Effect of carboxymethyl konjac glucomannan coating on curcumin-loaded multilayered emulsion: stability evaluation
Luhui Wng,Xinru Zhng,Junxi Xio,*,Jiyi Shi
a College of Food Science and Engineering,Qingdao Agricultural University,Qingdao 266109,China
b College of Food Science and Engineering,Ocean University of China,Qingdao 266109,China
c College of Food Science and Engineering,Nanjing University of Finance&Economics,Nanjing 210023,China
Keywords:Curcumin Multilayered emulsion Layer-by-layer assembly Carboxymethyl konjac glucomannan Electrostatic interaction
ABSTRACT The stability against various environmental stresses of the curcumin-loaded secondary and tertiary emulsions that was emulsified by whey protein isolate (WPI) and coated by chitosan (CHI),carboxymethyl konjac glucomannan (CMKGM),or their combination through layer-by-layer assembly was investigated.Generally,the multilayered emulsions were destabilized in high NaCl concentrations or medium pH that could interrupt the electrostatic interaction between the three polyelectrolytes or deprotonate CHI,indicating that electrostatic interaction played an important role in the stability of emulsions.Compared with the primary emulsion that was solely stabilized by WPI,extra coating with CHI and CMKGM generally increased the stability of the emulsion against repeated freezing-thawing,improved the retention of curcumin against heating,UV irradiation,and long-term storage,and the effects were more remarkable in the tertiary emulsion with CMKGM locating in the outmost layer.Since CMKGM has shown the colon-targeted delivery potency,the multilayered emulsions assembled by layer-by-layer deposition,especially the tertiary emulsion,could be used as an effective carrier for the targeted delivery of curcumin.
1.Introduction
Nutraceuticals are bioactive compounds contained in foods at relatively low levels that may be benef icial to maintain human health and prevent certain chronic diseases.Curcumin is the primary active ingredient of the perennial herbCurcuma longa(turmeric),which has been traditionally used as a nutritional supplement and herbal medicine in many Asian countries for thousands of years [1].Recent studies have demonstrated that curcumin has a wide spectrum of antioxidant,anti-inflammatory,anticancer,antimicrobial,wound healing,and neurodegenerative disease prevention activities [2,3].However,the utilization of curcumin in functional foods is limited.On the one hand,curcumin has an extremely low solubility and dissolution rate in aqueous media due to strong inter-and intramolecular hydrogen bonding [4];on the other hand,curcumin is photosensitive and precautions must be taken during its handling to avoid degradation [5].
To effectively exert functions,a variety of emulsion-based delivery systems,including conventional emulsions,multilayered emulsions,and multiple emulsions,have been developed to encapsulate bioactive components,among which,the multilayered emulsions fabricated by layer-by-layer (LbL) deposition have been proved more stable against environmental stresses than conventional single-layered emulsions [6,7].The LbL assembly technique enables one to control the characteristics of coatings,such as composition,net charge,layer thickness,permeability,and environmental responsiveness [8,9]and the resultant emulsions can be engineered to have desirable repulsive electrostatic and steric forces to prevent droplet aggregation and protect labile compounds from environmental stresses [10].
The coating biopolymer greatly affects the stability,absorption rate,and release pattern of the payload in multilayered emulsions [11,12].Konjac glucomannan (KGM) is a neutral polysaccharide derived from the tubers ofAmorphophallus konjacK.Koch.It is a traditional food in China and Japan and has been widely used as a gelling agent,thickener,film former,and stabilizer in the food industry.Due to its high viscosity,biodegradability,gelation ability,and film-forming capability,KGM has also found applications in the pharmaceutical,biotechnological,and fine chemical industries [13].KGM cannot be hydrolyzed by the digestive enzymes in the upper gastrointestinal tract,but can be degraded by theβ-mannanase produced by colonic microflora.Because of this unique property,KGM is widely used as an integrating agent,coating,and skeleton material for colontargeted delivery formulations [14].However,KGM does not carry any charges,which limits its application in the fabrication of delivery systems through electrostatic interaction-based techniques,such as LbL assembly.
Carboxymethyl konjac glucomannan (CMKGM) is a negatively charged derivative of KGM and has improved water solubility,swelling rate,and stability compared with its native form [15,16].Our previous research revealed that CMKGM maintained similar resistance to proteolytic enzymes and susceptibility toβ-mannanase with the native KGM and could associate with oppositely charged polymers [17,18],making it a promising component of colontargeted delivery systems that are fabricated through electrostatic interaction.Curcumin has been incorporated to many colon-targeted delivery systems to treat colon cancer and inflammatory bowel diseases [19].KGM has already been incorporated into the curcumin emulsion emulsified by whey protein isolate (WPI),which conferred a controlled and sustainable payload release from the emulsion [20].However,the utilization of CMKGM as a coating to fabricate multilayered curcumin emulsions has not been concerned yet.
In preliminary works,we have successfully produced curcuminloaded multilayered secondary and tertiary emulsions with CMKGM located in the outmost layer.Since the stability of emulsions against environmental stresses is of great significance for their practical applications,the aim of this study was to evaluate the effects of medium pH,ionic concentration,heating,freezing-thawing,and ultraviolet (UV) irradiation on the stability of the emulsions and/or the loaded curcumin.The long-term storage stability of the emulsions was evaluated as well.This work could provide useful information for the practical application of CMKGM-coated curcumin multilayered emulsions in the food industry.
2.Materials and methods
2.1 Materials
Curcumin with 98% purity was purchased from Beijing Solarbio Science&Technology Co.,Ltd.(Beijing,China).Medium chain triglyceride (MCT) was obtained from Shanghai Leaping Industrial Co.,Ltd.(Shanghai,China).WPI was purchased from Nanjing SenBeiJia Biotechnology Co.,Ltd.(Nanjing,China).Chitosan(CHI) with viscosity average molecular weight 150 kDa and 93.9% degree of deacetylation was bought from Shandong Lake Crustacean Products Co.,Ltd.(Qingdao,China).CMKGM with degree of substitution 0.55 was synthesized in the same procedure as described in a previous literature [17].All other reagents were of analytical or higher grade.Deionized water was used throughout the study.
2.2 Preparation of multilayered curcumin emulsions
In this work,curcumin was used as the payload,WPI was used as the emulsifier,CHI was used as the positively charged coating,and CMKGM was used as the negatively charged coating.The biopolymers were added in different sequences to form primary,secondary,and tertiary emulsions in the conditions optimized in preliminary works,which were stable and homogenous without aggregation or flocculation.
2.2.1 Solution preparation
An approximately 0.1 g curcumin was dispersed in 10 mL MCT oil and the suspension was stirred overnight to get the homogeneous 1 g/100 mL curcumin solution.The 0.2 g/100 mL CHI solution was prepared by dissolving the solute in 1% (V/V) acetic acid.The 1.0 g/100 mL WPI and 0.4 g/100 mL CMKGM solutions were prepared by dissolving the solutes in deionized water.All the solutions were stored at 4 °C until use.
2.2.2 Preparation of the primary emulsion
The 1.0 g/100 mL WPI solution was adjusted to pH 7.0 and then blended with the 1 g/100 mL curcumin solution in volume ratio 9:1.The mixture was dispersed with an Ultra-Turrax T25 high-speed homogenizer (IKA Labotechnik,Staufen,Germany) at 11 000 r/min for 3 min to generate the primary solution.
2.2.3 Preparation of the secondary emulsion-CMKGM
The 0.4 g/100 mL CMKGM solution was mixed with the primary emulsion in equal volume ratio,which was then adjusted to pH 4.0 and magnetically stirred at 300 r/min and room temperature for 30 min to allow the adsorption of CMKGM onto the primary emulsion surface.The resultant secondary emulsion with CMKGM locating in the outmost layer was designated as the secondary emulsion-CMKGM.
2.2.4 Preparation of the secondary emulsion-CHI
The primary emulsion was mixed with the 0.2 g/100 mL CHI solution in equal volume ratio.Then,the mixture was adjusted to pH 5.0 and magnetically stirred at 300 r/min and room temperature for 30 min to allow the adsorption of CHI onto the primary emulsion surface.The resultant secondary emulsion with CHI locating in the outmost layer was designated as the secondary emulsion-CHI.
2.2.5 Preparation of the tertiary emulsion
The 0.1 g/100 mL CMKGM solution was mixed with the secondary emulsion-CHI in equal volume ratio,which was then magnetically stirred at 300 r/min and room temperature for 30 min to allow the adsorption of CMKGM onto the surface of the secondary emulsion to generate the tertiary emulsion.
2.3 Emulsion characterization
2.3.1 Particle size and ζ potential measurement
The particle size andζpotential were determined by dynamic light scattering using a Nano ZS90 Zetasizer (Malvern Instruments Ltd.,Worcestershire,UK).Prior to analysis,the emulsions were diluted with ultrapure water in a volume ratio 1:1 000 to avoid multiple scattering effects and then measured at 25 °C in triplicate.
2.3.2 Stability index
Fresh emulsions were poured into plastic centrifuge tubes at room temperature.The heights of emulsified phase (He) after specific treatment and heights of total formulation (Ht) of the original emulsion were recorded.The stability index was calculated according to the following equation:
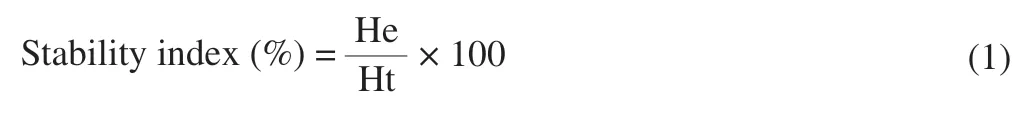
2.3.3 Curcumin content analysis
The content of curcumin in the emulsions was determined by a UV/VIS-spectrophotometer (Unocal,UV 2000,Japan).To extract curcumin,the emulsion of 1 mL was mixed with 4 mL of ethyl acetate,vortexed thoroughly,and centrifuged at 4 000 r/min for 10 min.The organic phase was collected,vacuum evaporated to remove ethyl acetate and the residual curcumin was then dissolved in ethanol and subjected to absorbance measurement at 425 nm.The curcumin content was calculated by comparing with the standard curve plotted based on the absorbance of a series of standard curcumin solutions in ethanol at 425 nm.The retention of curcumin in emulsions was obtained according to the following equation:

2.4 Emulsion stability evaluation
2.4.1 Against pH variation
The emulsions were adjusted to pH 3.0-8.0 by adding HCl or NaOH solution,which were then immediately subjected to particle size andζpotential measurement.
2.4.2 Against ionic strength
The emulsions were added with NaCl powder to generate NaCl concentrations ranging from 0 to 250 mmol/L and stirred until NaCl completely dissolved.Then,the emulsions were subjected to particle size andζpotential measurement.
2.4.3 Against heating
The emulsions were incubated in 30,60,and 90 °C water bath for 30 min and then immediately taken out and cooled to room temperature.Theζpotential,particle size,and stability index of the emulsions were immediately measured and the retention of curcumin was determined as well.
2.4.4 Against freezing-thawing
The emulsions were frozen in a -18 °C freezer for 22 h and then thawed by placing them in a 30 °C water bath for 2 h.This process was repeated once and the resultant emulsions in each cycle were immediately subjected to particle size,ζpotential,and curcumin retention measurement.
2.4.5 Against UV irradiation
A UV lamp was applied to mimic the UV rays of sunlight.The emulsions were transferred to transparent glass vials and exposed to UV illumination at room temperature in a dark box for 72 h.The distance between the bottom of the vials and the UV lamp was 10 cm with the lamp power set to 20 W.In the 3rd,10th,16th,24th,36th,48th,and 72ndh of the period,a small portion of the emulsions was sampled for particle size,ζpotential,and curcumin retention measurement.
2.4.6 Against storage
The emulsions were placed in transparent glass bottles and stored at room temperature for 28 days.In the 1st,3rd,5th,7th,9th,11th,14th,21st,and 28thdays of the period,a small portion of the emulsions was taken out and subjected to particle size,ζpotential,stability index,and curcumin retention measurement.
2.5 Statistical analysis
All the experiments were performed in triplicate and values were expressed as means ± SD.Differences between mean values were compared using the one-way analysis of variance (ANOVA),followed by the post hoc Tukey’s test with SPSS 16.0 and were statistically significant atP<0.05.
3.Results and discussion
3.1 Effect of medium pH on emulsion stability
The medium pH determines the charging status of biopolymers and could affect the stability of multilayered emulsions that are fabricated through electrostatic interaction.Hence,the effects of medium pH value on the stability of the emulsions were investigated first in this work.
As shown in Fig.1a,the primary emulsion showed the least particle size of 133 nm and 142 nm respectively in pH 7.0 and 8.0 and the largest particle size of 755 nm in pH 5.0.This could be related to the charging status of the emulsion.ζpotential is a measure of the effective electric charge on emulsion surface.It is closely related to the pH of the surrounding environment and the composition of the surface layer and greatly affects the stability of emulsions,that is,a higherζpotential confers greater repulsion between charged emulsion droplets and consequently less aggregation.According to Fig.1b,the primary emulsion was positively charged in pH 3.0 and 4.0 withζpotential 23 mV and 7 mV respectively,but became negative in pH 5.0 (-11 mV) and higher.This result was consistent with the fact that the isoelectric point (pI) of WPI was between pH 4.0 and 5.0 [21]and confirmed that WPI successfully adsorbed to the emulsion interface.At its pI,WPI carried the least charge and consequently conferred the weakest electrostatic repulsion,which caused emulsion aggregation;as the medium pH value deviated from the pI of WPI,the electrostatic repulsion between emulsion droplets increased and the particle size declined consequently.
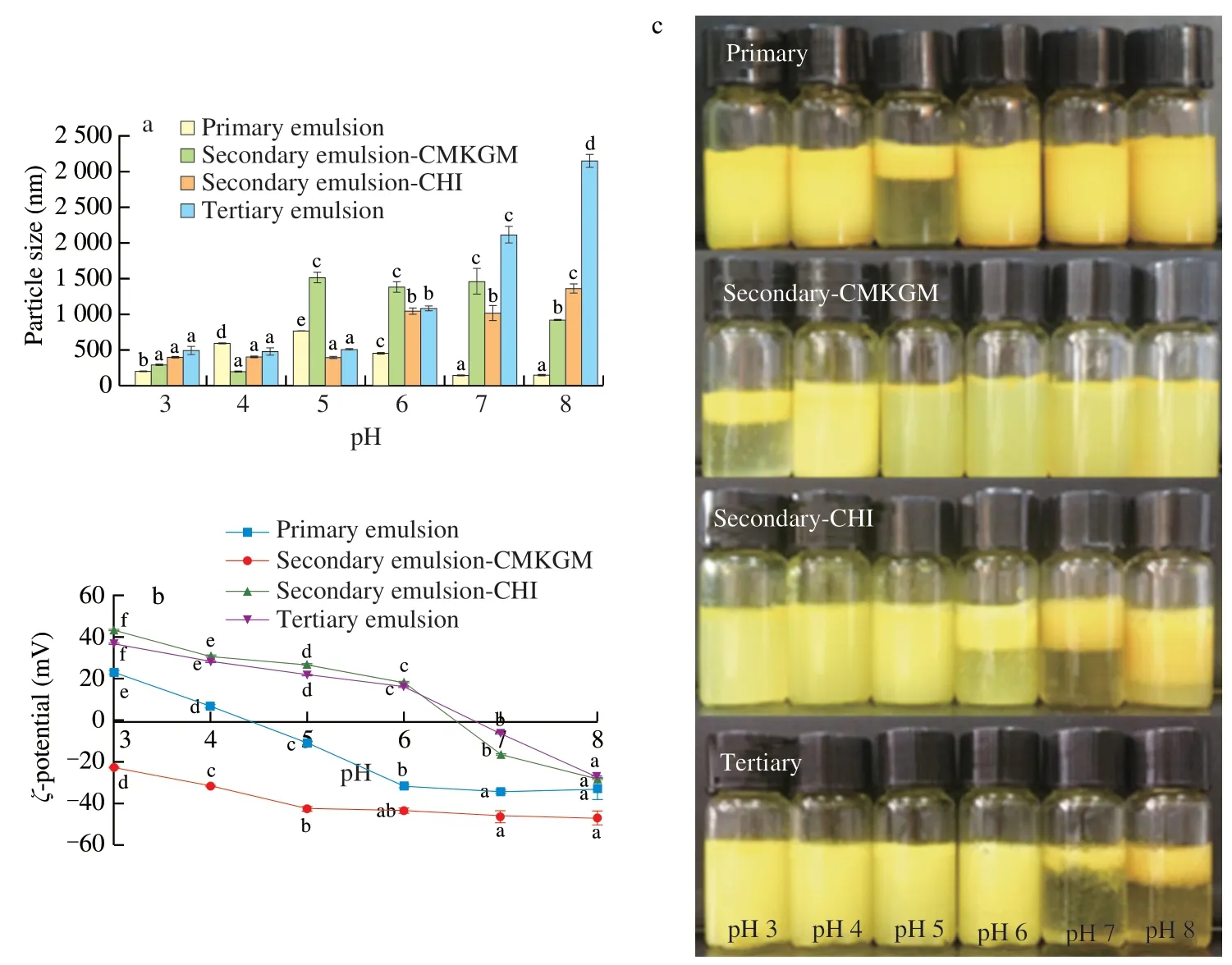
Fig.1 Effect of medium pH on the particle size (a),ζ potential (b),and appearance (c) of the curcumin-loaded multilayered emulsions.Comparisons were performed between values of the same series and values without same letter(s) were significantly different at P <0.05.
The secondary emulsion-CMKGM showed the least particle size of 191 nm in pH 4.0,in which CMKGM and WPI were oppositely charged;as the pH value increased to 5.0,the particle size increased sharply to 1 507 nm and then remained nearly constant in higher pH,but showed no significant change in pH 3.0.By checking theζpotential illustrated in Fig.1b,it might be speculated that CMKGM associate loosely with the WPI-stabilized primary emulsion and its coating could not completely prevent the contact of the inner primary emulsion with the surrounding medium;in this case,WPI became negatively charged in pH higher than its pI and the associated CMGKM detached from the primary emulsion as a result of the electrostatic expulsion between the two polyanions,thus leading to aggregation of the emulsion droplets.Fig.1c demonstrated that obvious emulsion layer could still be identified in pH 3.0,and it was unstable in other pH except pH 4.0.Hence,the secondary emulsion-CMKGM also showed poor stability against medium pH variation.
Regarding the secondary emulsions -CHI,its particle size was not affected in pH 5.0 (390 nm) and lower (395 nm);nevertheless,as the medium pH increased to 6.0 and higher,the particle size increased significantly to around 1 000 nm and the greatest particle size occurred in pH 8.0,reaching 1 357 nm.It could be seen from Fig.1b that the emulsion was positively charged in pH 6.0 (18 mV)and lower,implying that CHI successfully attached to the surface of the primary emulsion droplets;as the pH increased to 7.0 and 8.0,the emulsions became negatively charged withζpotential of -17 mV and-28 mV respectively,indicating that CHI detached from the primary emulsion and WPI got exposed.It might be speculated that CHI attached loosely to the primary emulsion and precipitated in neutral and alkaline media,leading to its detachment from the emulsion droplet surface.The partial desorption of CHI led to bridging flocculation and increased particle size of the emulsions.As can be seen in Fig.1c,the emulsions were still homogenous in pH 3.0-5.0;as the medium pH value increased to 6.0,serious phase separation occurred;after the pH value further increased to 7.0,both phase separation and oiling off could be identified;as the pH value further increased to 8.0,mild phase separation occurred.A similar work also reported that the carotenoid emulsion stabilized by soybean protein isolate and coated by CHI exhibited good stability in pH 3.0-6.0 but suffered serious aggregations in higher pH [22].
For the tertiary emulsion,its particle size was larger than that of the secondary emulsion -CHI in the entire pH range investigated(480-3 143 nm) and this could be ascribed to the successful adsorption of CMKGM onto the secondary emulsion.The particle size of the tertiary emulsion was not significantly different in pH 3.0 (485 nm),4.0 (477 nm),and 5.0 (497 nm),but increased greatly in higher pH and the largest size of 3 143.2 nm was seen in pH 8.0(Fig.1a).As shown in Fig.1b,the tertiary emulsion was positively charged in pH 6.0 (17 mV) and lower.This was because that the secondary emulsion-CHI was only partially covered by CMKGM;as the medium pH value increased to 7.0 and 8.0,the exposed CHI got unprotonated and the negative charge of CMKGM dominated as a result.Fig.1c revealed that the tertiary solution was stable and homogeneous in pH 3.0-6.0,but suffered serious aggregation in pH 7.0 and 8.0.Hence,Fig.1 revealed that the tertiary emulsion had better stability in acidic media than the other two secondary emulsions and electrostatic interaction played an important role in the stability of all multilayered emulsions.
3.2 Effect of ionic strength on emulsion stability
During food processing,NaCl or other salts are usually added for various purposes.Since ions could shield the electrostatic interaction between biopolymers,the stability of the emulsions against various ionic strengths deserves special attention.As showed in Fig.2a,the particle sizes of all of the emulsions generally increased with NaCl concentration rise and that of the primary,secondary-CMKGM,secondary-CHI,and tertiary emulsions increased by 8.6,3.9,2.1,and 1.3 times in the presence of 250 mmol/L NaCl compared with corresponding values in the absence of NaCl,reaching 265,981,1 439,and 1 642 nm,respectively.The same trend has also been reported in a previous work that NaCl increased the particle size of tuna oil emulsified by Tween-80 and/or coated by CHI [23].
In contrast,theζpotential of the multilayered emulsions decreased only slightly with NaCl concentration rise (Fig.2b).A similar variation was also seen in the curcumin emulsion stabilized by WPI and coated by CHI [24].Such a variation implied that,in contrast to medium pH variation,the presence of NaCl possibly did not cause serious detachment of the outmost coating from the emulsion droplets and the increase of the particle size in the presence of NaCl might be caused by the weakened electrostatic repulsion between the emulsion droplets contributed by the shielding effect of NaCl.
As can be seen in Fig.2c,the presence of NaCl greatly affected the appearance of the multilayered emulsions.The two secondary emulsions suffered serious phase separation in higher NaCl concentrations,but the tertiary emulsion was more stable without obvious phase separation.No oiling off was seen in all the multilayered emulsions.It is interesting that the primary emulsion showed no obvious changes in appearance and was consistent with its slight particle size variation revealed in Fig.2a,which highlighted that electrostatic interaction played an important role in the stability of the multilayered emulsions.
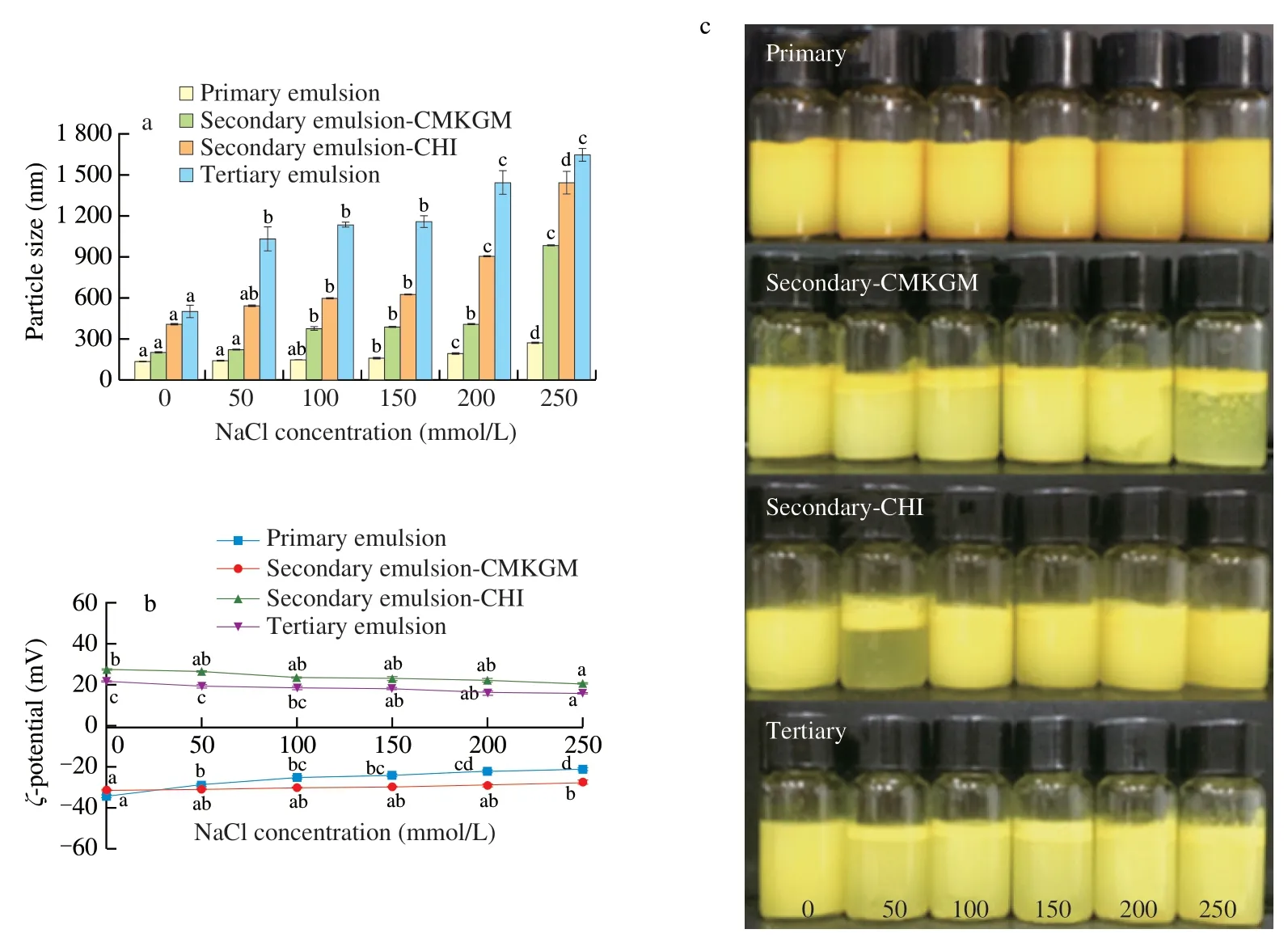
Fig.2 Effect of NaCl concentration on the particle size (a),ζ potential (b),and appearance (c) of the curcumin-loaded multilayered emulsions.Comparisons were performed between values of the same series and values without same letter(s) were significantly different at P <0.05.
3.3 Effect of heating on emulsion stability
The emulsions were incubated in 30,60,and 90 °C water bath for 30 min and then cooled to room temperature to reveal their thermal stability.As can be seen in Fig.3b,there was no significant changes in theζpotential of all the emulsions,indicating that no detachment of the outmost coating occurred to the multilayered emulsions.A same result has also been observed in the tuna oil emulsion that was stabilized by lecithin and coated by CHI [25].
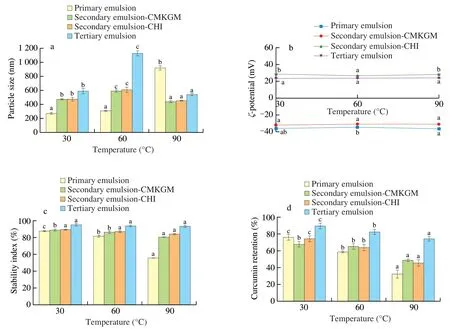
Fig.3 Effect of heating on the particle size (a),ζ potential (b),stability index (c),and curcumin retention (d) of the curcumin-loaded multilayered emulsions.Comparisons were performed between values of the same series and values without same letter(s) were significantly different at P <0.05.
Regarding the primary emulsion,its particle size was not affected when heated at 30 or 60 °C and maintained in around 280 nm;however,as the temperature further increased to 90 °C,the particle size increased greatly to 920 nm (Fig.3a).This result was consistent with a similar work that boiling significantly increased the size of curcumin emulsion droplets stabilized by WPI due to the denaturation of WPI and consequently the aggregation of the oil droplets [26].The stability of the emulsion at 30 and 60 °C might be related to the denaturation temperature of WPI at around 78 °C [27],which meant that the former two temperatures were incapable of inducing WPI aggregation and the emulsion size was not affected as a result.
The particle size of the multilayered emulsions displayed different sensitivity to heating.As the temperature increased from 30 to 60 °C,all the three multilayered emulsions exhibited significant particle size increase and the tertiary emulsion suffered the greatest increase from 476 nm to 608 nm;however,as the temperature further elevated to 90 °C,their particle sizes decreased sharply to 451 nm,which was comparable with that at 30 °C (Fig.3a).A similar trend was also seen in the curcumin-loaded secondary emulsion that was emulsified by WPI and coated by medium-or high-methoxyl CHI,which exhibited increased particle size upon heating at 63 °C for 30 min,but displayed significantly decreased size after heating at 100 °C for 10 min [24].This could be related to the temperaturedependent solubility of MCT.High temperatures increased its water solubility and hence extracted it from the emulsions.After returning to room temperature,the extracted MCT could form nanoparticles with the biopolymers,thus reducing the mean size.
As can be seen in Fig.3c,the primary emulsion was unstable against heating at 90 °C as evidenced by the significantly decreased stability index,which was consistent with the results in Fig.3a;while for the multilayered emulsions,though they underwent serious particle size variation as revealed in Fig.3a,they exhibited good stability and no marked decrease in stability index was observed upon heating,indicating good thermal stability of the emulsions.
Since curcumin is sensitive to heating,its retention was measured as well.As can be seen in Fig.3d,the curcumin retention of all the emulsions decreased significantly upon heating,but extra coating with CMKGM and/or CHI could provide effective protection.The tertiary emulsion conferred the highest curcumin retention in all the three temperatures,and both the secondary emulsions provided comparable protection at 60 and 90 °C.After incubation at 90 °C for 30 min,the retention of curcumin in the primary,secondary-CMKGM,secondary-CHI,and tertiary emulsions were 32.3%,48.8%,45.4%,and 74.3% respectively,implying that multilayered emulsion could effectively improve the thermal stability of curcumin and the effect was positively related to the number of layers.In a previous study,it was found that the addition of KGM to the curcumin emulsion stabilized by WPI increased the retention of curcumin upon heating at 85 °C for 90 min [20].Hence,the current work revealed that the carboxymethyl modification and electrostatic interaction did not impair the thermal protection ability of KGM.
3.4 Effect of freezing-thawing on emulsion stability
The emulsions were subjected to two freezing-thawing cycles consisting of freezing at -18 °C for 22 h followed by thawing at 30 °C for 2 h.Freezing-thawing significantly increased the particle size of all the emulsions (Fig.4a),but showed no effect on theirζpotentials(Fig.4b),implying that though emulsion aggregation occurred,no detachment or dissociation of biopolymers occurred.There were a 5.5,3.5,2.9,and 1.8 fold increase in particle size after the first cycle in the primary,secondary-CMKGM,secondary-CHI,and tertiary emulsions respectively compared with their original values;after the second freezing-thawing cycle,the particle size of all the emulsions increased except the secondary emulsion-CHI,which showed slight decrease,but all the multilayered emulsions exhibited smaller particle sizes than the primary emulsion,indicating that extra coating increased the freezing-thawing stability of the curcumin-loaded emulsions.The appearances of the emulsions after two freezing-thawing cycles were demonstrated in Fig.4d.As can be seen,serious oiling off was observed in the primary emulsion after the second freeze-thaw cycle,but was not seen in the secondary and tertiary emulsions,further confirming that extra coating improved the freeze-thaw stability of the emulsions.A similar result has been in a previous work that the primary tuna oil stabilized by lecithin suffered great particle size increase and oiling off after two freeze-thaw cycles,but oiling off disappeared after coating by CHI [25].
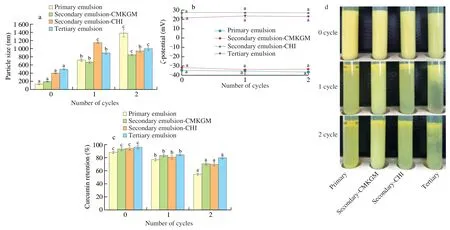
Fig.4 Effect of freeze-thaw cycles on the particle size (a),ζ potential (b),curcumin retention (c),and appearance (d) of the curcumin-loaded multilayered emulsions.Comparisons were performed between values of the same series and values without same letter(s) were significantly different at P <0.05.
The aggregation and even oiling off of emulsions upon freezingthawing could be related to the crystallization of either water in the aqueous phase or the payload in the oil phase,which could disrupt the interfacial membrane of emulsion droplets and make them more prone to coalescence and oiling off [28].Fig.4a demonstrated that coating increased the particle size of the curcumin-loaded emulsions.Since the same primary emulsion was used for multilayered emulsion preparation,the increased particle size could be ascribed to the increased thickness of the interfacial membrane,which provided extra protection against the injury of the crystals generated during freezing.The improved freezing-thawing stability upon coating by polysaccharides had been found in another similar work [9].
It can be seen from Fig.4c that the curcumin retention of the multilayered emulsions decreased with the increase of freezingthawing times.After two freezing-thawing cycles,the curcumin retention was the lowest (48.6%) in the primary emulsions and the highest (73.9%) in the tertiary emulsion.These results indicated that curcumin could be lost during freezing and thawing,but extra coating with CMKGM and/or CHI could provide effective protection.Biopolymers probably improved the freezing-thawing stability of the multilayered emulsions by increasing the osmolyte concentration in the aqueous phase,thereby reducing its crystallization temperature and minimizing the amount of ice crystals formed,which reduced the injury to emulsion membrane.This phenomenon would have increased the volume fraction of unfrozen aqueous phase available to the oil droplets,thereby causing less droplet-droplet interactions [25].
3.5 Effect of UV irradiation on emulsion stability
UV irradiation is a simple and effective sterilization method during food and beverage processing.Because curcumin is sensitive to UV irradiation,the stability of the emulsions against exposure to UV was examined.The particle size of the primary,secondary-CMKGM,secondary-CHI,and tertiary emulsions generally increased with exposure time elongation and reached 1 729.4,1 276.2,1 415.3,and 1 496.4 nm after UV irradiation for 72 h,which were 14.6,6.2,3.6,and 3.1 folds of the corresponding fresh emulsions respectively(Fig.5a);meanwhile,theζpotentials of the multilayered emulsions decreased significantly after UV treatment (Fig.5b).Similar variations in particle size andζpotential have also been reported in the UV-radiated curcumin emulsions stabilized by the lecithin-Tween 80 mixture and coated by CHI [24].A previous research has found that both proteins and CHI could be degraded by UV rays,which could alter their functional groups and charge distribution [29].UV irradiation produces radicals in the solution,which may oxidize or react with the charged groups,thus ‘neutralizing’ the charge located on the surface of emulsion droplets and leading to reduced net charge.The reduced charge weakened the electrostatic repulsion between emulsion droplets and caused aggregation.

Fig.5 Effect of UV irradiation on the particle size (a),ζ potential (b),and curcumin retention (c) of the curcumin-loaded multilayered emulsions.Comparisons were performed between values of the same series and values without same letter(s) were significantly different at P <0.05.
Fig.5c illustrated that the curcumin content in all the emulsions decreased significantly as UV irradiation proceeded and the highest loss occurred in the primary emulsion,in which only 8.9% curcumin was retained after exposure for 72 h.Extra coating greatly improved the retention of curcumin and the highest value of 57.9% was seen in the tertiary emulsion,followed by 50.2% of the secondary emulsion-CMKGM and 47.9% of secondary emulsion-CHI in sequence,indicating that the multilayered structure could effectively protect curcumin from photochemical oxidation induced by UV irradiation.The same increased stability of curcumin against UV radiation has also been observed in the secondary emulsion that was emulsified by Tween 80-lecithin mixture and coated by CHI [24]and the secondary emulsion that was emulsified by kafirin and coated by carboxymethyl CHI [30].
3.6 Storage stability
The multilayered emulsions were stored for 28 days at 25 °C and the changes in particle size,ζpotential,stability index,and curcumin retention were measured periodically to evaluate their storage stability.As can be seen in Fig.6a,all the emulsions showed increased particle size with storage proceeding and the trend was more obvious in the primary emulsion.At the end of the storage period,the particle sizes of the primary,secondary-CMKGM,secondary-CHI,and tertiary emulsions reached 387,282,442,and 606 nm respectively.Theζpotential measurement agreed with the particle size measurement well (Fig.6b),that is,theζpotential of the multilayered emulsions remained nearly constant,which provided sufficient repulsive force between the emulsion droplets;while that of the primary emulsion kept decreasing from -34 mV to -8 mV,which induced aggregation that was responsible for the increased size.This result was consistence with a similar work that the multilayered emulsion emulsified byβ-lactoglobulin and coated by citrus pectin was more stable than that solely stabilized byβ-lactoglobulin [31].
The stability indexes of all emulsions decreased with storage proceeding (Fig.6c).After storage for 28 days,the stability index of primary,secondary-CMKGM,secondary-CHI,and tertiary emulsions decreased to 70.1%,84.7%,80.1%,and 86.2% respectively.Fig.6d demonstrated the percentages of curcumin retained in the emulsions.Generally,the content of curcumin in the emulsions decreased with storage time elongation.At the end of 28 d,up to 34.9% curcumin was lost in the free form,while that of the primary,secondary-CMKGM,secondary-CHI,and tertiary emulsions were 25.9%,20.9%,22.0%,and 16.5% respectively.Hence,coating with polysaccharides could greatly improve the storage stability of curcumin and the effect was more obvious in the case of more layers.
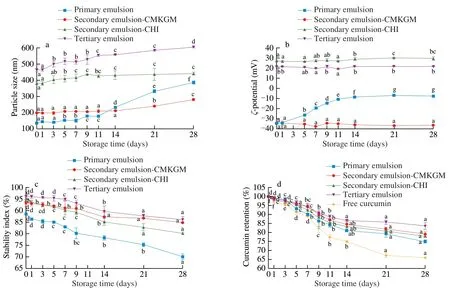
Fig.6 Effect of storage periods on particle size (a),ζ potential (b),stability index (c) and curcumin retention (d) of the curcumin-loaded multilayered emulsions.Comparisons were performed between values of the same series and values without same letter(s) were significantly different at P <0.05.
4.Conclusions
The electrostatic interaction between WPI,CHI,and CMKGM greatly influenced the stability of the curcumin-loaded multilayered emulsions fabricated by LbL assembly.The presence of NaCl or medium acidities that conferred same charges to the involved polyelectrolytes or deprotonated CHI could destabilize the multilayered emulsions.Compared with the starter primary emulsion emulsified by WPI,coating by CMKGM or CHI improved the retention of curcumin in the emulsion upon heating,UV radiation,freezing-thawing,or long-term storage and the improvement was notably significant in the case of the former two stresses due to the barrier effect of the emulsion membranes,but extra coating the secondary emulsion-CHI with CMKGM further enhanced the protection.Hence,the multilayered emulsions could find applications in formulations that might undergo an acidic environment,heat sterilization,or cold storage and their delivery potency should be further evaluated by cellular andin vivoanimal studies to validate their potency as a carrier for curcumin.
Conflict of interest
The authors declared no conflicts of interest to this work.
Acknowledgment
We thank the financial support from the Natural Science Foundation of Shandong Province (ZR2015CM037) and the National Science Foundation of China (31571890).
- 食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Biological factors controlling starch digestibility in human digestive system
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
- Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil

