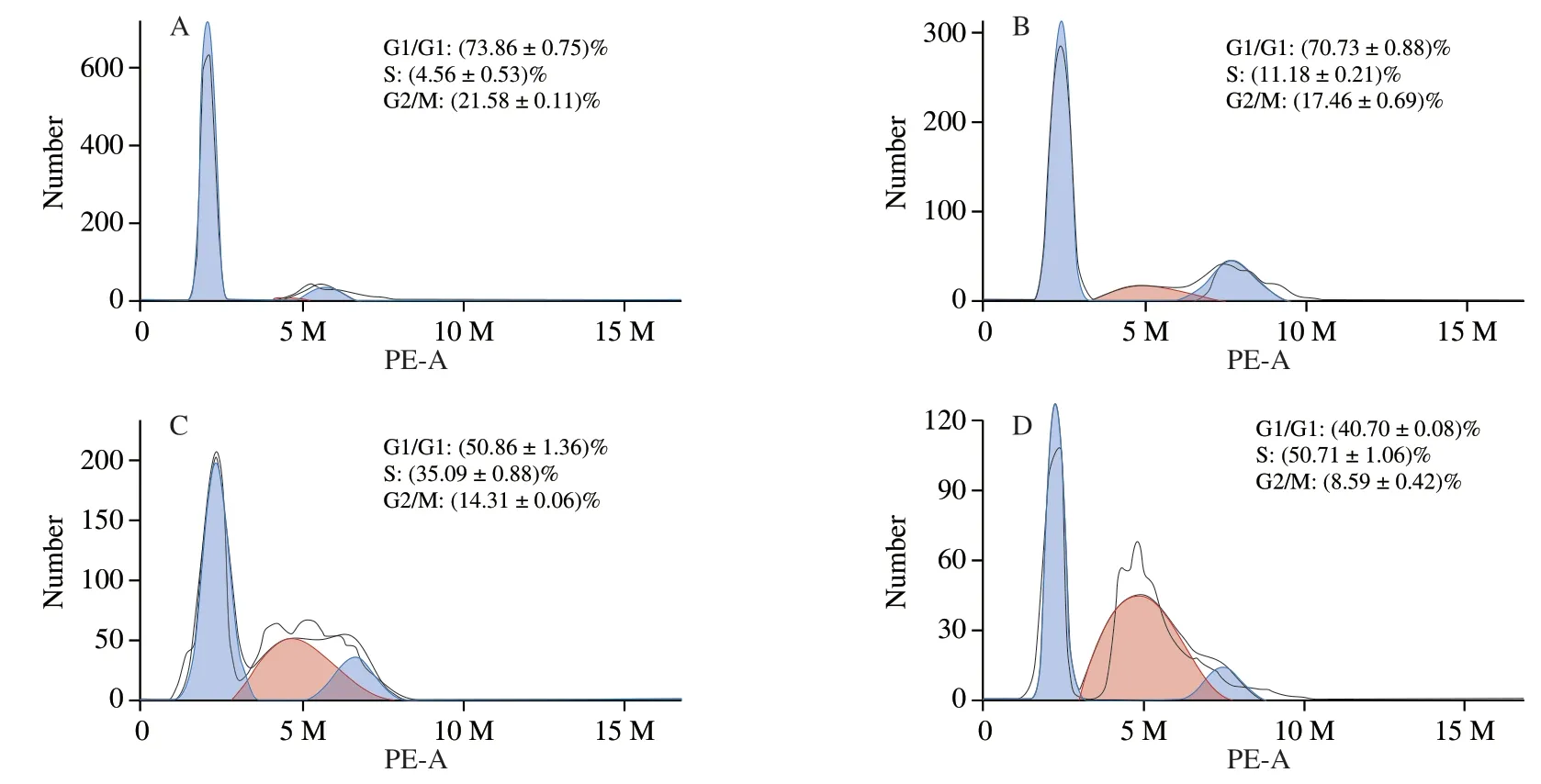
Fig.4 Cell cycle distribution of HCT116 cells treated with different concentrations of UGP1 for 48 h.(A,B,C,and D is the final concentrations of 0,25,50,150 μg/mL,respectively.)
3.4 Caspase activity induced by UPG1 in HCT116 cell
Caspase proteins are at the core of apoptotic machinery and play essential roles in the execution of apoptosis.According to the results of apoptosis assay,we hypothesized that UGP1 might promote the activation of the caspase-dependent cell apoptosis pathway.Therefore,we set out to analyze the ability of UGP1 to modulate caspase-3,caspase-8 and caspase-9 in Fig.5.The caspase-8 activities were elevated slightly by UGP1 with the increase of concentration from 25 μg/mL to 150 μg/mL,indicating that with the concentration increased,caspase-8 receptor pathway was slightly induced by UGP1.After the caspase-9 protein,which is always relevant to the mitochondrial apoptosis pathway,is activated by signal,the caspase-3(the reaction caspase protein) is activated,which take on the downstream effect on apoptosis activity [21,22].Both caspase-3 and caspase-9 were induced by UGP1 with a dose-dependent manner.The protein level of caspase-3 and caspase-9 were 2.43-fold and 1.74-fold,respectively,compared with the control.The results indicated UGP1 mainly activated caspase-3 and caspase-9 mitochondrial pathway.
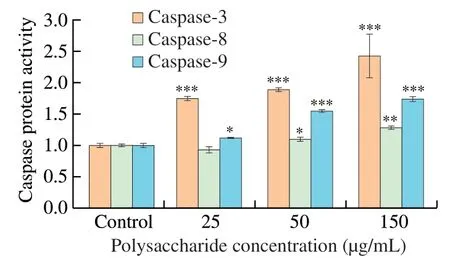
Fig.5 Caspase protein activity of HCT116 cells treated with different concentrations of UGP1.Values were significantly different compared with the control group using the t-test: ***P <0.001 **P <0.01,*P <0.05.
3.5 Apoptosis-associated protein expression induced by UPG1 in HCT116 cell
The results of Western blot assay in HCT116 were demonstrated in Fig.6.Protein level of p53 increased dramatically in 50 and 150 μg/mL treatment group.The protein level in 150 μg/mL was 1.53-fold of that in control.The Bax protein increased in a dose-dependent manner.The protein level in 150 μg/mL was 1.75-fold of that in control.Whereas the protein expression level of Bcl-2 decreased by 32.95% in a dose-dependent manner compared with the control group.Thus,UGP1 might cause cell apoptosisin vitroby promoting the upregulation of p53 and Bax protein expression and the down-regulation of Bcl-2 protein expression.
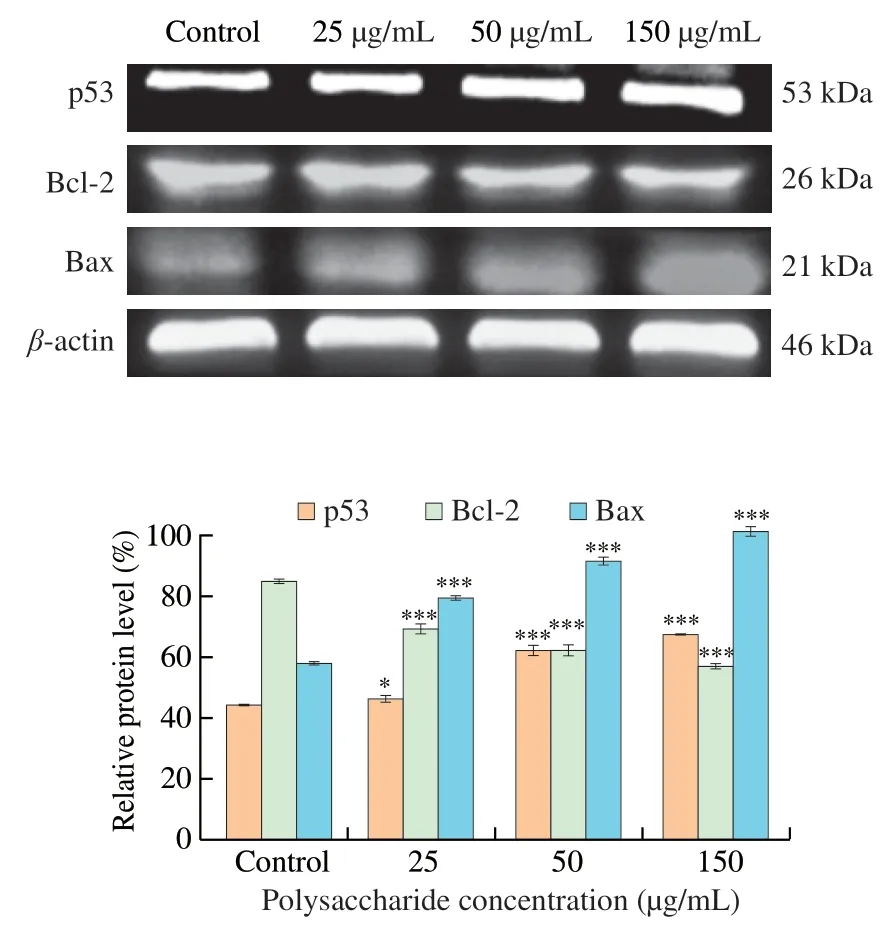
Fig.6 Apoptosis-related proteins expression in HCT116 cells treated with different concentrations of UGP1.Values were significantly different compared with the control group using the t-test: ***P <0.001 **P <0.01,*P <0.05.
3.6 Immunomodulatory activity in vitro
Studies on the antitumor activity of polysaccharides focus on the direct killing and immunomodulatory pathways.To investigate whether UGP1 can act as an immunomodulatory substance,the effects of UGP1 on the viability of RAW264.7 cells and the TNF-α secretions in RAW264.7 cells were analyzed.As shown in Fig.7A,compared with the control group,UGP1 was nontoxic to RAW264.7 cells from 25 μg/mL to 200 μg/mL,and significantly increased the rate of RAW264.7 cell proliferation.The highest proliferation rate of 50.36% (P<0.01) was found at 50 μg/mL.Activated macrophages can release several cytokines and other substances,and these cytokines can directly induce the tumoricidal activity of macrophages [23].Fig.7B showed that UGP1 enhanced the production of TNF-α in a dose-dependent manner.When UGP1 concentration was 200 μg/mL,the secretion of TNF-α was 2.4-fold of that in control,and there was no significant difference with that of LPS group.This suggested that UGP1 might stimulate the TNF-α production by RAW264.7 cells.These results indicated that UGP1 had beneficial effects on immunomodulatoryin vitroand might stimulate the secretions of cytokines to exert antitumor effect.
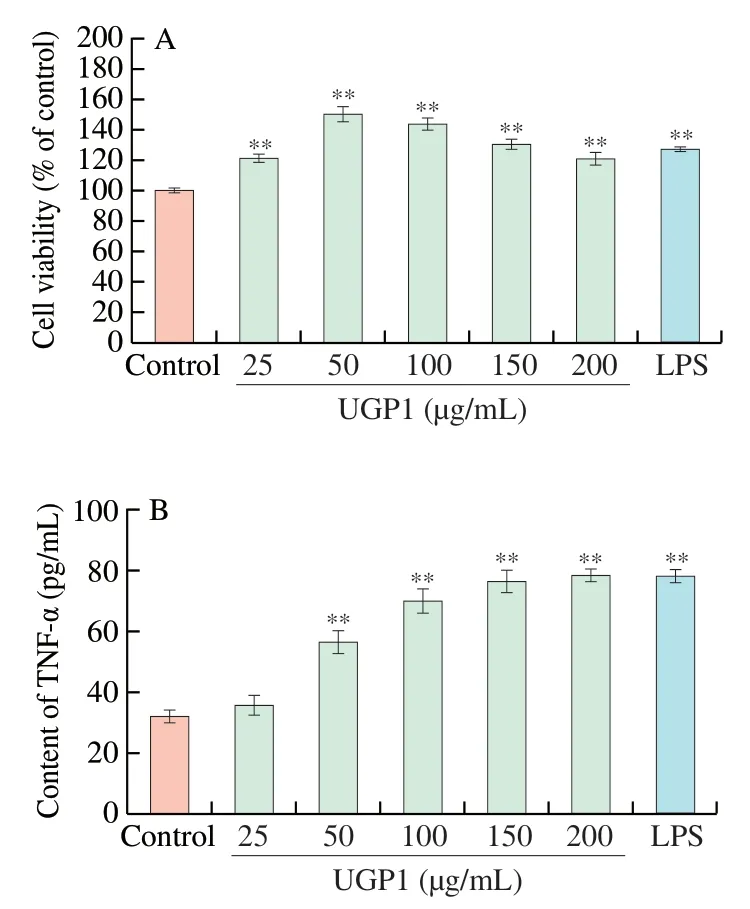
Fig.7 Effect of UGP1 on the proliferation of RAW264.7 cells (A) and on TNF-α secretion in RAW264.7 cells (B).Values were significantly different compared with the control group using the t-test: **P <0.01.
3.7 Effect of UGP1 on colon tumor growth in vivo
Antitumor effect of UGP1 was explored in athymic nude mice.There was no difference in body weight between each group (Fig.8A),indicating UGP1 had no severe side effects on mice.However,the body weight of DOX group at day 27 was significantly different from that of model group,which might be due to drug toxicity of DOX in the process of inhibiting tumor growth.UGP1 treatment remarkably prevented the progression of tumor growth in nude mice in Figs.8B-D.The tumor volume of UGP1 treatment groups (20,80 and 200 mg/kg) were significantly (P<0.05,P<0.01 andP<0.01)decreased compared to the model group after 27 days treatment.The inhibition ratios of UGP1 were (36.55 ± 15.72)%,(61.10 ± 7.62)% and (65.85 ± 7.97)%,respectively,compared with model group.DOX,an antitumor medicine,used as positive control exhibited inhibition ratio of (81.97 ± 4.62)% at the concentration of 10 mg/kg.In addition,the photographs of nude mice and tumors took at day 27 also obviously displayed the retard of tumor growth.As shown in Fig.8E,the live,spleen and kidney index raised after the treatment of UGP1 compared with model group.As an immune organ,the weight and organ index of spleen are in response to the nonspecific immunity of the organism [24].The spleen index was significantly increased after treatment with low (P<0.05),medium (P<0.05) and high(P<0.01) concentrations of UGP1,which indicated that UGP1 could act as an immune system activators to enhance spleen weight.Conversely,DOX suppressed the growth of liver and kidney significantly(P<0.05),indicating that DOX had toxicity on liver and kidney.
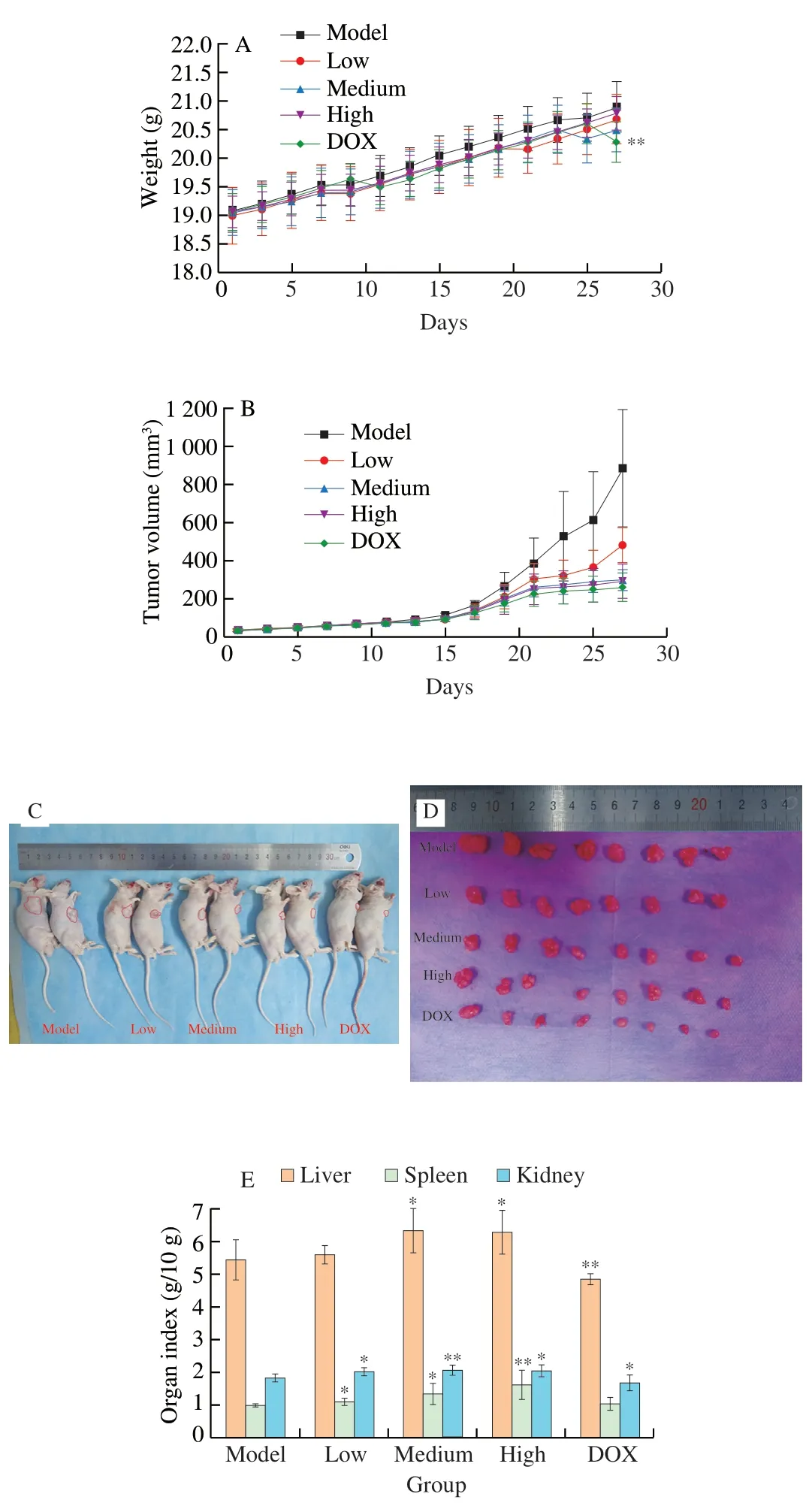
Fig.8 In vivo antitumor efficacy of UGP1 tested in HCT116 tumor-bearing nude mice.(A) Body weight changes of HCT116 tumor-bearing nude mice.(B) The growth curves of tumor volume inoculated on nude mice treated with DOX or different concentrations of UGP1.(C,D) The images of representative mice and tumors in each group taken at the end of treatment.(E) Organ indices of HCT116 tumor-bearing nude mice.Values were significantly different compared with the control group using the t-test: **P <0.01,*P <0.05.
Morphological changes of tumor xenografts using H&E staining also examined whether apoptosis was involved in arrest of tumor growth.As Fig.9 showed,there were typical morphological characteristic of cell apoptosis in treatment groups,such as shrinkage of the cell and the nucleus,as well as condensation of nuclear chromatin.However,chromatin condensation and nuclear shrinkage did not appear in control group.Moreover,UGP1 induced apoptosis effect in a dose-dependent manner.Altogether,these results demonstrated UGP1 exerted its antitumor activity in colon cancer by promoting cell apoptosisin vivoand had no damage on organism compared with DOX.
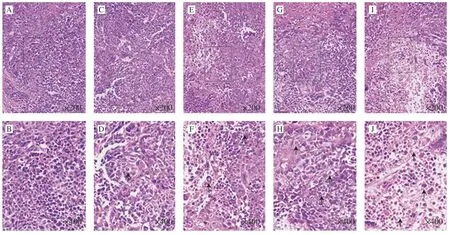
Fig.9 Tumor morphology of HCT116 tumor tissues stained by HE and viewed.(A,C,E,G,I) Model,low-dose,medium-dose,high-dose,DOX group captured at 200 × (B,D,F,H,J) Model,low-dose,medium-dose,high-dose,DOX group captured at 400 ×.
3.8 Apoptosis-associated protein expression induced by UGP1 in vivo
To verify the role of apoptosis pathway in the mechanism of UGP1 against colon cancer tumor,the expression level of proapoptotic and anti-apoptotic proteins (p53,Bax and Bcl-2) in tumor tissues were analyzed by western blot assay.The expression level tendency of these proteins were similar with those in HCT116,as shown in Fig.10.After 27 days treatment,there was a dose-dependent increase in the expression of p53 and Bax proteins and decrease in the expression of anti-apoptotic protein Bcl-2.The expression of p53 protein in the high-dose group (200 mg/kg) was 1.41-fold of that in the model group,while there was no significant difference (P>0.05)between the p53 protein content in the low-dose group (20 mg/kg) and the model group.The content of Bax protein in low-dose,mediumdose (80 mg/kg) and high-dose groups were significantly higher(P<0.001) than that in model group.Especially,the protein level in high-dose group was 2.01-fold of that in model group.Conversely,the protein content of Bcl-2 in medium and high dose groups were significantly lower (P<0.001) than that in model group,and the protein content of Bcl-2 in high dose group was 26.08% lower than that in model group.Altogether,these results demonstrated that UGP1 inhibited tumor growth by inducing apoptosis of tumor cells,which accompanied with increased expression of p53 protein and ratio of Bax/Bcl-2.
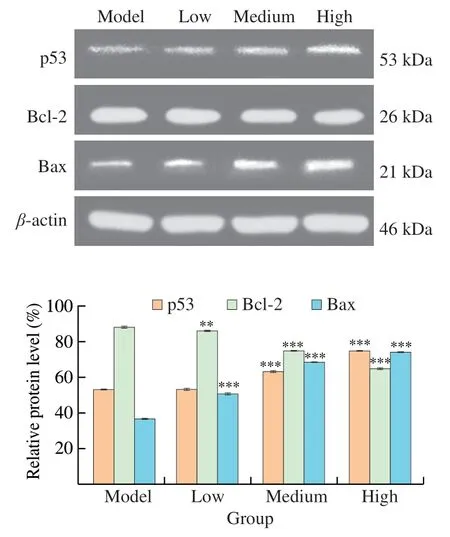
Fig.10 Apoptosis-related proteins expression of HCT116 tumor-bearing mice treated with different concentrations of UGP1.Values were significantly different compared with the control group using the t-test: ***P <0.001**P <0.01,*P <0.05.
3.9 Effect of UGP1 on cytokine production in vivo
Many research reported the polysaccharides could exert the antitumor activities by simulating the immune response of the host organism [4].TNF-α,IL-6 and IL-2 are the major pro-inflammatory cytokines that stimulate the innate immunity,and regulate cancer growth.The content of TNF-α,IL-6 and IL-2 increased with a dosedependent manner after treatment with various concentration of UGP1 in Fig.11.After treatment with 200mg/kg UGP1,the content of TNF-α,IL-6 and IL-2 were (81.74 ± 14.04),(43.31 ± 11.42)and (22.38 ± 7.58) pg/mL,which were remarkly higher than the control group.However,the secretion of TNF-α,IL-6 and IL-2 was not significantly increased after DOX treatment.TGF-β is found to be elevated in cancer cells and has been associated with higher tumor grade,increased invasiveness,and metastatic progression [25].The content of TGF-β significantly decreased with the 200 mg/kg UGP1 treatment,compared to the model group,while the TGF-β secretion in DOX positive group had no significant change.It appeared that DOX did not affect the content of TGF-β in serum.bFGF exists a high level in serum of patient with cancer [26],which was relevant to mature of new blood vessels.The results demonstrated that the high doses of UGP1 (200 mg/kg) induced the decrease of bFGF expression significantly,which was accordant with the effect of the DOX group.These results indicated that UGP1 inhibited the expression of TGF-β and bFGF,thus promoting the immunity activities for tumor suppress.In general,UGP1 showed a better activity in regulating the secretion of serum immune cytokine to inhibit tumor growth than DOX.
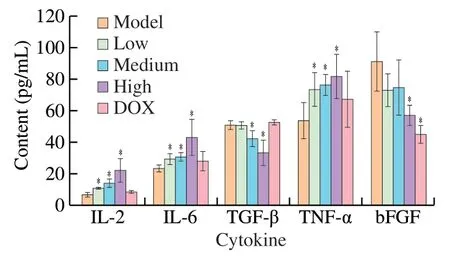
Fig.11 Cytokine levels in HCT-116 tumor-bearing mice.Values were significantly different compared with the control group using the t-test: *P <0.05.
4.Discussion
The antitumor mechanism of polysaccharides demonstrated various aspects,including directly killing tumor cells,inducing apoptosis and immune function improvement.In previous study,we evaluated the antitumor activities of UGP1 from ginger by MTT assay and concluded the results that UGP1 could inhibit the proliferation of HCT116.The antitumor mechanism of UGP1 was further evaluated in this article.
In vitroassay,we evaluated the apoptosis effect of UGP1 on HCT116 tumor cells by measuring the change of cell cycle arrest,the activity of apoptosis-associated protein (caspase-8,caspase-3,caspase-9),the expression level of protein p53 and protein Bax combined with apoptosis-inhibitory protein Bcl-2.The results demonstrated UGP1 arrested cell cycle at S phase,which was relevant to DNA replicate.Many research studied polysaccharides could induce cell cycle arrest by downregulate the cyclin family protein and the effect might be induced by upregulating the p21 and caspase-3 [27].At the same time,p53 protein can control cell proliferation and growth through the regulation of cell cycle.When the tumor grows large,it restricts the blood supply,causes tissue hypoxia,and activates the expression of p53 gene.p53 protein and its downstream processes can stop the cells at the cell cycle checkpoint or lead the cells to apoptosis.The high expression of p53 protein activates the transcription of p21 gene and further blocks the cell cycle.In our study,the effect of UGP1 on cell cycle arrest might be induced by upregulate of p53 and caspase-3 protein.
There are two major signaling routes in mammalian cells apoptosis,the intrinsic and extrinsic pathway.The extrinsic pathway involves signal transduction from caspase-8 and death receptors,and activates the intrinsic pathway of mitochondrial pro-apoptotic events [18,28,29].Intracellular anti-apoptotic depends on the antiapoptotic Bcl-2 and pro-apoptotic Bax protein levels.Bax and Bcl-2 are both B-cell lymphoma 2 (Bcl-2) family members [30].Healthy cells have high relative amounts of free anti-apoptotic Bcl-2 family members to bind and sequester pro-apoptotic Bax.Whereas,freeing Bax,a requisite gateway to mitochondrial dysfunction and cell death in response to diverse stimuli,enhances mitochondrial cytochrome C release and induce caspase cascade activation [29].The cytochrome C combines with caspase-9 and activates the level of caspase-9 [31].In this article,we studied whether these two kinds of apoptosis pathway were induced by polysaccharide.The results demonstrated that level of caspase-8 and caspase-9 improved by UGP1 in a dosemanner,while the improvement of caspase-8 was not so significant than that of caspase-9,suggesting polysaccharide might induce the intracellular pathway.We then evaluated the level of Bax and Bcl-2 protein,the result demonstrated an increase of Bax protein followed by a decrease of Bcl-2 protein in a dose-manner,further verifying the polysaccharide induced an intracellular apoptotic pathway.Caspase-3 protein is a terminal/executioner caspase protein and plays a crucial role in apoptosis [32].Caspase-3 and caspase-9 protein level improved in our study,indicating the caspase-3 might be activated by caspase-9 and executed a series of apoptosis result.
Protein p53 has been shown to be involved in various forms of apoptosis induced by cellular ‘stress’ and studied showed the lack of p53 contributes to resistance of cells to DNA-damaging agent [33].The p53 directly binds to and inhibits antiapoptotic protein Bcl-2,and also can trigger oligomerization of proapoptotic protein Bax,thereby stirring up the activation of the mitochondria-dependent apoptotic signaling pathway [15,34].In this study,we found the level of p53 protein improved after treated with UGP1,indicating the activation of p53 might occur in the apoptosis processin vitro.
Here we estimated the antitumor activities of UGP1in vivo.The tumor growth could be efficiently decreased after oral administration of UGP1,indicating that UGP1 had antitumor activitiesin vivo.Then we questioned whether the antitumor activities could be related to the function of p53 and Bcl-2 family.After treatment,the expression of p53 protein and Bax protein increased while the expression of Bcl2 protein decreased in a dose-dependent manner,in accordance with the tendencyin vitro.Some studied suggested polysaccharides could reduce Bcl-2 expression [35-37].And some research suggested a negative correlation between cytokine secretion levels and Bcl-2 protein levels,indicating pro-inflammatory IL-6 cytokines secretion decreased Bcl-2 levels [36].In this article,the Bcl-2 protein level might be co-induced by p53 and IL-6,while the specific pathway was needed to be discussed further.In brief,UGP1 can induce tumor cell apoptosis and inhibit tumor development by regulating the expression of anti-apoptotic and pro-apoptotic proteins.
We further investigated whether UGP1 could increase the immune activities to exert antitumor activities.Many polysaccharides were reported to modulate cytokine responses of activated lymphocytes and inflammatory cytokines,such as TNF-α,IL-12 and IFN-γ [38,39].The results suggested that UGP1 promoted the proliferation of RAW264.7 cells and increased the secretion of cytokines TNF-αin vitro,which owned immunomodulatory and anti-tumor activity.Then we estimated several pro-inflammatory cytokines,such as the TNF-α,IL-6 and IL-2,and anti-apoptosis cytokines,such as the TGF-β and bFGF in serum.TNF-α is one of the most important pro-inflammatory cytokines secreted by stromal cells,and promotes destruction of the tumor vasculature,which causes indirect necrosis of tumor cells [40].IL-6 is also a T-cell and macrophages-derived protein with proinflammatory cytokinase and it induced inflammation by increasing the synthesis of acute-phase proteins and nertropils in bone marrow [41].IL-2,seen as the canonical T-cell growth factor,stimulates natural killer cells (NK) to proliferate and B cells to divide and to produce antibody for the induction and the resolution of inflammatory immune responses [42].In this study,the polysaccharide UGP1 could induce the secretion of TNF-α,IL-6,and IL-2,which might enhance the immune activities to acquire antitumor activities.
TGF-β and bFGF play an important regulatory role to promote neovascularization,apart from the well-known effects such as a fibrogenic mediator in other organs,breakdown of extracellular matrix proteins and fibroblast chemotaxis.TGF-β and bFGF could cause immune escape in tumor microenvironment,and promote tumor growth and metastasis [43-45].The results demonstrated UGP1 could suppress the level of TGF-β and bFGF to decrease the angiogenesis,re-epithelization and granulation tissue formation in tumor tissues.In conclusion,UGP1 could inhibit tumor angiogenesis,improve tumor immune microenvironment and inhibit tumor growth by inhibiting the secretion of TGF-β and bFGF.
5.Conclusion
In this article,we discussed the antitumor activities of UGP1in vitroandin vivoand found the UGP1 induced S-phase cell cycle arrest,enhanced the expression of Bax and p53 protein,inhibited the expression of Bcl-2 protein,and activated the downstream protein caspase-9 and caspase-3 expression.In addition,the UGP1 increased the secretion of pro-inflammatory cytokines and decreased the secretion of pro-tumor cytokines.We concluded UGP1 could inhibit the growth of human colon cancer by inducing tumor cell apoptosis and regulating the activity of immune system.This study provided a theoretical basis for the possible use of ginger polysaccharides in colon cancer treatment and enriched the antitumor research of naturally active polysaccharide.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research,authorship,and/or publication of this article.
Acknowledgments
This study was supported by the National Key Research and Development Project “Modern food processing and food storage and transportation technology and equipment” (2017YFD0400203).
- 食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Biological factors controlling starch digestibility in human digestive system
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
- Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil

