Complexation with pre-formed “empty” V-type starch for encapsulation of aroma compounds
Jingyi Zhou,Lingyan Kong*
Department of Human Nutrition and Hospitality Management,The University of Alabama,Tuscaloosa,35487,USA
Keywords:Starch“Empty” V-type Aroma Inclusion complex Encapsulation
ABSTRACT Aroma compounds are low-molecular-weight organic volatile molecules and are broadly utilized in the food industry.However,due to their high volatility and evaporative losses during processing and storage,the stabilization of these volatile ingredients using encapsulation is a commonly investigated practice.Complexation of aroma compounds using starch inclusion complex could be a potential approach due to the hydrophobicity of the left-handed single helical structure.In the present study,we used starch of three different V-type structures,namely V6h,V7,and V8,to encapsulate six different aroma compounds,including 1-decanol (DN),cis-3-hexen-1-ol (HN),4-allylanisole (AN),γ-decalactone (DA),trans-cinnamaldehyde (CA),and citral (CT).The formed inclusion complexes samples were characterized using complementary techniques,including X-ray diffraction (XRD) and differential scanning calorimetry (DSC).The results showed that upon complexation with aroma compounds,all V-subtypes retained their original crystalline structures.However,different trends of crystallinity were observed for each type of the prepared inclusion complexes.Additionally,among three V-type starches,V6h-type starch formed inclusion complexes with aroma compounds most efficiently and promoted the formation of Form II complex.This study suggested that the structure of aroma compounds and the type of V starch could both affect the complexation properties.
1.Introduction
Aroma compounds are low-molecular-weight organic volatile molecules that are mostly from the chemical classes of alcohols,aldehydes,ketones,and esters [1,2].Many aroma compounds are used in food products due to their desirable scent and health-promoting properties,such as antibacterial,antifungal,and antioxidant properties [3,4].In addition,aroma compounds are commonly employed in non-food industries,such as cosmetics and personal care.However,given its high volatility and evaporative loss during processing and storage,the stabilization of these volatile ingredients using encapsulation is a widely investigated practice [5,6].These aroma compounds’ utility could be largely enhanced through encapsulation approach by reducing evaporation,preventing volatile loss,and enhancing stability during storage and application [7,8].
The amylose component of starch is well-known to form inclusion complexes (ICs) with various types of molecules.When forming the ICs,the amylose helices tend to stack together to form a V-type crystalline structure to entrap the guest molecules [9].The binding forces of the host-guest structure are mainly weak bonds,including hydrogen bonds,Van der Waals force,and electrostatic interactions [6,9,10].Based on the size and shape of the guest molecules and also the water bound to the glucose units,the single helical structure could be in the form of either V6-,V7-,or V8-type,which contains 6,7,or 8 glucose units per turn,respectively.When small molecules,e.g.,iodine [11],linear alcohols [12],and fatty acids [13,14],are entrapped,amylose can form 6-fold single helical structure (V6).Helices of larger dimensions could be obtained when bulkier molecules get entrapped,such astert-butanol [15]and 2-propanol [16],which could result in the formation of 7-fold single helices (V7).Even larger or bulkier molecules,e.g.,1-naphthol and quinolone [17],could facilitate the arrangement of V-type structure with a 8-fold single helical structure (V8).The inner cavity diameter of the V-type structures ranges 5.4-8.5 Å and the outer cavity diameter varies from 13.0 to 16.2 Å,respectively,allowing them appropriate to encapsulate guest molecules with different chemical structures [10,18-20].
Although there are reports on the encapsulation of aroma compounds into amylose and starch,the formation process all required high temperature,which may influence the stability of the heat-sensitive aroma compounds [2,4,21,22].As compared to the traditional complexing methods,such as water method and DMSO method,the novel approach does not require high temperature and long processing time.In addition,encapsulating aroma compounds into V-type starches with different diameters and/or structuralcomplexation ability relationship have rarely been investigated,to our knowledge.Accordingly,in this study,a novel complexing method,the preformed “empty” V-type method,was applied to form ICs with a wide variety of aroma compounds that have different structures,including 1-decanol (DN),cis-3-hexen-1-ol (HN),citral (CT),4-allylanisole (AN),γ-decalactone (DA),andtrans-cinnamaldehyde(CA).Based on their structures,selected aroma compounds can be classified into either linear or cyclic (Table 1).Among the linear aroma compounds,including DN,HN,and CT,DN has a C10linear alkyl structure,HN has a double bond and a shorter linear segment,while CT has more double bonds and a branched structure.Among the selected cyclic aroma compounds,DA has the longest linear segment that attached to a lactone ring.When comparing AN with CA,although they both consist of a benzene ring,CA’s benzene ring is attached to an unsaturated aldehyde,whereas AN’s benzene ring is substituted with a methoxy group and an allyl group.
The present work aimed to investigate the complexation ability of the guest aroma compounds as well as the subsequent physicochemical properties of the ICs.This investigation will enhance our understanding of the starch-aroma complexation as a function of guest structural characteristics and expedite the practical application of ICs in the food industry.Such ingredient could possibly regulate the storage stability and release profiles of aroma compounds under various circumstances in food formulations.
2.Materials and methods
2.1 Materials
High amylose maize starch (HAMS;Hylon VII) was kindly provided by Ingredion (Bridgewater,NJ,USA).All of the selected aroma compounds including,DN (8034630100),HN (STBB7845),CT (STBG4262V),AN (MKCH7037),DA (MKBW9719V),and CA(MKCD4749) were purchased from MilliporeSigma (Burlington,MA,USA).Table 1 lists the physicochemical properties and odor descriptors of the guest molecules evaluated in this study.Ethanol,acetone,dimethyl sulfoxide (DMSO),and DrieriteTMdesiccant were obtained from VWR International (Radnor,PA,USA).Salicylic acid andtert-butanol were obtained from Thermo Fisher Scientific(Waltham,MA,USA).All other reagents were of analytical grade.
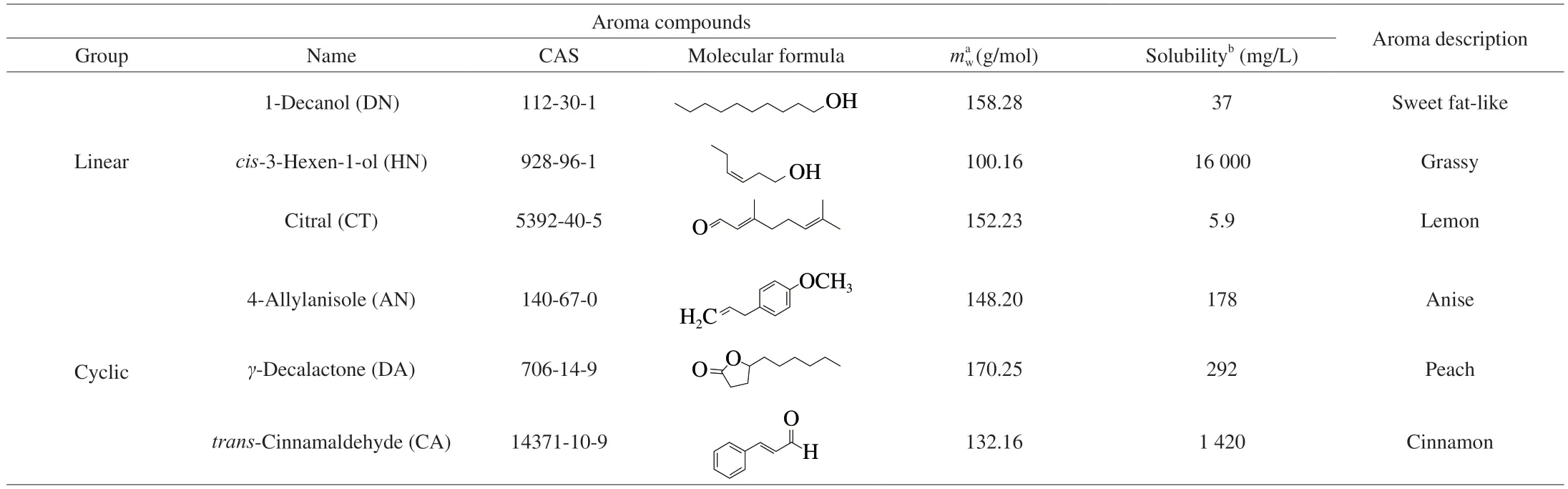
Table 1 Physicochemical properties and odor descriptors of guest aroma compounds evaluated in this study.
2.2 Preparation of V-subtype of HAMS
The “empty” V-starch was prepared according to an established method [23]with slight modifications.HAMS (5%,m/V) was dissolved in 95% (V/V) aqueous DMSO solution in a boiling water bath with constant stirring for at least 30 min.The hot dispersion was mixed into 2.5 vol of pure ethanol at 20 °C with vigorous stirring.The mixture was centrifuged (3 000 r/min,10 min) and the supernatant was discarded.The precipitate was washed twice with pure ethanol,followed by annealing at 75 °C in 40% (V/V) aqueous ethanol solution.The mixture was then washed with pure ethanol twice and finally dried in a vacuum desiccator to obtain the V6a.The V6hsample was obtained by conditioning the V6asample in a chamber containing supersaturated NaCl solution (aw=0.75) for 2 days.
The V7-type HAMS was obtained by heating the V6hsample in 50% (V/V) aqueoustert-butanol (V6h: aqueoustert-butanol=1:8,V/V)at 90 °C for 30 min with stirring at each 10 min interval.The suspension was then cooled down to room temperature,centrifuged,washed with aqueous 40% (V/V)tert-butanol twice,and vacuum dried in order to evaporatetert-butanol.The V8-type HAMS was producedby heating the V6h-HAMS with salicylic acid (V6h:salicylic acid=1:1,m/m) in a sealed pressure vessel at 150 °C for 1 h.The product was then washed with excess acetone to remove all salicylic acid and vacuum dried.The V8-type sample was then obtained by conditioning the dried sample in a desiccator containing saturated NaCl solution(aw=0.75) for 2 days.
2.3 Inclusion complexation with aroma compounds
Guest aroma compounds (0.02 g) and the preformed different“empty” V-subtype starch samples (0.1 g) were mixed together.The mixture was kept at 70 °C for 10 min.The mixture was then washed with pure ethanol at 20 °C and vacuum dried in a desiccator for 48 h.The resulted samples were weighed and pulverized into fine powders for further analysis.
2.4 Complex characterization
2.4. Wide angle X-ray diffraction (XRD)
Wide angle XRD patterns were obtained with a Bruker D8 Discover X-ray diffractometer (Bruker Corporation,Billerica,MA,USA) for HAMS inclusion complex samples.The ground samples were exposed to Co Kα radiation (1.79 Å).Data obtained using the Co Kα radiation source were converted to Cu Kα radiation based 2θvalues from 4° to 30°.
2.4.2 Differential scanning calorimeter (DSC)
Approximately 2 mg of dry sample was weighed using a Mettler-Toledo XP2U ultra-microbalance (Mettler-Toledo International Inc.,Columbus,OH,USA) into an aluminum pan (TA Instruments,New Castle,DE,USA).Deionized water was added to obtain a 10% (m/V)dispersion.An empty pan was used as the reference.The sample pans and reference pan were hermetically sealed.After the pans were equilibrated to 10 °C,two heating scans were conducted from 20 to 120 °C at 5 °C/min,separated by cooling at 5 °C/min in a Discovery DSC 250 DSC (TA Instruments,New Castle,DE,USA).
2.5 Statistical analysis
All characterization experiments were conducted in duplicates.The results are reported as means ± standard deviation,and significant differences between groups were analyzed using one-way analysis of variance (ANOVA) followed by Tukey multiple comparison test.Statistical analysis was conducted using GraphPad Prism software(San Diego,CA,U.S.A.).Means followed by the same lower-case letter are not significantly different atα=0.05.
3.Results and discussions
3.1 Characterization of HAMS V-subtypes
XRD patterns were obtained to confirm the formation of various preformed “empty” V-subtypes of HAMS and their corresponding diffraction peaks are shown in Fig.1.As compared with raw HAMS,all prepared V-type HAMS samples,including V6h,V7,and V8,exhibited higher crystallinity and shift of diffraction peaks.The observed characteristic patterns resembled the pseudo-hexagonal orthorhombic profiles of V6Ias proposed by Le et al.[24].The V6htype starch showed major diffraction peaks at 2θ=13.0° and 19.9°.These values agree with those previously reported work [23,25].Comparing to V6allomorph,the diffraction peaks shifted to lower Bragg angles for V7-and V8-type HAMS,which could indicate the expansion of V6-type structure to entrap the largertert-butanol and salicylic acid molecules,respectively.According to Le et al.[26],Uchino et al.[27],and Shi et al.[25],here we designate the formed V7-and V8-type HAMS as pseudo-hexagonal samples that comprise 7-and 8-fold helices.The V7-type starch showed major diffraction peaks at 2θ=12.9° and 18.0°.This diffraction pattern was consistent with previous reported V7[28],V7a[25],or V7I[26].Characteristic reflections for the V8-type starch appeared at 2θ=12.7° and 16.4°,which were also close to the reported V8[28]or V8h[25].
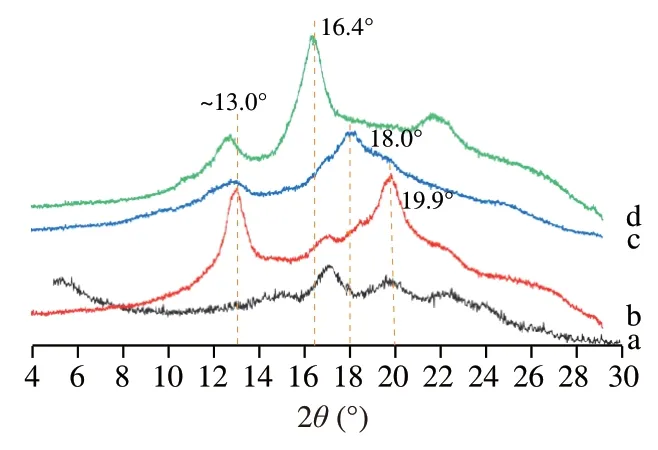
Fig.1 X-ray diffraction patterns of (a) raw high amylose maize starch(HAMS) and preformed “empty” (b) V6h-,(c) V7-,and (d) V8-type starches used to form inclusion complexes in this study.
3.2 HAMS-aroma compound inclusion complexes
3.2.1 V6h-type HAMS ICs
3.2.1.1 Crystalline structure
The crystalline structure of starch-aroma complexes was characterized by XRD,which can provide the evidence of ICs’formation.Raw HAMS without the inclusion of aroma compounds served as the reference.The diffraction diagrams of the reference and of the V6h-aroma complexes are presented in Fig.2A and corresponding percent crystallinities of complexes are shown in Table 2.As shown in Fig.2A,all V6hICs samples retained V6htype structure upon complexing with V6h-type HAMS which major diffraction peaks at around 13° and 20°,corresponding to the hydrated V6(V6h) or V6Istructure [29],while significant difference in crystallinity among V6h-type IC samples were observed,with V6h-DN IC being the highest at 48.73% and V6h-CT IC the lowest at 37.13% .Among complexes prepared with linear and/or branched alkyl aroma molecules,including DN,HN,and CT,their characteristic peaks at 13° and 20° indicated the hydrophobic interactions between the linear alkyl chains of DN,HN,or CT,and the internal helical cavity,which induced the encapsulation of the guest compounds within the central cavity of the single helical structure of V-type starch [30].For DN and HN complexes,the results are consistent with previous reports,which observed the same characteristic peaks for linear alcohols inclusion complexes with starch [2,12,31-33].For CT complexes,the same characteristic peaks at 13° and 20° were also observed for other types of starch-linear aldehyde ICs,such as 1-decanal,decanoic acid,and hexanal [2,34].
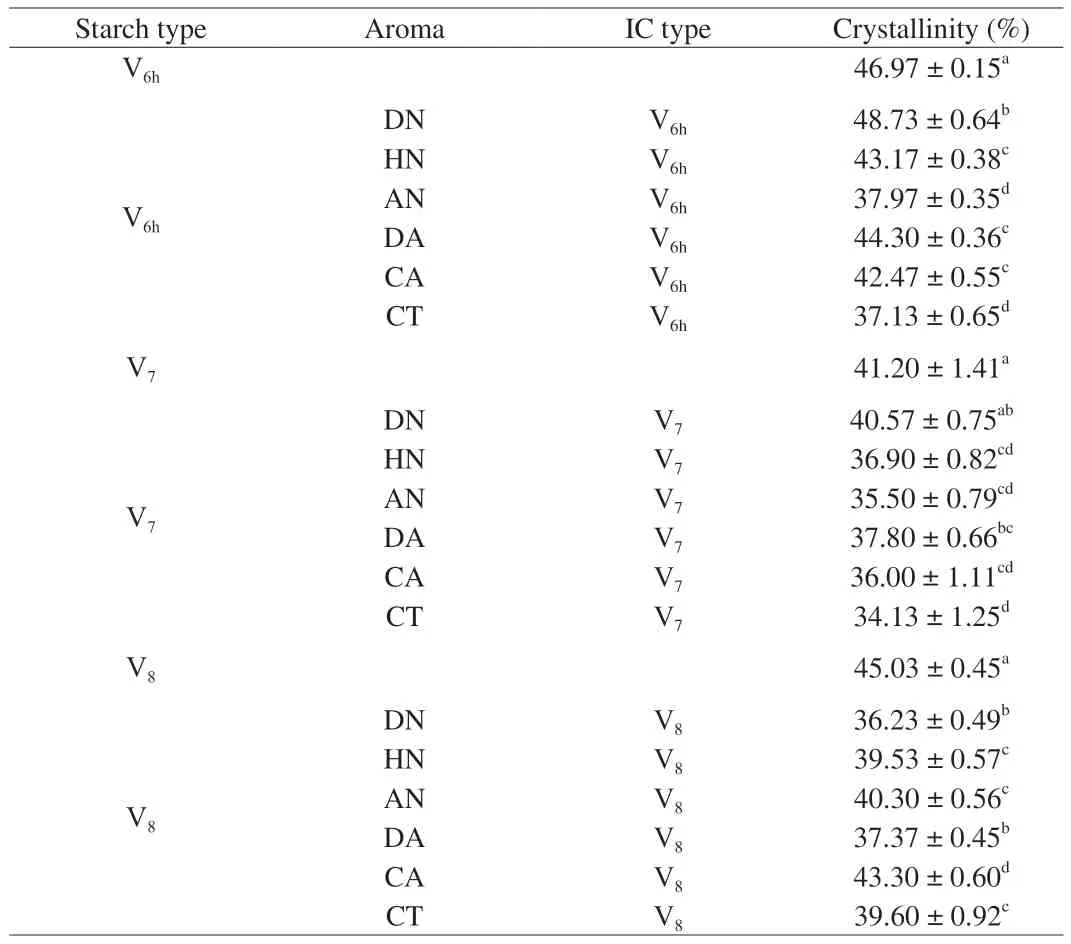
Table 2 Inclusion complexes’ type,and crystallinity of V6h-,V7-,and V8-type high amylose maize starch (HAMS) inclusion complexes prepared with DN,HN,AN,DA,CA,and CT.
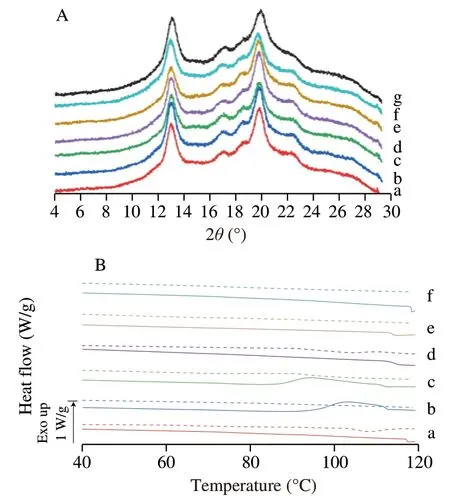
Fig.2 (A) XRD patterns and (B) DSC thermograms of V6h-type high amylose maize starch (HAMS) inclusion complexes prepared with (a) DN,(b) HN,(c) AN,(d) DA,(e) CA,and (f) CT.The XRD pattern of (g) the raw V6h-type HAMS is shown in Fig.2A.
Similar to linear and branched aroma compounds,V6h-type ICs prepared with AN,DA,and CA,also did not show new characteristic peaks,indicating no structure alteration on V6hcrystalline structure upon complexing with cyclic aroma compounds.The result for complexes with DA was consistent with a previous report of Heinemann et al.[35],that observed XRD characteristic peaks at 13° and 20° for DA ICs prepared with freeze-dried potato starch.Although some previous works showed that amylose-lactone complexes exhibited either single V6III-or V7-type,or mixed pattern with V6-and V7-type crystalline structure [36,37],the hydrophobic interaction between DA’s C6alkyl aliphalic chain and HAMS’ helical cavity could be strong enough to stabilize the formation of IC without changing the performed V6h-type structure.Accordingly,it is assumed that the linear segment of DA could be wrapped within the V6h-type HAMS central cavity.Limited studies have been done to examine the formation of starch-CA ICs up till the present study.Based on the existing studies,Tian et al.[38]employed ultrasound treatment to from IC between HAMS and CA,complementary techniques,including Fourier-transform infrared (FT-IR) spectroscopy and thermogravimetric analysis (TGA),were used and confirmed the successful encapsulation of CA into HAMS matrix.Such results indicated the ability of HAMS to act as an encapsulating material to entrap CA inside its central cavity.Similar to CA,no studies have been conducted related to starch-AN ICs based on current literature search.However,the short C3alkyl aliphalic chain in AN may not facilitate its residence within V6h-type HAMS helical cavity.
3.2.1.2 Thermal properties
Thermoanalysis is generally used to detect and characterize starch-guest inclusion complex formation and previous research has shown that variations in peak temperature (Tp) and enthalpy(ΔH) of endotherms could reflect the structure,composition,and crystallinity of V-type starch [13,25,39].As shown in Fig.2B and Table 3,no endotherms were observed for all V6h-type ICs during the first-run heating process.However,the complexes with DN,HN,and DA showed an endothermic phase transition during the secondround heating cycle,but not in other V6hIC samples.The V6hICs samples prepared with DN,HN,and DA showed a endothermic peak temperature at 108.30 °C (ΔH=6.69 J/g),102.88 °C (ΔH=3.06 J/g),and 101.98 °C (ΔH=3.77 J/g),which correspond to the formation of Form II complex,that has comparatively high thermostability and high crystallinity [4,13,29].In addition,comparing to HN and DA,the longer linear C10alkyl-chain of DN would require less space when forming inclusion complex with starch,so that is only resided the helical cavity of the V6h-HAMS,which could elicit to tight stacking between the helices and thus result in a compact crystalline structure.Such compact crystalline structure would require more energy during the dissociation process,which could further explain the fact that the V6h-DN had the highest dissociation temperature and highest enthalpy as compared to V6h-HN and V6h-DA ICs.The result was also consistent with its crystallinity pattern observed by XRD that V6h-DN has the highest crystallinity when comparing with V6h-HN and V6h-DA ICs.No endothermic phase transition was observed for ICs during the first-run heating process could be due to the water addition during the DSC testing sample preparation process,which could lead to ICs’ dissociation.However,during the first-round cooling and second-round heating processes,the decomplexed samples may then recrystallized and form the more crystalline structure,which could the melt during the second-run heating process within the 100-115 °C range [22].Unlike DN and DA ICs,an exothermic phase transition at around 102 °C was also observed for V6h-HN IC,which could indicate the crystallization of new complexes [40].Together,these results indicated that the melting of V6hcomplexes prepared with DN,HN,and DA,were reversible when samples were heated to temperatures lower than 120 °C.As compared to V6h-HN IC,although V6h-AN IC also showed an exothermic phase transition during the firstrun heating cycle,it did not promote formation of new crystallized complex,and thus,no endotherm was observed during the secondround heating process.In addition,no phase transitions were shown in either V6h-CA or V6h-CT ICs.In conjunction with their XRD patterns,it can be concluded that there is no structural change to V6h-type starch even if ICs with CA or CT were formed.
3.2.2 V7-type HAMS ICs
3.2.2.1 Crystalline structure
XRD patterns of V7-type HAMS ICs and raw HAMS (served as reference) were obtained to confirm the formation of complexes.The diffraction diagrams of the reference and of the V7-aroma complexes are presented in Fig.3A.It shows that the complexation with six selected aroma compounds did not change the V7-type HAMS structure,which could be because its cavity does not need to expand to complex with the aroma molecules.However,all V7-aroma ICs had a relatively lower crystallinity as compared with the“empty” V7HAMS,and such decreased crystallinity could be owing to either the site of aroma molecules been entrapped,or the loss of water molecules that stabilize the helical structure,and thus lead to less regular packed or distorted structure [25].Interestingly,the trend of V7-aroma ICs’ crystallinity was the same as those of the prepared V6h-aroma IC samples,which follows the order of DN >DA >HN >CA >AN >CT (Table 2).Such trend could be because of the length and structure and alkyl aliphalic chain.To be specific,among all six selected aroma compounds,DN has the longest linear alkyl chain,thus could stably reside within the V7HAMS helical cavity without interfering with its structure.In comparison with DN,although the linear C6alkyl chain of DA could help to stabilize its residence in the helical cavity though hydrophobic interaction,the lactone rings may not be able to be wrapped by or may alter the structure V7’s helical cavity.Accordingly,DA molecules might be present both within helical cavity,between helices,and perhaps within amorphous regions [4,16,25,41].In addition,although HN also has a C6alkyl chain,the decreased crystallinity could be possibly caused by itscisconfiguration,which can disrupt the linear shape of HN,the leaf alcohol,and thus cannot be fully contained within the V7HAMS central cavity.While for CA,AN,and CT,since the complexation did not alter the structure of the V7-type HAMS,their cyclic and/or branched structures may not be able to be incorporated into the helical cavity,and resulted in being entrapped with amorphous regions [42],which could give rise to lower crystallinity.
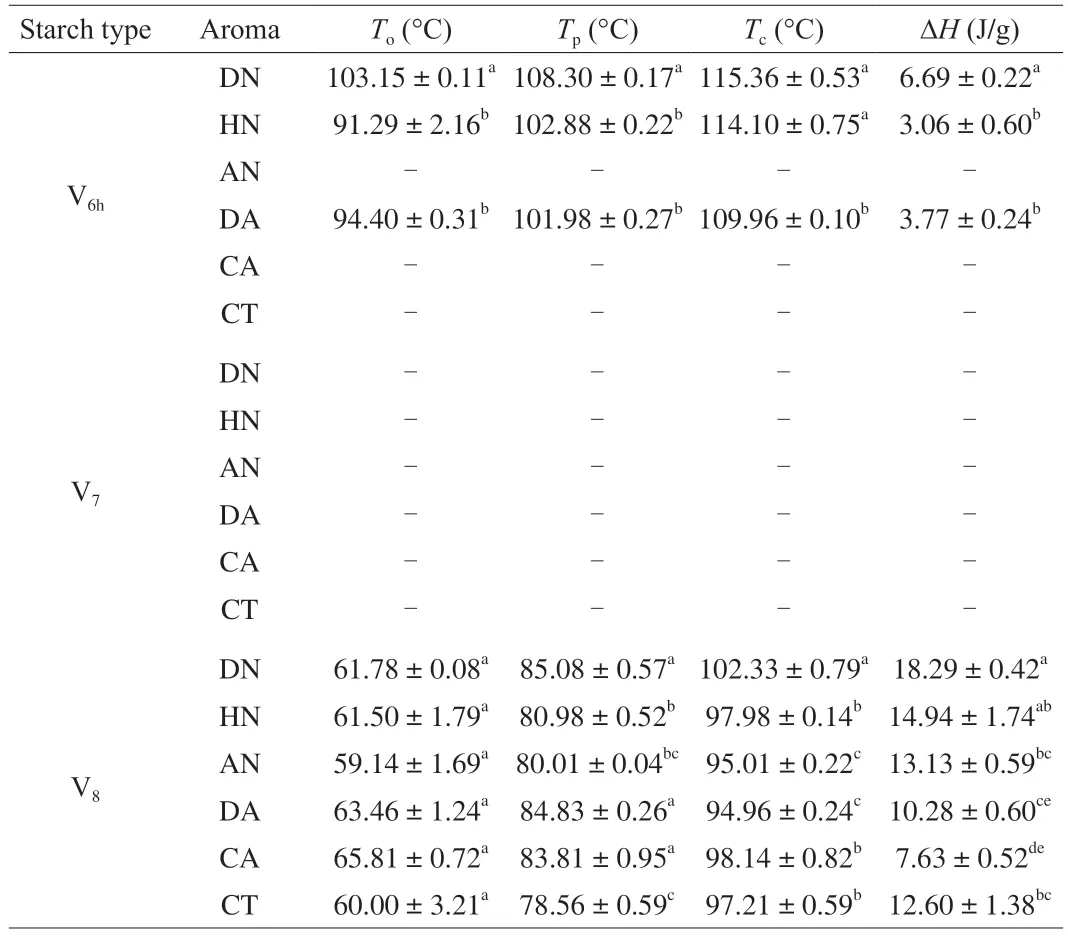
Table 3 Thermal properties of V6h-,V7-,and V8-type high amylose maize starch(HAMS) inclusion complexes prepared with DN,HN,AN,DA,CA,and CT.
3.2.2.2 Thermal properties
Thermal properties of V7-aroma ICs were evaluated and shown in Fig.3B and Table 3.As shown in the figure,no endotherms were observed for all V7-type ICs during both the first-run and the secondrun heating processes.In spite of this,a broad exothermic phase transition ranging from 90 °C to 108 °C was observed for aroma compounds that have linear carbon chain length longer than 6 Cs,which include,DN,DA,and CT.Such broad exotherms could be responsible for the rearrangement and partial recrystallization of starch helices [40].The results for V7-DN and V7-DA correspond to their XRD patterns of higher crystallinity,but not for V7-CT sample.Additionally,no phase transitions were found in V7-HN,V7-AN,and V7-CA ICs.In tandem with their XRD patterns,it can be supposed that there is no structural change to V7-type starch even though ICs were formed between V7HAMS and HN,AN or CA.Further validation methods such as GC-MS should be employed to verify whether these aroma compounds were successfully encapsulated by preformed “empty” V7-type HAMS.
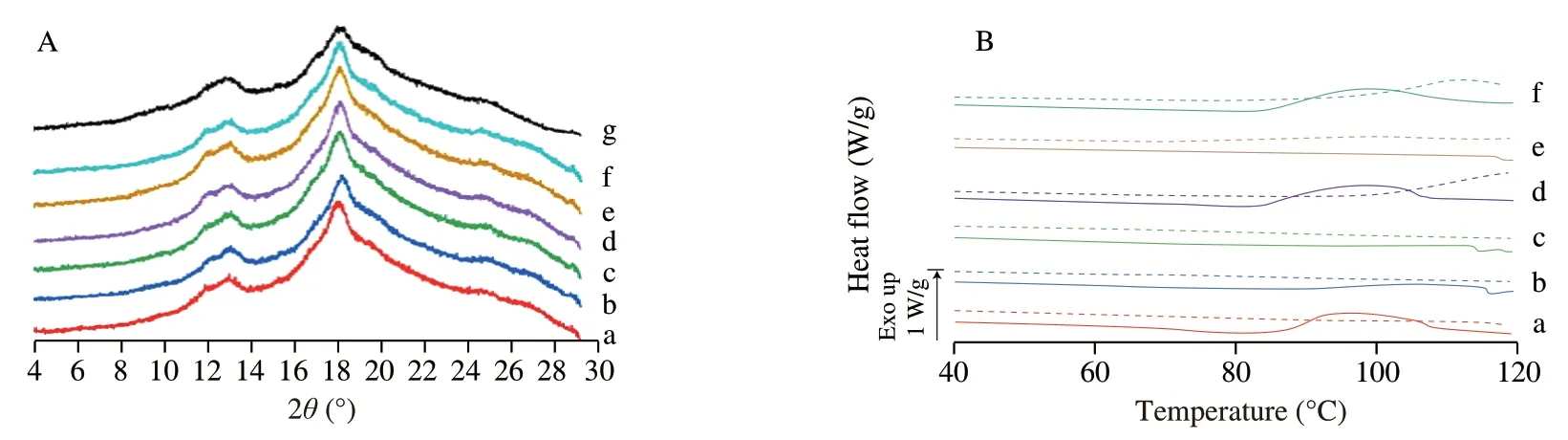
Fig.3 (A) XRD patterns and (B) DSC thermograms of V7-type high amylose maize starch (HAMS) inclusion complexes prepared with (a) DN,(b) HN,(c) AN,(d)DA,(e) CA,and (f) CT.The XRD pattern of (g) the raw V6h-type HAMS is shown in Fig.3A.
3.2.3 V8-type HAMS ICs
3.2.3.1 Crystalline structure
XRD patterns of the V8-type HAMS ICs are shown in Fig.4A.Compared with the “empty” V8-type HAMS sample,although the diffraction peaks of prepared ICs were not shifted,the peaks were less sharp and experienced significant lower crystallinity (Table 2).Such trend could be due to either the amount of aroma molecules that entrapped inside the V8helices,or the insufficient interactions between aroma compounds and the preformed “empty” V8-type HAMS that has a large central cavity,which agrees with the previous studies [25].However,the crystallinity trend did not follow the same pattern as those of the V6h-and V7-type ICs,which follows the order of CA >AN >CT >HN >DA >DN instead (Table 2).This phenomenon can be explained by the fact that the large cavity of the V8-type HAMS would provide more space and facilitate the encapsulation of cyclic aroma compounds and/or acyclic (or branched) aromatic molecules,including CA,AN,and CT.Correspondingly,these three types of molecules would present both within and between helical cavities,and perhaps within amorphous regions.While,the linear alkyl molecule,such as DN and HN,and lactonic molecule that has a relative long linear alkyl chain,DA,may not stably reside within the V8HAMS’central cavity.As a consequence,they may only present between helices and/or within amorphous regions,and thus lead to relatively lower crystallinity.
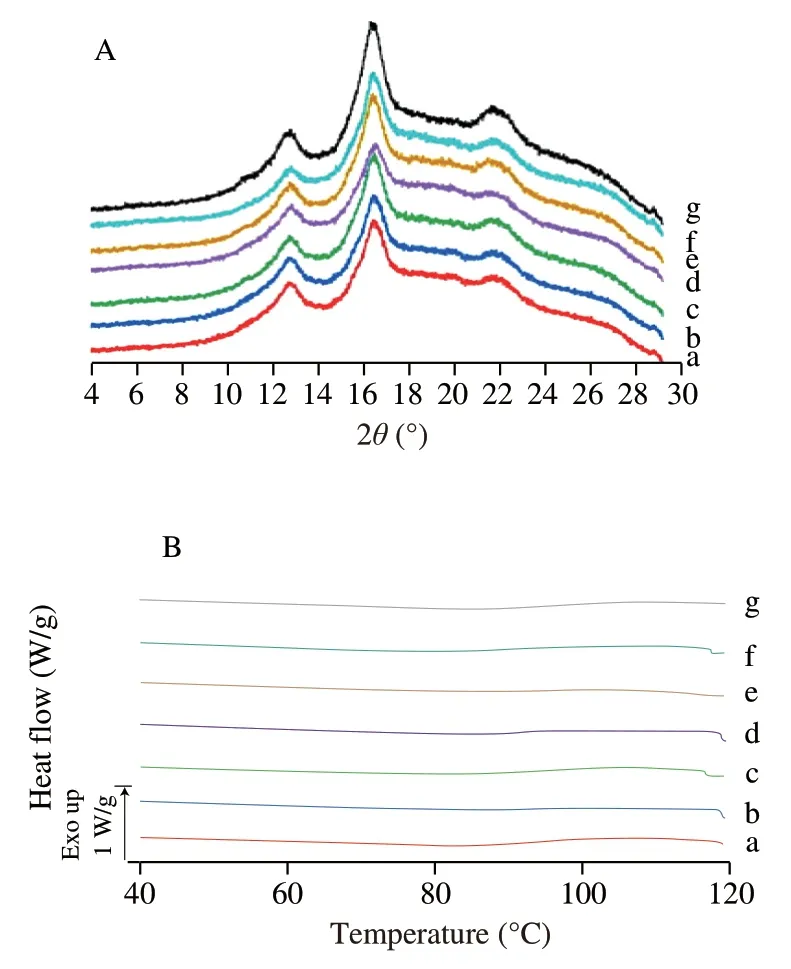
Fig.4 (A) XRD patterns and (B) DSC thermograms of V8-type high amylose maize starch (HAMS) inclusion complexes prepared with (a) DN,(b) HN,(c) AN,(d) DA,(e) CA,and (f) CT.The XRD pattern of (g) the raw V6h-type HAMS is shown in Fig.4A.
3.2.3.2 Thermal properties
Fig.4B and Table 3 show the melting thermograms and dissociation temperatures of the V8HAMS ICs that were evaluated by DSC.The thermogram (Fig.4B(g)) of the reference (V8HAMS)only showed a very broad phase transition at aTparound 86 °C,which could correspond to the residual HAMS-salicylic acid IC from the V8-type HAMS preparation process,particularly during the washing and extraction process [25].However,the thermal behaviors of prepared V8-aroma ICs were very similar to that of the reference and the analysis curves did not show any visible phase changes correspond to the melting of ICs (Fig.4B(a-f)).Although there were no structural changes to V8-type starch,assumptions such as,ICs were not successfully formed between V8HAMS and selected aroma compounds,still cannot be drawn,as ICs may possibly be formed without changing V8HAMS’ structure.Consequently,verification of the encapsulation process could be further examined by other techniques,for example,GC-MS,to quantify the amount of aroma compounds that present in the prepared V8IC samples.
4.Conclusion
To conclude,this study successfully prepared a series of preformed V-type starches that have different cavity sizes (V6h,V7,and V8) and demonstrated the capability of these V-subtype starches to molecularly encapsulate the selected different aroma compounds,including DN,HN,AN,DA,CA,CT,in ICs.Upon complexation with these aroma compounds,all V-subtypes retained their original crystalline structures.Although no crystalline structure changes were observed for each type of V-type ICs,different trends were observed for each type of the prepared ICs.For ICs prepared with V-type starches that have relatively smaller central cavities,the crystallinity of the IC samples followed the order of DN >DA >HN >CA >AN >CT,whereas the V8-type ICs had the trend of CA >AN >CT >HN >DA >DN.In addition,V6htype starch formed IC with aroma compounds more efficiently in comparison to V7-type and V8-type starches,as V6hHAMS was the only type of V-type starch that promoted the formation of Form II complex,that has higher crystallinity and higher thermal stability.This study elucidated that both types of V-type starch and the structure of aroma compound could affect the encapsulation procedure and starch-aroma IC formation.Starch-aroma IC could affect the properties of starch-containing foods and could also possibly control the release characteristics of aroma compounds under various circumstances in food formulations.Hence,future research is planned to study ICs’ release behaviors under various parameters,which could include different temperature,storage,and pH conditions.
Conflicts of interest
The authors confirm that they have no conflict of interest to declare for this publication.
Acknowledgments
This project is funded by the USDA National Institute of Food and Agriculture,Agriculture and Food Research Initiative Program,Competitive Grants Program award from the Improving Food Quality(A1361) program FY 2018 as grant # 2018-67017-27558.
- 食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Biological factors controlling starch digestibility in human digestive system
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
- Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil

