3D printed lactic acid bacteria hydrogel: cell release kinetics and stability
Yifei Liu,Xinto Yin,Xiudong Xi,Zhen Liu,Lifei Chen,Mingsheng Dong,*
a College of Food Science and Technology,Nanjing Agricultural University,Nanjing 210095,China
b Institute of Agricultural Product Processing,Jiangsu Academy of Agricultural Sciences,Nanjing 210014,China
Keywords:3D printing Bioink Living hydrogel Lactic acid bacteria Culture starter
ABSTRACT In this study,a new type of 3D printed living biological hydrogel was developed by integrating lactic acid bacteria (LAB) into biocompatible and non-toxic polymer materials.Interestingly,the living materials loaded with LAB can be freeze-dried and reused for more than 100 times.The bio-hydrogel can be used to co-culture different LAB and keep its fermentation performance stable in long-term use.The release kinetics model and response surface method were used to simulate and optimize the bacteria release mode in the bio-hydrogel.The results show that the release of bacteria from hydrogel is regulated by the coupling of Fickian diffusion and polymer swelling.The stability of LAB hydrogel was evaluated by reuse experiments.The images of confocal microscopy and scanning electron microscope showed that the bacteria with high cell viability were distributed in the hydrogel and intact structure of the living hydrogel was maintained after 100 times of reuse as yoghurt starter.In conclusion,the 3D printed LAB bio-hydrogel developed in this study has the advantage of reuse and sustainability,which is expected to open up a new way for the preparation of food culture starter.
1.Introduction
As an important branch of 3D printing,biological 3D printing is based on the principle of “Additive manufacturing” (AD) [1],the main content of this technology is to process and manufacture 3D structures of active materials,including biomaterials,growth factors,cells based on computer-aided design (CAD) model [2,3].Biological 3D printing possesses good spatio-temporal controllability [3],and can carry out multi-cell and multi-level personalized matching according to the needs of cells after packaging.3D biological printing was originally designed to produce tissues and organs for organ transplants,with the development of biological printing technology,the application f ield has also been expanded,which can be used to predict the drug response of diseases by rapidly constructing tissues with accurate cell distribution [4].At present,although there are several types of 3D printing technologies available (including extrusion,inkjet,stereo lithography and laser assisted) [5],extrusion 3D printing is more attractive because of its versatility and cell compatibility.Extrusion 3D bioprinting,also known as direct ink writing (DIW)[6],in the process of 3D biological printing,bioink in syringe is driven by compressed air,mechanical piston or mechanical screw through nozzle or needle,and then deposited on the construction platform layer by layer.One of the advantages of this technology is the ability to easily add additional syringes to the printer,alternately delivering different bioinks or the same type of ink,but encapsulating different types of cells.This is particularly useful for creating complex multi-tier and multi-area structures.In addition to the requirements of printing technology and equipment,the bioink is very important [7],which is an important hub to connect 3D printers and cells.The bioink must not only guarantee the stability and accuracy of the printing process [8],but also protect the cells from damage during the printing process and provide a good environment for the growth of the cells,to ensure that the cells are fully differentiated and grown after printing [9].Among many materials,hydrogels are a kind of hydrophilic gel with three-dimensional network structures,which can maintain a certain shape and three-dimensional network spatial structure and swell rapidly in water [10].One of the main application fields of hydrogels is the controlled release of molecules and cells [11].As a biomaterial scaffold,it has significant advantages.It can provide a similar intracellular environment,allowing nutrients to flow in and waste diffusion out of the gel [12].It can be used as a carrier for microbial reproduction and product fermentation [13].Schaffner et al.[14]developed a biocompatible bioink with optimized rheological properties,which can obtain complex shapes and material composition by direct 3D printing,and make it possible to digitally manufacture bacterial biomaterials through the metabolic reaction of microorganisms.
Starter refers to the bacteria and other microorganisms used in the production of fermented products such as yogurt [15].When the starter is inoculated into the raw material,the microorganism can propagate rapidly under certain conditions,making the original product have certain acidity,flavor and other characteristics due to the produce of metabolites by microorganism fermentation.And it prolongs the preservation time of the product,and improves the nutritional value of the product at the same time.However,there are still limitations in the production and utilization of starter.Traditional subculture starter requires large-scale culture of microorganisms in order to be used in fermentation.Therefore,it is easy to be polluted and difficult to preserve and passage,which resulting in a lot of waste [16].Although the direct vet set (DVS) starter simplifies the production process and improves the quality of the starter.However,the use of direct vet set starter is more expensive for enterprises in many countries.Therefore,it is necessary to develop a kind of starter which is flexible,portable and can be used continuously to reduce the cost.As a kind of cell immobilization technology,microencapsulation of probiotics and starter is widely used [17-19],but there is little about the use of 3D printing to make a food starter.Compared with microencapsulation technology,3D printing can customize personalized models according to the requirements of microbial growth conditions,and provide a more suitable environment for their growth and reproduction [20].For example,through the different demand for oxygen,to combine different microorganisms,in order to achieve the directional synthesis of products.
The release rate of cells in hydrogels is very important for the efficient use of hydrogels,because it can improve the fermentation efficiency of hydrogels as starter.Therefore,it is necessary to study the cell release kinetics under different conditions in order to understand the response of hydrogels to environmental changes,especially in hydrogels sensitive environments.Carrier is one of the important components of cell-controlled release process,and the release mechanism based on polymer carrier includes diffusion-based release,swelling-controlled release,dissolution-controlled release and so on [21].Through the mathematical model of release kinetics,the release mechanism of cells can be predicted,the release conditions can be optimized according to the release mechanism,and the release rate of cells can be improved.
In this research,hydrogels were used as the main material of bioink,and lactic acid bacteria (LAB) hydrogel was prepared by biological 3D printing technology.The release kinetic model was employed to predict the bacteria release mechanism,so as to optimize the release conditions.LAB hydrogel was used to ferment yogurt,the long-term stability of the hydrogel was tested and the advantages of 3D bioprinting technology in multi strains mixed fermentation were confirmed.
2.Materials and methods
2.1 Materials,chemicals,and strains
Polyether (molecular weight about 12 600 g/mol) is noncytotoxic [22]and purchased from Sigma-Aldrich (Shanghai,China).Isocyanoethyl methacrylate,dibutyltin dilaurate,dichloromethane,methanol,ether,iodine,starch,trichloroacetic acid,2,3-butanedione,o-phenylenediamine,2-hydroxy-4’-(2-hydroxyethoxy)-2-methylpropiophenonewere all analytical grade and purchased from Aladdin (Shanghai,China).Propidium Iodide and SYTO were purchased from Nanjing KeyGen Biotech Co.Ltd.(Nanjing,China).
Lactobacillus helveticusMB2-1,Streptococcus thermophilusCQ43 were isolated from Sayram ketteki yoghurt of southern Xinjiang,China.Lactobacillus bulgaricusCICC6045 was purchased from Shanghai Preservation Biotechnology Center (Shanghai,China).
2.2 Synthesis of polymers
Polyether (60 g) was dissolved in dichloromethane (600 mL)under N2,dibutyltin dilaurate (0.6 mL) and ethyl methacrylate-2-isocyanate (3.5 mL) were added,the reaction was carried out under dry N2and stirring at 30 °C.After 2 days,the mixture was terminated by adding methanol (60 mL),and the mixture was concentrated at 30 °C by a rotary evaporator Hei-VAP Advantage (Heidolph,Germany).The mixture was stirred in ether (2 L),the precipitate was obtained by centrifugation (5 000 r/min),and then was washed twice with ether.The excess ether was volatilized,and the polymer powder was obtained and be stored at 4 °C in the dark.
2.3 Rheological evaluations
The polymer was dissolved in sterile deionized water with a mass fraction of 20%,25%,30% and 35% (m/m) respectively,mixed and cooled overnight below 4 °C.After dissolving,1% (m/m) bacterial fermentation broth was added to the 30% (m/m) mixed solution and fully mixed at 4 °C,and mark it as 30% (m/m)+.A Discovery HR10 mixed rheometer (TA Instruments,State of Delaware,America)was used with plate–plate geometry (25,0.5 mm distance).For all materials,rotational shear-viscosity measurements were performed in flow mode with shear rate ranging from 0.01 to 1 000 s−1or to the highest shear rate achievable before material was lost out of the plate–plate system.Rotational recovery measurements were performed to characterize the materials recovery behavior by applying a low shear rate of 0.01 s−1for 200 s,following by a high shear rate at 895 s−1for 100 s and finally a low shear rate of 0.01 s−1for 200 s.
2.4 Preparation of bioinks
The polymer was dissolved in sterile deionized water at 30% (m/m),and the mixture was uniformly mixed and cooled overnight below 4 °C to promote the complete dissolution of the polymer.After dissolving,0.1% (m/m) 2-hydroxy-2-methylacetophenone and 1% (m/m)L.helveticusMB2-1 (at least 1 × 106CFU/mL) were added and fully mixed at 4 °C.
2.5 3D printing and culture of LAB hydrogel
Put the above bioink into the extruded 3D printer cartridge.All printing was performed using a FOODBOT-D1 3D printer,from Hangzhou Panda Technology Co.(Hangzhou,China).CAD models were designed in 123D Design.G-code commands for the printer were generated using Slic3r.All printing was performed using a print temperature of 25 °C,a print speed of 20 mm/s,and a 1 mm inner diameter nozzle attachment.The formed hydrogel was irradiated under 365 nm ultraviolet light for 60 s.Wash the hydrogel with sterile water for 3 times.(The ultraviolet light of 365 nm belongs to long-wave ultraviolet,and short-time irradiation has no effect on the survival rate of bacteria.)
LAB hydrogel can be used for experimental test and yoghurt fermentation after being cultured in aseptic milk at 42 °C for 12 h.
2.6 Bacterial release ability of different model hydrogels
The LAB hydrogel was divided into two groups.Group A was 3D printed grid hydrogel with length and width of 20 mm and height of 2 mm.Group B is a mold-machined cylinder with a radius of 8 mm and a height of 6.5 mm (Fig.1d).Two groups of LAB hydrogels with the same quality were suspended in 4 °C aseptic saline for the same time,and 1 mL was sampled periodically by plate counting method to detect the change of bacterial density in the solution.Each sample was repeated an average of three times.
2.7 Release mechanism of LAB hydrogel cells
2.7.1 Dynamic swelling behavior
The LAB hydrogels were first placed into a liquid nitrogen tank for 10-15 min.After the freezing step,put the hydrogel in a vacuum freeze dryer (Ningbo Xinzhi freeze drying equipment Co.,Ningbo,China) and dry for 2 days [13].Freeze-dried hydrogels were weighed and place in normal saline and placed at 4 °C.The hydrogels were carefully removed from the solution specified time intervals,absorbed the water on the surface of the hydrogel and weighed.The swelling rate,q,was calculated as follows:

whereWsis the weight of the swollen hydrogel andWdis the weight of freeze-dried hydrogel.
2.7.2 Cell release kinetics
Three grams of LAB hydrogels were suspended in sterile saline solution at 4 °C for a period of time,a plate counting method was used to take 1 mL of samples periodically to detect the change of bacterial density in solution.There was an average of three repetitions per sample.The data of bacteria release were calculated according to the Higuchi equation [23],equation (2);Korsmeyer-Peppas equation [24],equation (3);Peppas-Sahlin equation [25],equation (4);Weibull equation [26],equation (5) using Origin 2019 software to fit.Nonlinear least squares fitting method was used to determine the parameters in each equation.

whereMtis the concentration of the bacteria released at timet,Mfis the concentration of the bacteria released at equilibrium,kandk1are constants incorporating the structural and geometric characteristics of the hydrogel,nis the release exponent describing the mode of the transport mechanism,mis the purely Fickian diffusion exponent for a system of any geometrical shape,ais the proportional parameter to describe the time dependence,andbis the shape parameter to describe the dissolution curve.
2.8 Optimization of bacteria release conditions in hydrogel
The LAB hydrogel was added into sterile milk at the ratio of 1:10(m/m) and cultured at 42 °C for 12 h.After culture,the hydrogel was taken out and the surface bacteria were washed off.The hydrogel was weighed.According to the characteristics of swelling at low temperature and shrinkage at high temperature.10% (m/m) sterile saline was added and placed at 4 °C for swelling.Put it in the water bath at different temperature to make the hydrogel shrink.To explore the effect of swelling and shrinkage of hydrogel on the release of bacteria.
2.8.1 Single factor experiment
The effects of swelling time,shrinkage time,shrinkage temperature on bacteria release ability in hydrogel were studied by single factor design.In detail,the single factor experiment was performed in a designed swelling time (range from 20 to 100 min),shrinkage time (range from 5 to 25 min),shrinkage temperature (range from 30 to 60 °C).One factor was changed,while the other factors kept constant in each experiment.The effect of each factor was evaluated by measuring the amount of bacteria release.
2.8.2 Response surface optimization
According to the single factor experimental results,a BBD with three-level and three independent variables (swelling time,A;shrinkage time,B;and shrinkage temperature,C) was employed in this optimization procedure.
2.9 Reuse of LAB hydrogel in fermented yogurt
Three grams of LAB hydrogel was added into the aseptic milk at the ratio of 10% (m/m) and activated at 42 °C for 12 h.After taking out the hydrogel and washing the surface bacteria with aseptic saline,adding 10% (m/m) aseptic milk and release cells under optimal conditions.Take out the LAB hydrogel and ferment the remaining milk at 42 °C until curdled.Stored at 4 °C for 24 h for further research.The hydrogel was reused according to the above method.The control group selected the direct vat set starter (The direct vat set starter is self-made in our laboratory) of corresponding strains for yoghurt fermentation,fermented at the same temperature until curdled,stored at 4 °C for 24 h,and then tested the fermentation performance.
After repeated use for 10 times,the LAB hydrogel was freezedried and stored at 4 °C for a month.One month later,the hydrogel was activated with aseptic milk and then reused,and the fermentation performance of the hydrogel after freeze-drying and storage was tested.After reusing for 20 times,the LAB hydrogel was stored at 4 °C for a week.One week later,the hydrogel was activated aseptically and then reused,and the fermentation performance of the hydrogel after low temperature storage was tested.
2.9.1 Determination of apparent viscosity
The apparent viscosity was measured directly by NDJ-8S type rotational viscometer (Shanghai,China).The measuring condition is the No.3 rotor,the rotational speed is 1.5 r/min,the torque is 20% -100%,and the measuring time is 90 s.
2.9.2 Determination of water holding capacity (WHC)
Adding 5 g fermented milk samples to the centrifuge tube,centrifuge at 3 000 r/min for 20 min at 4 °C,then discard the supernatant,and determine the remaining mass.The calculation formula of WHC of the sample is as follows:

whereWis the weight of the remaining yogurt after the supernatant is discarded,andW0is the weight of the yogurt.
2.9.3 Determination of acetaldehyde content
Trichloroacetic acid solution (0.16 g/mL) was mixed with yogurt sample at 1:1 (V/V) and centrifuged with 4 500 r/min for 10 min.The supernatant was filtered through filter paper.The clarified supernatant was used to determine the content of acetaldehyde and diacetyl.
Accurately take 2.00 mL 0.01 g/mL NaHSO3solution in triangular bottle,add 10 mL of the treated yogurt supernatant to mix,stand at room temperature for 1 h,add 1 mL 0.01 g/mL starch solution,drop 0.1 mol/L iodine solution,change to 0.01 mol/L iodine solution near the end point,and the solution happens to be purple,and remain unchanged for 30 s.Then add 20 mL 1 mol/L NaHCO3,shake to make the solution colorless,finally titrate with 0.01 mol/L iodine standard solution until it is just blue and do not change color for 30 s,record the volume of consumed standard iodine solution,and do blank experiment at the same time [27].
The calculation formula of acetaldehyde of the sample is as follows:
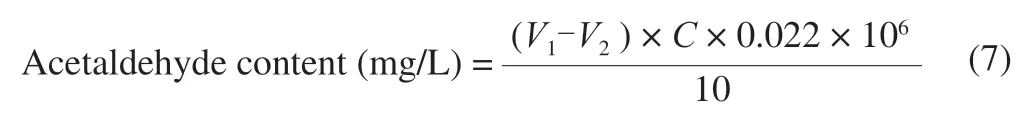
whereV1is Volume of I2standard solution consumed by sample titration,V2is Volume of I2standard solution consumed by blank titration,Cis 1/2 concentration of I2standard solution,and 0.022 is the basic unit of acetaldehyde chemical reaction.
2.9.4 Determination of diacetyl content
Established the standard curve of diacetyl concentration.The supernatant of the treated yogurt was diluted with 16% TCA solution and the volume was fixed to 10 mL.0.5 mL 1%o-phenylenediamine solution was added in the sample group,but not in the blank group.The reaction was statically placed 30 min under the condition of avoiding light after oscillation.4.0 mol/L hydrochloric acid was added to terminate the reaction,the sample group is added as 2.0 mL and the blank group is added as 2.5 mL.After mixing,the blank group was used as the reference solution,and the absorbance was determined by quartz colorimetric plate at 335 nm wavelength.The concentration of diacetyl is determined according to the absorbance value and the standard curve of diacetyl [28].
2.10 Spatially compartmentalized LAB co-culture to ferment yoghurt
Two tubes of bioink containingL.bulgaricusCICC6045 andS.thermophilusCQ43 were loaded into two cartridges of 3D printer and printed according to the preset procedure.The first layer isS.thermophilusand the second layer isL.bulgaricus(Fig.1a).After the hydrogel was pretreated,the yogurt was fermented by hydrogel repeatedly to determine its fermentation performance.In the control group,the two kinds of LAB were mixed in the ratio of 1:1 and added 10% to sterile milk for fermentation at 42 °C until curdled.Took the yogurt after the last round of fermentation as the starter and mix it with 40 g fresh sterile milk in the ratio of 1:10 for the next round of fermentation until curdled.And repeated the above process for yogurt fermentation.All the above fermented yogurt were determined the fermentation performance after stored at 4 °C for 24 h.
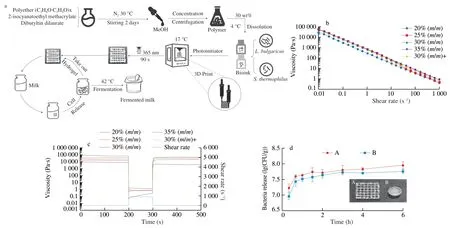
Fig.1 Overview of synthesis and application of LAB hydrogel based on 3D Printing.(a) Synthesis and application process of LAB hydrogel;(b) Shear-viscosity results for bioinks;(c) Recovery testing;(d) Bacterial release ability of different model hydrogels (A: 3D printed grid hydrogel with length and width of 20 mm and height of 2 mm;B: a mold-machined cylinder with a radius of 8 mm and a height of 6.5 mm).
2.11 Scanning electron microscopy and live/dead imaging
The LAB hydrogels which were used once and reused for 100 times were taken and washed off the surface bacteria with sterile water.Put them in liquid nitrogen for 10 min.Then the LAB hydrogel was sectioned with a frozen scalpel and freeze-dried.The cross sections and surface of dried hydrogels were sputtered and goldplated for 140 s,and the images were obtained by scanning electron microscope EVO-LS10 (Carl Zeiss AG,Baden Wurzburg,Germany).
After the LAB hydrogel was cultured and cleaned,a complete part of the hydrogel was stained.Living cell staining: Syto Green stain stock solution was diluted to 5 × 10−6mol/L and the sample was soaked for 30 min.Live/dead cell staining: Pi red stain stock solution was diluted to 2 × 10−4mol/L and the samples were immersed in the above two dye solutions for 20 min.The sample imaged using laser confocal microscope Ultra-View VOX (PerkinElmer,Massachusetts,America).
2.12 Statistical analysis
The experimental processing is repeated for 3 times,and the data processing and drawing are made by GraphPad 8.0.The results are expressed as the average ± standard deviation.One-way analysis of variance and Duncan’s multiple comparison tests were used to determine significant differences among means (P<0.05) by using SPSS version 25.0 (SPSS Inc,Chicago,IL,USA).Design expert 8.0.6 software was used for response surface analysis.
3.Results and discussion
3.1 Shear thinning character and recovery behavior
The shear viscosity profiles of a range of experimental samples were acquired from the rheology measurements (Fig.1b).All samples show shear thinning behavior,characterized by a decrease in viscosity over an increasing shear rate.This meets the material requirements of extruded 3D printers.
Recovery testing was undertaken to characterize the behavior of the materials post-printing.To mimic the shear conditions during printing,the materials were subjected to a low shear rate of 0.01 s−1to simulate at-rest conditions prior to extrusion.Subsequently,a shear rate of 895 s−1was applied to the samples for 100 s to simulate the shear forces in the needle tip during extrusion.And finally,a low shear rate of 0.01 s−1was again applied to measure the recovery of the materials.Fig.1c shows the recovery results for all samples,the printable concentration of 25%,30%,35% and 30% +show rapid recovery after application of the high shear rate,this allows the material to rapidly increase in viscosity after extrusion and maintain high shape fidelity.By comparison,the sample with 20% print concentration failed to recover its viscosity during the test time.In addition,the viscosity of 30% +sample is lower than that of 30% sample.Therefore,the addition of fermentation broth will reduce the printing effect of the sample,and the better printing effect can be achieved by adding 1% of the fermentation broth to the 30% sample.
3.2 Bacterial release ability of different model hydrogels
To demonstrate the advantage of 3D printing in microbial fermentation,the release of bacteria from different model hydrogels in aseptic saline was tested.As shown in Fig.1d,model A is a 3D printed grid LAB hydrogel,and model B is a cylindrical solid LAB hydrogel made by a mold.The number of bacteria released by the two models increased with time,but the number of bacteria released by the two models with the same quality was different.In the whole process of bacterial release,the number of bacteria released from model A was higher than that from model B.This shows that model B hinders the release of bacteria and the number of bacteria in model B is less than that of model A.
3.3 Release mechanism of LAB hydrogel
3D bioprinting is actually a kind of directional deposition cell immobilization technology,which limits the cells,such as microorganisms in a space with biological activity to perform fermentation.Compared with free cell culture,it has many advantages,including high cell densities,product yield improvement,lowered risk of microbial contamination,better process control and reproducibility [21].He et al.[29]developed a new type of double-net polymer carrier for the immobilization of bacteria [30].But few people use it for continuous inoculation (Fig.1a): after a simple incubation,continuous inoculation of the cell immobilization device,while maintaining the above advantages,but also to improve productivity and expand economic benefits.Therefore,it is very important to study the release of bacteria from hydrogel.
In the swelling controlled release system,the transport of solute in the polymer network is controlled by physical and chemical phenomena such as polymer water absorption,polymer chain swelling and relaxation,polymer dissolution/erosion and so on [31].The transmission of embedded cells in hydrogel network depends on environmental factors such as pH,temperature and ionic strength [11].Because the hydrogel used in this experiment has the characteristic of temperature-sensitive swelling,it will continue to swell below 4 °C as shown in Fig.2a.Therefore,the release kinetics of LAB hydrogel was studied at low temperature.
To explore the relationship between hydrogel swelling and bacteria release,we evaluated the weight swelling ratio of the hydrogel at different time points and the bacteria release at corresponding time points,Fig.2b showed that there was a positive correlation between the release rate of bacteria and the swelling of hydrogel.At 4 °C,the initial bacteria release of the hydrogel was faster,and then release rate decreased after 1.5 h.Similarly,the change of the weight swelling ratio of the hydrogel at 4 °C,which increased rapidly in the first 2 h and then decreases gradually.Therefore,swelling of the hydrogel is the limiting factor of bacteria release rate.That is,the highly expanded hydrogel contains a large amount of unbound water,which can release more solute [32].
Then,in order to further explore the release mechanism of bacteria in hydrogel,we used four common mathematical models to fit the hydrogel release data.As shown in Table 1,the fitting effect of Korsmeyer-Peppas,Peppas-Sahlin and Weibull model were better(R2>0.95).The fitting equation of Korsmeyer-Peppas model isF=0.444 3t0.506,n=0.506.For planar matrix,the release process depended on the swelling and diffusion of the polymer [21].
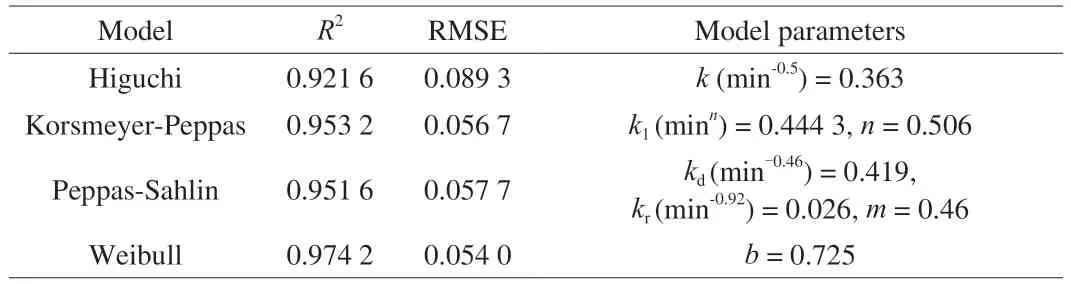
Table 1 Analysis of cell release data from LAB hydrogel using Higuchi equation,Korsmeyer-Peppas equation,Peppas-Sahlin equation and Weibull equation.a)
The relative contribution of diffusion and relaxation processes to the release mechanism could be analyzed by Peppas-Sahlin model.The pure Fickian diffusion index m of the hydrogel used in this experiment was 0.46,because the aspect ratio of the planar matrix was 8.3.The fitting equationF=0.419t0.46+0.026t0.92for Peppas-Sahlin model,in whichkd(min-0.46) >kr(min-0.92),also showed that the release behavior was dominated by diffusion [33],this is consistent with the result of Korsmeyer-Peppas model.While the post-polynomial of the Peppas-Sahlin model is expressed as a relaxation mechanism,indicating that the release process is controlled by the coupling of Fickian diffusion and matrix swelling [34].And with the increase of release time,the Fickian diffusion contribution (F) decreased,while the swelling diffusion contribution (R) gradually increased(Fig.2c).The results showed that with the increase of release time,the importance of diffusion limitation decreased and the relative importance of micelle structure change increased.
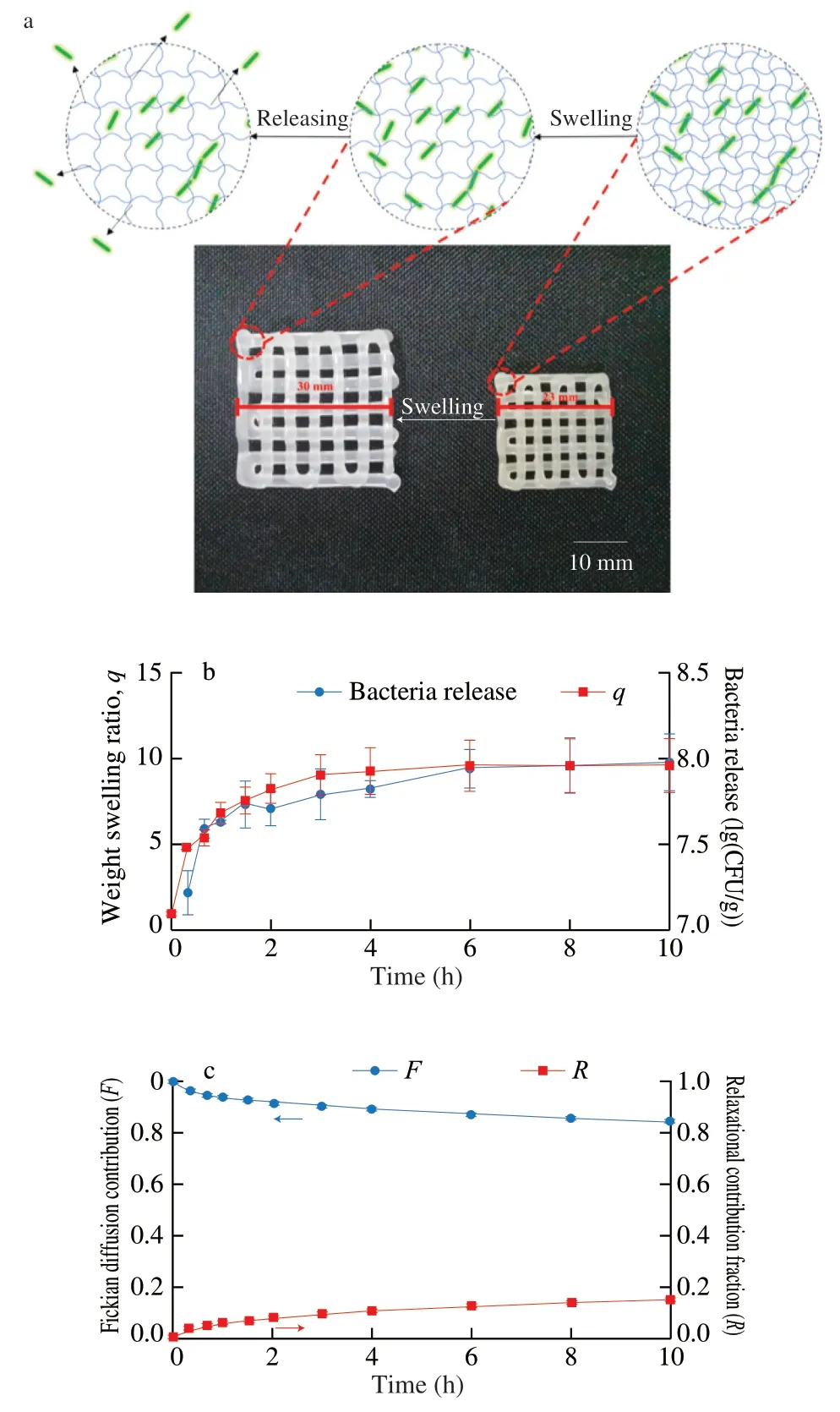
Fig.2 Cell release kinetics of LAB hydrogel.(a) Photograph of the printed 3D lattice before and after swelling at 4 °C,and schematic diagram of cell release;(b) Release of LAB hydrogel bacteria and dynamic swelling of hydrogel;(c) Fickian release contribution (F) and Relaxational release contribution fraction (R) of bacteria release from lactobacillus hydrogel.
TheR2of the fitting curve of the Weibull model was the highest and the RMSE was the lowest,indicating that the fitting effect of the model was the best.The fitting equationF=1 -exp (-0.582t0.725) and the shape parameterb=0.725 2 <0.75.It showed that the release rate curve is a parabola,with the extension of time,the release rate decreases and the release mechanism follows Fick diffusion law [26].
To sum up,it could be concluded that the release mechanism of bacteria in hydrogel was regulated by the coupling of diffusion and polymer swelling.
3.3 Optimization of bacteria release conditions in hydrogel
3.3.1 Single-factor release experiments
To obtain the optimal release conditions,several factors affecting the release rate were analyzed.According to the release mechanism of cells in the hydrogel,we selected swelling time,shrinkage time and shrinkage temperature that have great influence on the swelling ratio of hydrogel (Fig.3).As shown in Fig.3a,when the shrinkage time is 15 min and the shrinkage temperature is 37 °C,the longer the swelling time,the stronger the ability of releasing bacteria from the hydrogel.However,the release amount of the bacteria reached the maximum after 60 min,it shows that the maximum release amount of hydrogel has been reached.Then we studied the effect of shrinkage time on bacteria release in hydrogel (Fig.3b).When the swelling time is 30 min and the shrinkage temperature is 37 °C,with the increase of shrinkage time,the bacteria release in hydrogel increased and then decreased,and reached the maximum at 15 min,the release was(8.27 ± 0.18) lg (CFU/g).The decrease of post-release of 20 min may be due to bacteria adhere on the hydrogel surface passively due to hydrophobic interaction between the bacteria and the gel surface after reaching swelling equilibrium [12].Finally,we explored the effect of shrinkage temperature on it (Fig.3c).With the increase of shrinkage temperature,there was no significant change in the bacteria release of hydrogel at 30-42 °C.When the shrinkage temperature higher than 50 °C,the release amount of the bacteria decreased significantly,which was due to the damage of the bacteria caused by the higher temperature and the decrease of the activity.
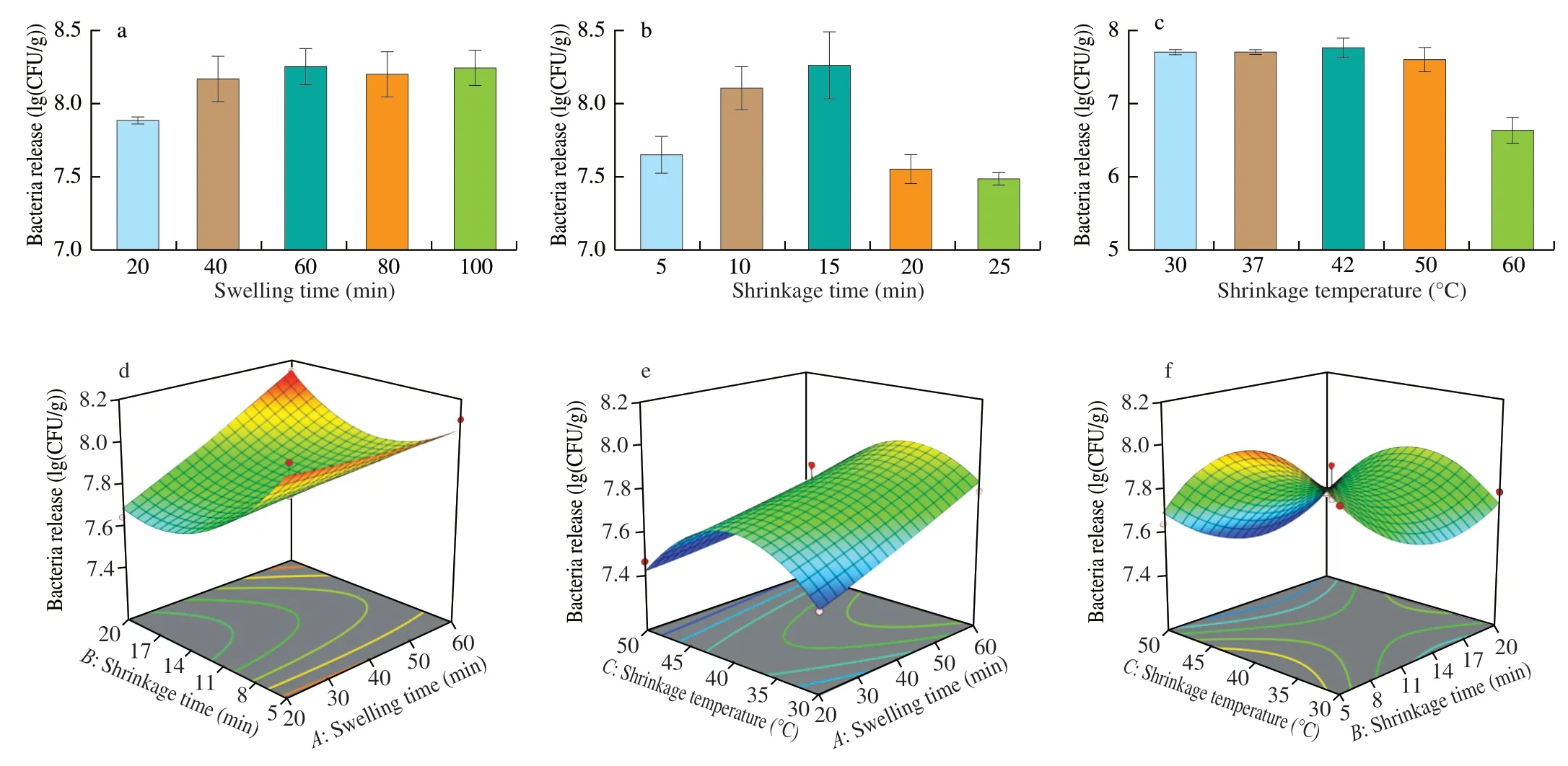
Fig.3 Effects of different factors on bacteria release in hydrogel.(a) Hydrogel release bacteria under different swelling time;(b) Hydrogel release bacteria under different shrinkage time;(c) Hydrogel release bacteria under different shrinkage temperature;(d) Swelling time and shrinkage time;(e) Swelling time and shrinkage temperature;(f) Shrinkage time and shrinkage temperature.
3.3.2 Bacteria release optimization using RSM
Based on the results of the single-factor experiments,swelling time (20-60 min),shrinkage time (5-20 min) and shrinkage temperature (30-50 °C) were selected for the optimization of the bacteria release (Table 2).A quadratic equation was used to establish a statistical model to confirm the optimum conditions and the response of the combined factors.By using multiple regression analysis on the experimental data,the bacteria release was obtained using the following quadratic equation:
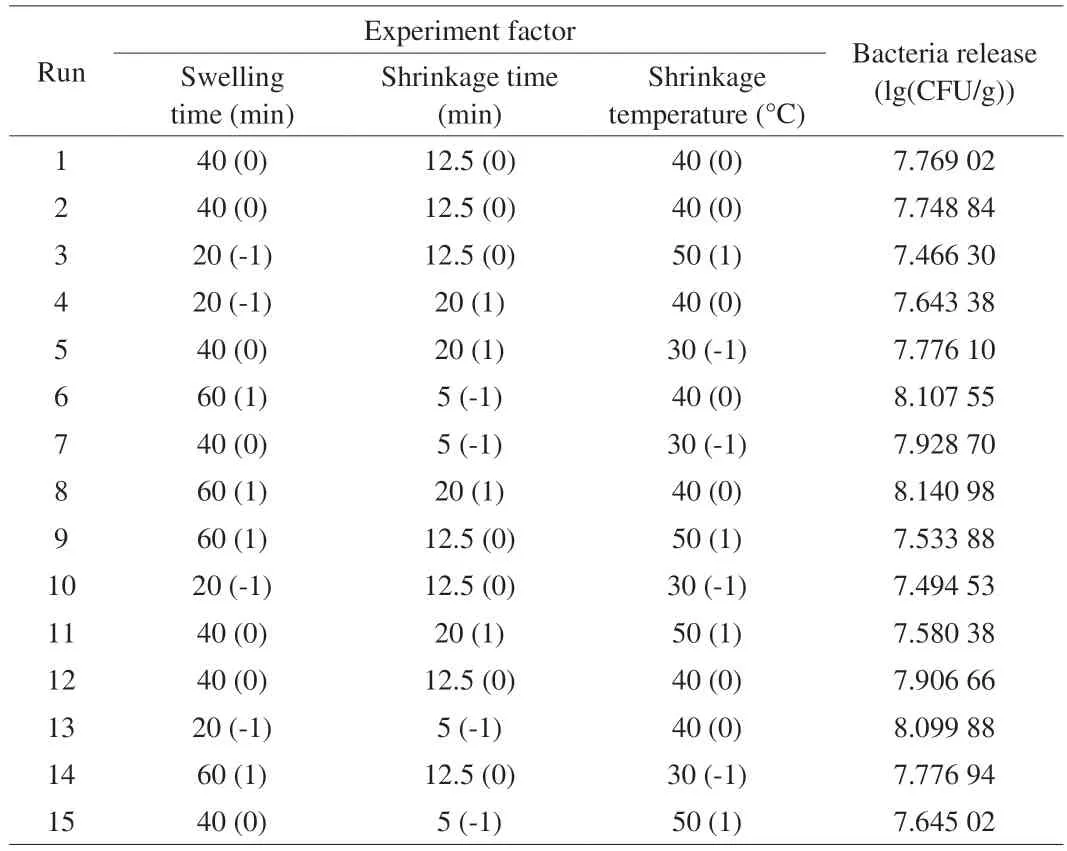
Table 2 Factors and levels used in response surface experiments.a)
Y=7.81+0.11A-0.080B-0.094C+0.12AB-0.054AC+0.022BC+0.013A2+0.18B2-0.25C2
whereYwas the predicted response;A,B,andCwere the swelling time (min),shrinkage time (min) and shrinkage temperature (°C).
The variance analysis and linear coefficient of the regression model,the coefficient of the two-item coefficient interaction and its significance test results are presented in Table 3.It could be seen from the analysis of table variance that theFvalue of the quadratic regression model was 13.02,P=0.005 7 <0.01,which indicated that the model reached a very significant level,and the misfit term was the variation of the data in the model,P=0.665 4 >0.05,the results showed that there was no significant difference in the inexact terms,the inexact factors existed in the test,which could fully reflect the actual situation,and the regression model was suitable.The determining coefficient of the test modelR2=0.959 1,which showed that the results of bacteria release were in good agreement with the predicted results of the model.The correction coefficientof the model was 0.885 4,and 88.54% of the experimental results were affected by experimental factors.Therefore,the results were reliable and this model could be used to analyze and predict the number of hydrogel release bacteria.In the analysis of variance of regression equation,Ftest could judge the influence of independent variables on dependent variables,and the order of influence of various factors on hydrogel release bacteria wasA>C>B,the swelling time had the greatest effect on hydrogel release bacteria,followed by the shrinkage temperature,and finally the shrinkage time.According to the regression equation and variance analysis,the effects of primary termA,BandCon bacteria release and the effect of interactive termABon bacteria release were significant (P<0.05).The effect of quadratic termsB2andC2on the number of viable bacteria were very significant(P<0.01).
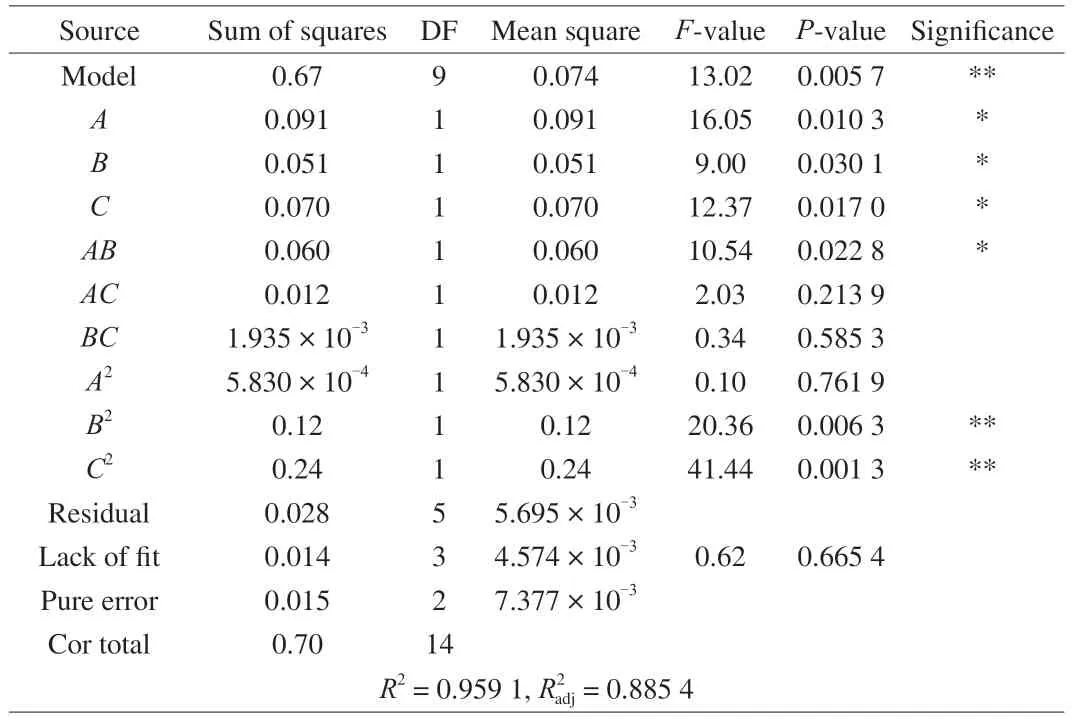
Table 3 ANOVA of the quadratic polynomial model and significance test.a)
The three-dimensional diagrams of the RSM model of bacteria release in the hydrogel are shown in Fig.3.When one factor was fixed at a centered level,the interaction between other factors could be reflected in the diagrams.The shape of the contour line was ellipse,which meant the interaction of the factors was significant,but the circle meant the interaction was not significant [35].From the 3D response surfaces of Fig.3d,there was a significant interaction between swelling time and shrinkage time.It could be seen from the three-dimensional diagram that the bacteria release has the maximum under the larger swelling time and contraction time.On the contrary,the interaction between swelling time and shrinkage temperature,shrinkage time and shrinkage temperature was not significant(Figs.3e and Fig.3f).
By using Design-Expert 12.0 software to optimize the process parameters,the optimum conditions for predicting hydrogel release bacteria were as follows: swelling time 60 min,shrinkage time 20 min,shrinkage temperature 37.52 °C,the most bacteria released were 8.163 lg (CFU/g).
In order to verify the reliability of the results obtained by the response surface method,the process conditions of the model optimization were verified.Considering the feasibility and convenience of the experiment,the process parameters were selected: swelling time=60 min,shrinkage time=20 min,shrinkage temperature=37.5 °C for 3 times,and the release bacteria of hydrogel was (8.123 ± 0.04) lg (CFU/g),which was close to that of the optimal combination,which indicated that the equation fitted well with the real test,and the optimized process was reliable and feasible.
3.4 Reuse of LAB hydrogel in yogurt fermentation
The fermentation of milk by LAB was verified to demonstrate the stability and repeatability of the hydrogel.We designed the technology of LAB hydrogel continuous inoculation to produce fermented milk(Fig.1a),aiming to maximize its production efficiency.As shown in Fig.4,LAB hydrogel was reused to obtain fermented milk,and its fermentation properties (WHC,apparent viscosity,diacetyl content and acetaldehyde content) were studied.Compared with the control group,the fermentation properties of LAB hydrogel did not change significantly after being reused for 100 times,indicating that LAB hydrogel not only has the same fermentation performance as direct vet set starter,but also can repeatedly prepare yoghurt for many times,it is economical and environmentally friendly.Qian et al.[36]used bioink composed of PEGDA,nano-cellulose and yeast freeze-dried powder for 3D printing and photocuring into hydrogel.After being reused for 4 months,the ethanol production activity did not decrease,indicating that the long-term use of light-cured hydrogel as biocatalyst is feasible.Therefore,the fermentation performance of LAB hydrogel has no significant effect after being used as starter for many times.In addition,the bacterial release effect of the hydrogel did not change significantly after repeated use,because the clotting time of each batch of yogurt was about 6-7 h.To explore the recoverability of after long-term storage,we evaluated the reuse of LAB hydrogel to produce fermented milk under different preservation conditions.First,the LAB bacteria hydrogel was freeze-dried and reactivated in milk after being stored for one month.In addition,the LAB hydrogel was reactivated in milk after being stored at 4 °C for a week.It can be found that the fermentation performance was not significantly affected in the subsequent reuse.He et al.[29]also tested the activity of hydrogel embedded withbacteria.After 4 weeks of storage,the activity of hydrogels did not decrease,but after a month of preservation,the activity of ethanol produced by freeze-dried hydrogel decreased,but it was not obvious.It shows that low temperature can preserve biomaterials for a long time and has no significant effect on the properties,which is consistent with the experimental results of this study.Therefore,we believe that these two methods have good preservation effect.
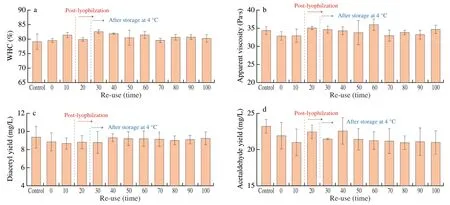
Fig.4 Fermentation performance of LAB hydrogel after repeated use.(a) WHC of fermented yoghurt;(b) Apparent viscosity of fermented yoghurt;(c) Diacetyl production of fermented yoghurt;(d) Acetaldehyde production of fermented yoghurt.
3.5 Spatially compartmentalized LAB co-culture to ferment yoghurt
In nature,mixed cultures often perform better than pure cultures [37].Therefore,different types of LAB are usually selected to ferment in appropriate proportion to produce yogurt with excellent characteristics [38].However,if there are no certain conditions,liquid cultures often fail over time.The immobilized cell technology can encapsulate microorganisms in polymer matrix as an alternative to suspension cell culture [30].To prove the excellent performance of the hydrogel,L.bulgaricusandS.thermophilus(two kinds of LAB commonly used in yogurt fermentation) were added to two tubes of bioink,and 3D printing technology was used to deposit alternately in the hydrogel grid.The fermentation performance of yoghurt was determined by repeatedly using compound LAB hydrogel to ferment yogurt.As shown in Fig.5,in 25 times of reuse,the fermentation performance of the compound starter did not change significantly,the WHC was maintained at 59% -66%,the diacetyl content was about 1.46-2.93 mg/L,and the acetaldehyde content was about 7.07-7.68 mg/L.After the two kinds of LAB were cultured in liquid mixture in the same proportion and fermented repeatedly,the indexes of yogurt gradually changed,in which the WHC,diacetyl content and apparent viscosity increased to a certain extent,and remained stable after the corresponding repetition times,while the acetaldehyde content decreased slightly.Some studies believe thatS.thermophilusis the main producer of diacetyl,while acetaldehyde is mainly produced byL.bulgaricus[39],andS.thermophiluscan produces more polysaccharides,which can improve the texture,WHC and apparent viscosity of yogurt [40].Some studies have shown that although there is a synergistic symbiosis betweenS.thermophilusandL.bulgaricus,in fact,there are still varying degrees of antagonism and inhibition between some strains [41].Therefore,it is likely thatS.thermophilusincreased andL.bulgaricusdecreased in the mixed starter.Therefore,the spatial separation of different bacteria by 3D printing LAB hydrogel can avoid their inhibition to a certain extent and make the microbial assembly more stable.
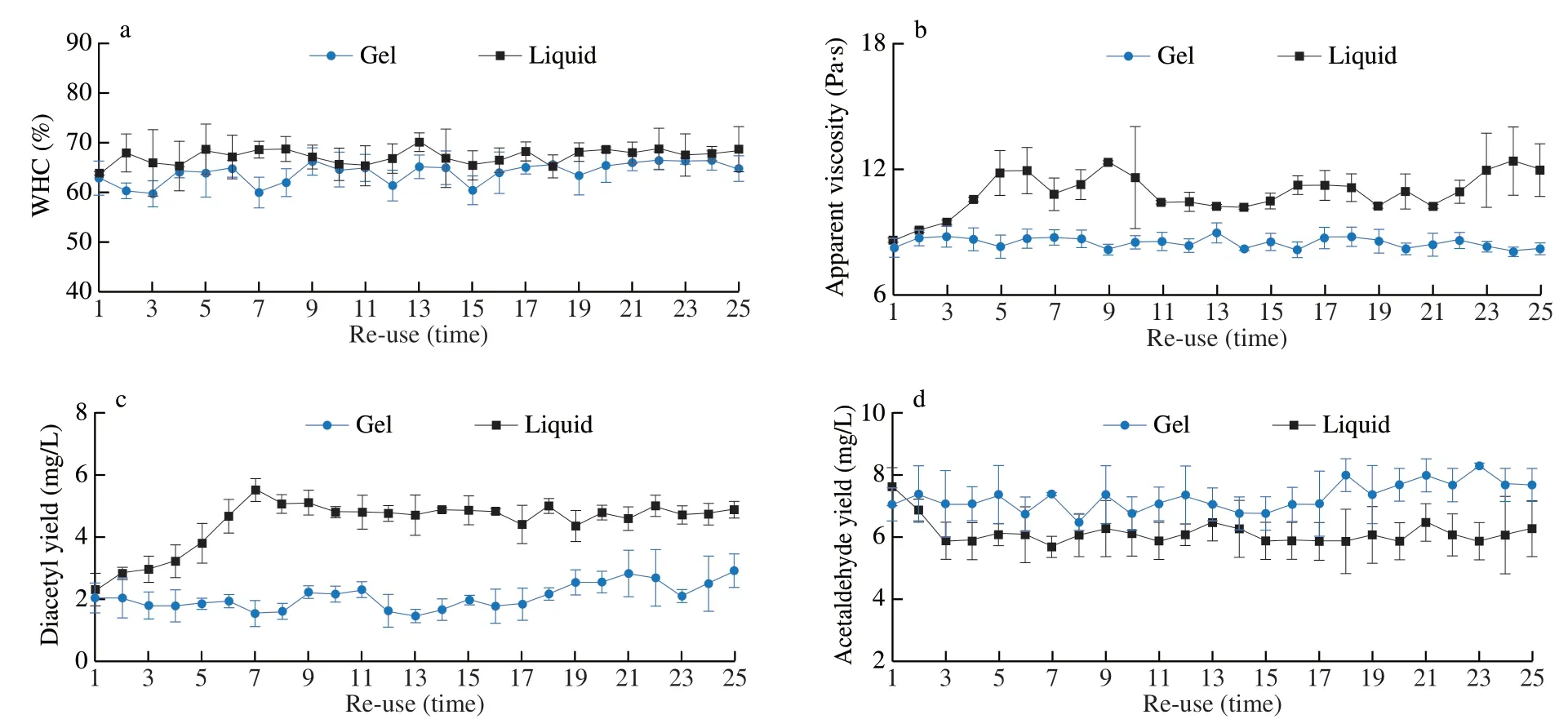
Fig.5 The fermentation performance of LAB hydrogel and liquid mixed starter culture after repeated used.(a) WHC of fermented yoghurt;(b) Apparent viscosity of fermented yoghurt;(c) Diacetyl production of fermented yoghurt;(d) Acetaldehyde production of fermented yoghurt.
3.6 Scanning electron microscope
To further demonstrate the reusability of LAB hydrogel,we used scanning electron microscopy to observe the surface structure of onceused hydrogel and reused hydrogel for 100 times (Fig.6).The surface of the hydrogel which is used only once was distributed with holes of various sizes (Fig.6a).Similarly,holes were also distributed on the surface of the hydrogel that has been reused 100 times (Fig.6b).The holes of the former were more three-dimensional,while those of the latter seemed to be filled with some materials.It was speculated that it may be a metabolite secreted by bacteria after repeated use.In addition,through the scanning electron microscope photos of the cross section of the hydrogel,we can see the internal structure of the hydrogel,which contained a large number of LAB (Figs.6c and 6d).The similarities in morphology and pore size of the hydrogel in both conditions implies that multiple rounds of fermentation do not affect the integrity of the hydrogel material.Calcium alginate hydrogel,as the most commonly used encapsulation platform for microbial cells,will break with the passage of time unless the concentration of calcium in the solution is regularly replenished [42].However,the hydrogel encapsulation device studied in this experiment does not need other additives and has the characteristics of strong stability.
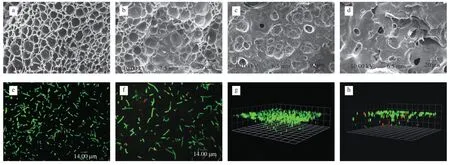
Fig.6 Scanning electron microscope and laser confocal imaging.(a) The surface of the hydrogel that has been used only once;(b) The surface of the hydrogel after 100 times of use;(c) Cross section of hydrogel that has been used only once;(d) Cross section of hydrogel after 100 times of use;(e) 2D photographs of living cells stained by confocal microscopy;(f) 2D photographs of living/ dead cell staining;(g) 3D photographs of living cells stained by confocal microscopy;(h)3D photographs of living/dead cell staining.
3.7 Live/dead imaging
To further explore the distribution of bacteria in this living material,we observed the live/ dead cells of LAB hydrogel by laser confocal microscope.As shown in Figs.6e and 6g,after staining with living cell stain,the 3D image of LAB hydrogel taken by confocal microscope showed that the bacteria were distributed in the hydrogel,but the bacteria density near the surface was significantly higher than that in the interior.After staining with living/dead cell stains,as shown in Figs.6f and 6h,dead bacteria and living bacteria were distributed everywhere in the hydrogel,but the closer to the interior,the fewer living bacteria and more dead bacteria.It can be inferred that the distribution of bacteria in the hydrogel was controlled by the gradient of nutrient concentration,the closer to the surface,the richer the nutrition is,and the higher the density of living bacteria was,which provided a theoretical basis for 3D printing of bacteria.This also explains that for hydrogels of the same quality,grid hydrogel made by 3D printing have a larger surface area and have more bacterial release than solid hydrogel made by models.Maximum bioconversion efficiency is achieved by printing hydrogel with a larger surface area.
4.Conclusions
In the present study,the bioink with printability,biocompatibility,stability and non-toxicity was prepared,and bacteria were added to it.The extruded 3D printer was used to print hydrogel embedded in live bacteria.Three common mathematical models were used to fit the bacteria release data of the hydrogel.The results showed that the bacteria release mechanism in the hydrogel is regulated by the coupling of Fickian diffusion and polymer swelling,and the bacteria release rate can be effectively controlled by changing the swelling rate of the hydrogel.After 100 times of yogurt fermentation experiments,there was no significant change in the fermentation performance of LAB hydrogel and the structure of the hydrogel had not been destroyed.In addition,the distribution of bacteria in the hydrogel was controlled by the gradient of nutrient concentration,and the activity near the surface was higher.Therefore,we can increase the hydrogel specific surface area by optimizing the 3D printed model,resulting in enhanced fermentation efficiency of LAB hydrogel.The results of coculture of different LAB showed that the fermentation performance of spatial isolation culture based on 3D bioprinted hydrogel was more stable than that of liquid mixed culture.It provides an appropriate growth environment for the co cultivation of different microorganisms and an unprecedented opportunity to improve the overall biocatalytic performance.Therefore,it has good application potential in fermentative production utilizing various microorganisms.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
This study was supported by Jiangsu Agriculture Science and Technology Innovatioin Fund (CX (21)2003).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.1016/j.fshw.2022.07.049.
- 食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Biological factors controlling starch digestibility in human digestive system
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
- Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil

