Effect of cooked rice with added fructo-oligosaccharide on faecal microorganisms investigated by in vitro digestion and fermentation
Fei Pei,Wen Li,Xiolei Ni,Xinyng Sun,b,Yijun Yo,Yong Fng,Wenjin Yng,Qiuhui Hu
a College of Food Science and Engineering/Collaborative Innovation Center for Modern Grain Circulation and Safety/Key Laboratory of Grains and Oils Quality Control and Processing,Nanjing University of Finance and Economics,Nanjing 210023,China
b Department of Food and Human Nutritionl Sciences,University of Manitoba,Winnipeg MB R3T 2N2,Canada
Keywords:Fructo-oligosaccharide In vitro Fermentation Rice Short-chain fatty acids Bacterial phase distribution
ABSTRACT In the present study,the effects of cooked rice (CR) with added fructo-oligosaccharide (FOS) on faecal flora were studied by a simulated in vitro digestion and fermentation method.The total carbohydrate content,pH,and s hort-chain fatty acids (SCFAs) were determined during in vitro digestion and fermentation.The change in the bacterial phase distribution after the fermentation was also analysed.The results showed that t he total carbohydrate content of the CR with added FOS (FCR) significantly decreased during the simulated digestion.Meanwhile,the pH of the FCR decreased and the SCFAs concentration increased significantly compared to those of the CR during the simulated fermentation.In addition,the FCR showed the advantage of promoting beneficial bacteria,such as Bifidobacterium and Lactobacillus,and inhibiting harmful bacteria,such as Bacteroides and Klebsiella compared to the CR.Therefore,the FOS as a prebiotic could be recommended to produce the high-quality healthy rice food.
1.Introduction
Ri ce is a common food and extensively grown worldwide [1].Rice is comprised of more than 90% starch,and its main constituent is starchy endosperm,which is rich in carbohydrates.However,rice rich in carbohydrates typically has a high glycaemic index (GI),i.e.,there is rapid release of glucose during digestion in the small intestine [2].Ingestion of this type of food periodically is related to type II diabetes and cardiovascular diseases [3],and there is increasing evidence that these health issues are related to the composition and richness of the gut microbiota [4].Therefore,to reduce the risk of disease,it is necessary to improve the cooking mode of rice to improve the ability to regulate the gut microbiota.
The intake of cooked rice (CR) has different effects on the gut microbiota under different conditions,such as rice varieties,growing environment,and processing methods.Some studies have shown that eating unpolished rice with high dietary fibre content can increase the total short-chain fatty acid content and beneficial bacteria in the gut [5].However,be cause of the poor taste,nutritional resistance,and low safety of unpolished rice,it cannot completely replace traditional ref ined staples [6].Concurrently,some physical treatments,such as ultra-high pressure [7],ultrasonic [8]and autoclaving treatments [9],or chemical pre-t reatment,including water gel addition [10],have been applied to rice cooking to improve taste and quality.However,these methods are high cost,complex processes,unsafe,lack functionality in gut microbiota regulation,and even adversely affect the taste [11].Therefore,it is highly significant to develop safe,probiotic,quality improvements and processes for rice processing methods.
In recent years,some functional sweeteners have been reported to improve the anti-retrogradation and edible quality of starch products.Fructo-oligosaccharide (FOS) is considered as prebiotics,which is currently defined as a substrate that is selectively utilized by host microorganisms.They confer health benefits,e.g.by the stimulation ofBifidobacteriaand the eventual formation of butyrate by cross-feeding mechanisms [12].FOS does not affect the original taste of food and is poorly digested in the gastrointestinal tract;however,they can selectively stimulate the growth and sustain the colonization of probiotic bacteria,particularlyBifidobacteriaandLactobacilli[13].Currently,FOS is extensively used in improving the retrogradation resistance of starch products,reducing the GI,and improving the edible quality.Park et al.[14]found that the addition of FOS to frozen dough improved the baking quality of frozen dough,increased the moisture content of bread,and decreased the hardness of bread crumbs.Angioloni et al.[15]showed that the addition of carboxymethylcellulose and FOS could improve the sensory of bread,reduce the hydrolysis of starch,and decrease the expected GI.Zeng et al.[16]showed that adding FOS could increase the water solubility of starch and interfere with the retrogradation of wheat starch during storage.In our previous study,it was proved that addition of FOS to rice can increase the resistant starch content,reduce itsin vitrodigestibility hydrolysis rate,and improve the ability of antiretrogradation and the edible quality.However,there is still a lack of research on the probiotic effect and regulation of gut microbiota duringin vitrofermentation of cooked rice with added FOS.
The objective of this research is to study the changes in the intestinal microorganisms in rice with the addition of the FOS after simulatedin vitrodigestion and fermentation method.Using anin vitrofermentation system,the digestion and fermentation behaviours,short-chain fatty acids (SCFAs) concentration,and pH were evaluated.The microbiota composition was analysed using a sequencing platform;specifically,the 16S DNA V4 fragment of the microbial DNA isolated from the fermentation broth was sequenced.
2.Materials and methods
2.1 Sample preparation
Rice variety,Kongyu 131,was obtained from the COFCO Nutrition and Health Research Institute,Beijing,China.The FOS and glucose were purchased from Yuanye Bio-Technology Co.Ltd.,Shanghai,China.The rice was soaked in 1.6% FOS solution and 1.6% Glu solution,respectively,with a water to rice ratio of 1:1.4.The soaked rice was steamed with the soaked liquid in a steamer for approximately 35 min,following which the cooked rice was removed and mashed for subsequent use.
2.2 In vitro digestion
According to the methods of Zhao et al.[17]and Ayimbila et al.[18],the process ofin vitrodigestion was divided into three stages: oral(0–1 h),stomach (1–3 h),small intestine (3–6 h).Approximately 12 g of the sample was taken in a triangular flask,to which 150 mL of artificial saliva (2.38 g of Na2HPO4,0.19 g of KH2PO4,8 g of NaCl,and 1 L of ultra-pure water) was added,and the pH was adjusted to 7.Alpha-amylase was added to achieve 200 U enzyme activity.Following this,5 mL of artificial gastric juice (0.1 mol/L hydrochloric acid,containing 0.54 g of pepsin) was added,and the pH was adjusted to 2.The system was cultured in a 37 °C constanttemperature water bath oscillator at 200 r/min for 2 h to simulate gastric digestion.The pH of the enzymatic hydrolysate was adjusted to 6.8 using 6 mol/L NaOH,following which 25 mL of artificial intestinal fluid (0.11 g pancreatin and 0.7 g pig bile salt dissolved in 25 mL of 0.5 mol/L NaHCO3) was added.Culturing was performed in a 37 °C constant-temperature water bath oscillator at 200 r/min for 3 h.Following mixing,the mixture was transferred into dialysis bags with a molecular weight cut off at 1 kDa,and dialysis was conducted overnight against 0.01 mol/L NaCl.The sample was collected and freeze dried for the subsequent experiments.
2.3 Composition determination of digestion
The total carbohydrate composition was determined by the phenolsulfuric acid method [19].It can accurately and rapidly determine the total soluble carbohydrate in solution [20].The FOS content was determined by high-performance liquid chromatography with an evaporative light-scattering detector (HPLC-ELSD).The specific conditions were as follows.Separation was achieved on a Sugar D column (250 mm × 4.60 mm,5 μm),and the mobile phase,flow rate,sample size,and column temperature were acetonitrile:water (75:25,V/V),1 mL/min,20 μL,and 25 °C,respectively.All the samples and the standards were filtered through a 0.45 μm Millipore membrane before use.
2.4 Anaerobic culture
Growth medium: The method used in this study was according to Guergoletto et al.[21]and Pham et al.[22]with some modifications.The pH of the anaerobic medium was adjusted to 7.
Faecal inocula: Three healthy volunteers,aged from 22 to 24 years,not suffering from gastrointestinal discomfort or other health problems and not consuming any antibiotics,other drugs,or dietary supplements that might affect gastrointestinal functions and intestinal bacteria,were selected for the study.Faecal samples were collected in sterile vials,diluted with 30 mL of 10% (V/V) Dulbecco’s phosphate buffered saline to obtain a 20% (m/m) solution,and homogenized.The microorganism suspension was collected by centrifugation(550 ×gfor 5 min) to obtain the final human faecal inoculum.
Fermentation:in vitrofermentation was performed in an anaerobic environment for 48 h.Specifically,0.3 mL of the faecal suspension and 2.7 mL of the growth medium were added to a centrifuge tube [17].Each experiment was replicated independently five times.Three replicates of each sample were set and anaerobically cultured for 48 h,following which the samples were collected.
2.5 Measurement of pH
At the culture times of 0,6,12,24,36,and 48 h,the cultured samples were placed in an ice-water bath for 20 min.The supernatants were collected by centrifugation (8 000 r/min,15 min) and transferred to 10-mL centrifugal tubes.A PHS-3C pH meter was used to measure the pH of the supernatants.The measurement was repeated thrice for each sample.
2.6 Determination of SCFAs concentration
After the culture of 0,6,12,24 and 48 h,the culture samples were centrifuged (4 800 ×g,15 min),and the supernatant was collected.The concentration of SCFAs was determined by gas chromatography(GC) according to Ji et al.[23]with some minor modifications.Agilent 7890A GC system equipped with a HP-INNOWax column(30 mm × 0.32 mm,0.50 μm) and a flame ionization detector were used for chromatographic separation.The SCFAs were identified according to the retention time of each reference standard.The concentration of total and individual SCFAs were calculated based on the calibration curves made.
2.7 Cell DNA extraction and high-throughput sequencing
The cells were mechanically lysed using a glass bead breaker,and DNA was extracted using the Fast DNA Spin Kit for Soil(MP Biomedicals company,America).High-fidelity polymerase chain reaction (PCR) was conducted to amplify bacterial 16S rDNA hypervariable region 4 (V4) with primers.High-throughput sequencing was performed on an Illumina MiSeq platform using the 2 × 250 bp paired-end method after the library was quantified,mixed,and quality checked.
2.8 Statistical analysis
All the experiments were repeated at least thrice.Means and standard deviations were calculated.The results were analysed by SPSS version 23 software for Windows (SPSS Inc.,Chicago,IL,USA).Duncan tests at a significance level ofP<0.05 were performed for the significance analysis.
3.Results and discussion
3.1 Total carbohydrate change during in vitro digestion
CR undergoes a series of digestive procedures,such as amylase [24],pepsin [25],and pancreatin [26]to digest and break down.Accordingly,it produces glucose,amino acids,fatty acids,and other components in the body.In this study,three different treatments including CR,CR with added FOS (FCR),and CR with added glucose (GCR) were investigated.The corresponding changes in the total carbohydrate concentration in the digestive fluid duringin vitrodigestion are shown in Fig.1.In the simulated oral digestion phase(0–1 h),the rice began to digest rapidly,and the total carbohydrate content increased rapidly with the addition of amylase.Concurrently,there was no significant difference (P<0.05) in the total carbohydrate content of the three groups.However,in the gastric simulation digestion phase (1–3 h) and the small intestine (3–6 h),the total carbohydrate content of the FCR was significantly lower (P<0.05)than those of the GCR and CR.This might be due to the addition of the FOS,which has been found and proved to delay the digestibility of ricein vitroin line [27,28].
Table 1 lists the FOS composition obtained by simulating the oral,gastric,and intestinal environments.It could be seen that the main components of the FOS were 1-kestose (GF2),nystose (GF3),and fructofuranosylnystose (GF4).Moreover,there was no difference among the different digestion phases (P>0.05) during the simulatedin vitrodigestion,which suggests that little FOS could be digested.This illustrates that the added FOS could not be consumed during digestion and could directly play a role in gut health.
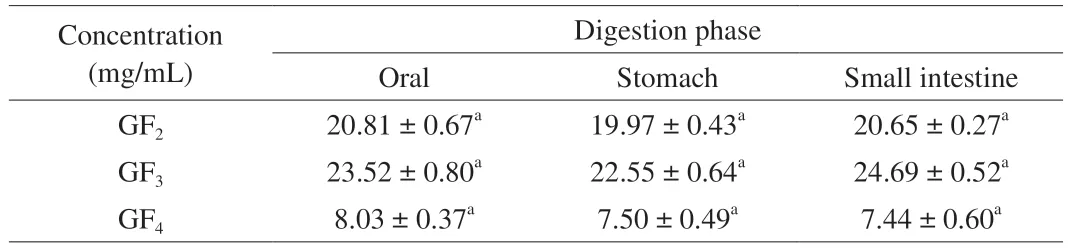
Table 1 Changes in FOS during different phases of in vitro digestion.Oral means in the 0–1 h digestion period,Stomach means in the 1–3 h digestion period,and Small Intestine means in the 3–6 h digestion period.Different lowercase letters mean significantly different (P <0.05).
3.2 pH variation during fermentation
After the simulated digestion,the samples were freeze-dried,added to the medium,and then fermented in an anaerobic environment.The pH changes of the medium during 48 h of the anaerobic culturing are shown in Fig.2.It could be seen that the pH value decreases significantly (P<0.05) from 0 h to 12 h,and subsequently flattens out from 12 h to 48 h during the fermentation.This trend was consistent with the finding of Xiong et al.[29],who reported that the pH in the faecal culture of a novel polysaccharide fromSargassum thunbergiiinitially exhibited a significant decline during fermentation from 0 to 24 h and subsequently increased slightly.The decrease in the pH may be due to the fermentation products,such as acetic acid and propionic acid from the gut microbiota in the faecal inoculum [30].Compared to the pure fermentation medium (Blank),the pH of the fermentation liquids of the three experimental groups were significantly lower(P<0.05),among which,the GCR had the lowest pH.Glucose,as a monosaccharide,could be directly used by intestinal microorganisms to produce metabolites rapidly and reduce the pH of a fermentation liquid,which might be the reason for this phenomenon.Concurrently,the pH of the FCR was significantly lower (P<0.05) than that of the CR after fermentation for 24 h.It is known that an appropriate lowering of the pH can inhibit the proliferation of undesirable pathogens and affect the activities of microbial enzymes [31].The decreased pH in the fermentation broth caused by the FOS and glucose may be beneficial to the probiotic bacteria colonizing in the intestinal lumen and have an advantageous effect on human intestinal health.
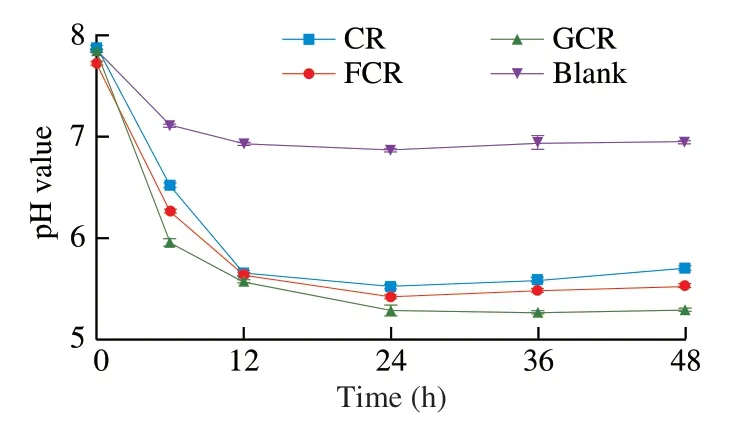
Fig.2 Changes in the pH value of the fermentation liquor during in vitro fermentation.
3.3 Production of SCFAs during fermentation
SCFAs are the main metabolites produced during the fermentation of carbohydrates,and their concentrations are considered to be important reflections of the gut microbiota activity [32]and the factor of pH reduction in a fermentation environment [33].Thus,the changes in the SCFAs content were analysed in the different treatment groups during the fermentation.As shown in Fig.3,it could be seen that the SCFAs content in all the groups increased during the fermentation.However,compared to the Blank,the increase in the total SCFAs content in the other groups was significantly faster (P<0.05).Rice is rich in carbohydrates,which interact directly with gut microbes,leading to the production of key metabolites,such as SCFAs [34].After 48 h of fermentation,the total SCFAs content of the GCR was the highest.Glucose is one of the most easily used carbon sources,and it can be easily employed by intestinal microorganisms to rapidly produce metabolites [35].This increases the total SCFAs,which was the main reason for this phenomenon.However,it is absorbed directly by the small intestine in the human body.The total SCFAs content of the FCR was significantly higher (P<0.05) than that of the CR.This was because the undigested polysaccharide can be used as the main carbon and energy sources of gut microbiota,stimulating the growth of SCFAs while producing them [36].FOS,as the indigestible oligosaccharide,can significantly increase the content of resistant starch,which can regulate the metabolic activities of intestinal microbiota,thus increasing the yield of SCFAs [37].
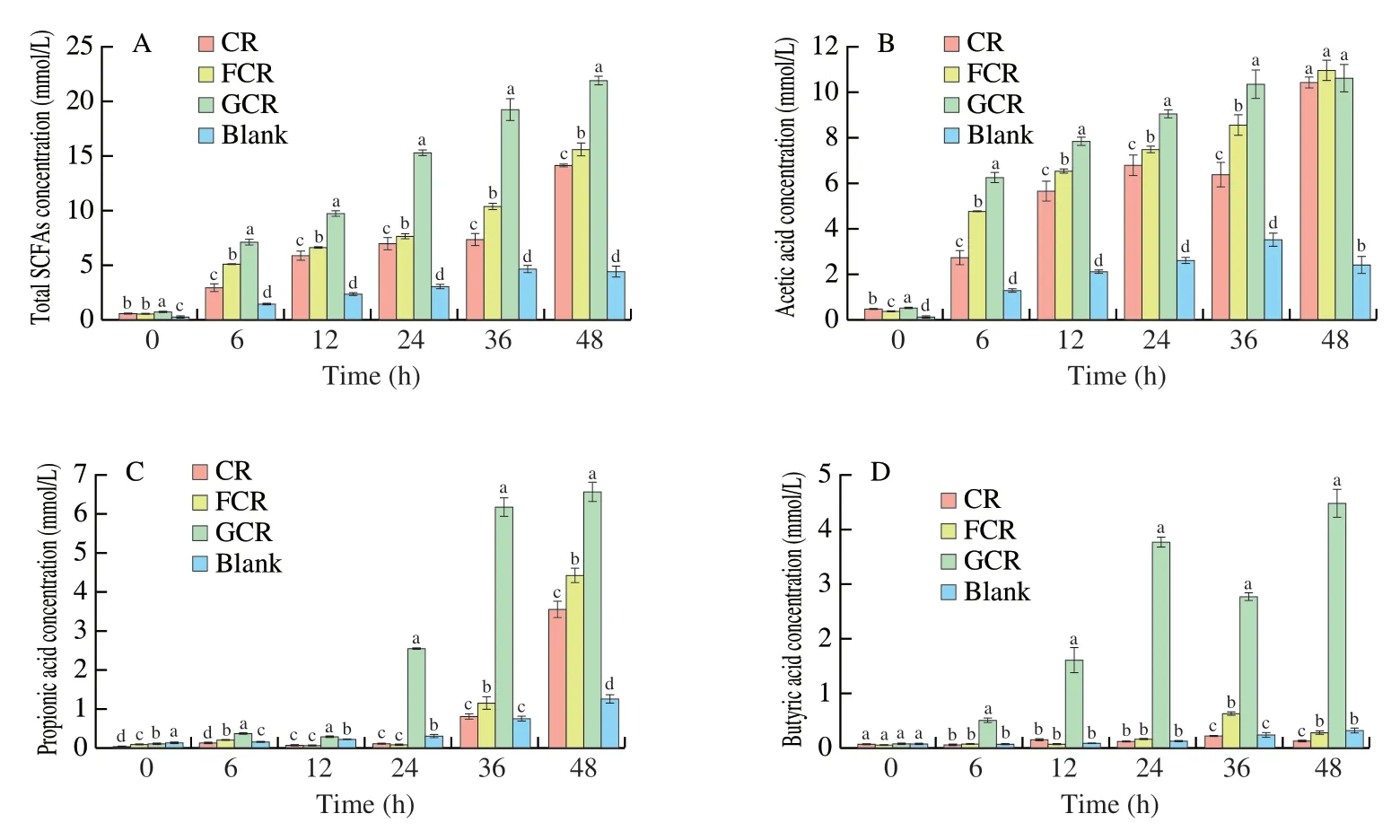
Fig.3 Changes in the total SCFAs (A),acetic acid (B),propionic acid (C),butyric acid (D) of the fermentation liquor during in vitro fermentation.Values are expressed as the mean standard deviation.Different lowercase letters at the same fermentation time mean significant difference (P <0.05).
In addition,the analysis of the acetic acid,propionic acid,andn-butyric acid changes during the 48 hin vitrofermentation are shown in Figs.3B–3D,respectively.It could be seen that the content of acetic acid in the FCR and GCR were significantly higher (P<0.05)than those in the CR within 6–36 h of the fermentation.Moreover,there was no difference (P>0.05) in the acetic acid content at 48 h(Fig.3B).In addition,the contents of propionic acid and butyric acid of the FCR and the GCR increased during the fermentation,and their contents of 36 h and 48 h were significantly higher(P<0.05) than those in the CR.Meanwhile,the propionate and butyrate play important roles in the liver and colonic mucosa functioning,respectively [38].Although the GCR produced a higher SCFAs concentration than the FCR,the former contained glucose,which is more easily utilized by putrid bacteria,and it also increased the risk of type 2 diabetes [3].Overall,these results indicated that the addition of the selected FOS could increase the total and individual SCFAs production.This was in correspondence with the fermentation of non-digestible carbohydrates and prebiotic ingredients (such as FOS and inulin) by intestinal bacteria,eventually accelerating the production of SCFAs [39].
3.4 Intestinal microbe composition analysis
In this research,to further investigate the structural changes of the faecal microbiota in the fermentation liquors produced by different treatments,high-throughput sequencing was used and the difference in the microbiota in the phylum and genus level were analysed.As shown in Fig.4,the microbiota in the phylum level mainly included Proteobacteria (42.99% –48.49%,such asKlebsiellaandEscherichia),Bacteroidetes (25.07% –36.99%,such asBacteroidesandParabacteroides),Firmicutes (8.86% –14.82%,such asClostridium_XIandLactobacillus),and Actinobacteria (0.09% –11.39%,includingBifidobacteriumandCollinsella).At the phylum level,Actinobacteria was increased by the FCR fermentation,whereas Firmicutes showed an opposite behaviour compared to the other groups (Fig.4A).This was related to the changes in the gut microbiota at the genus level.At the genus level,the CR groups (CR,FCR and GCR) could reduce the relative abundance ofClostridium_XIandKlebsiella(Figs.5C and 5D)(P<0.05) compared to the Blank,and promote the relative abundance ofBifidobacteriaandLactobacilli(Figs.5A and 5B) (P<0.05).This result agrees with that of Ayimbila et al.[18]according to which the colonic fermentation of rice hydrolysates could enhance the growth of probiotic bacteria,includingBifidobacteriaandLactobacilli.It was reported thatClostridium_XIandKlebsiellamay be related to sepsis and eventually lead to death and human nosocomial infections [40].However,BifidobacteriumandLactobacillus,as the most important probiotics, could provide numerous health benefits to the human intestinal tract [41].The reason for these phenomena could be the presence of resistant starch in CR.Resistant starch can achieve the bioactive functions of modulating the growth and metabolic activity of gut microbiota [37]and increase the content ofBifidobacteriaandLactobacilli[42]during the fermentation period.
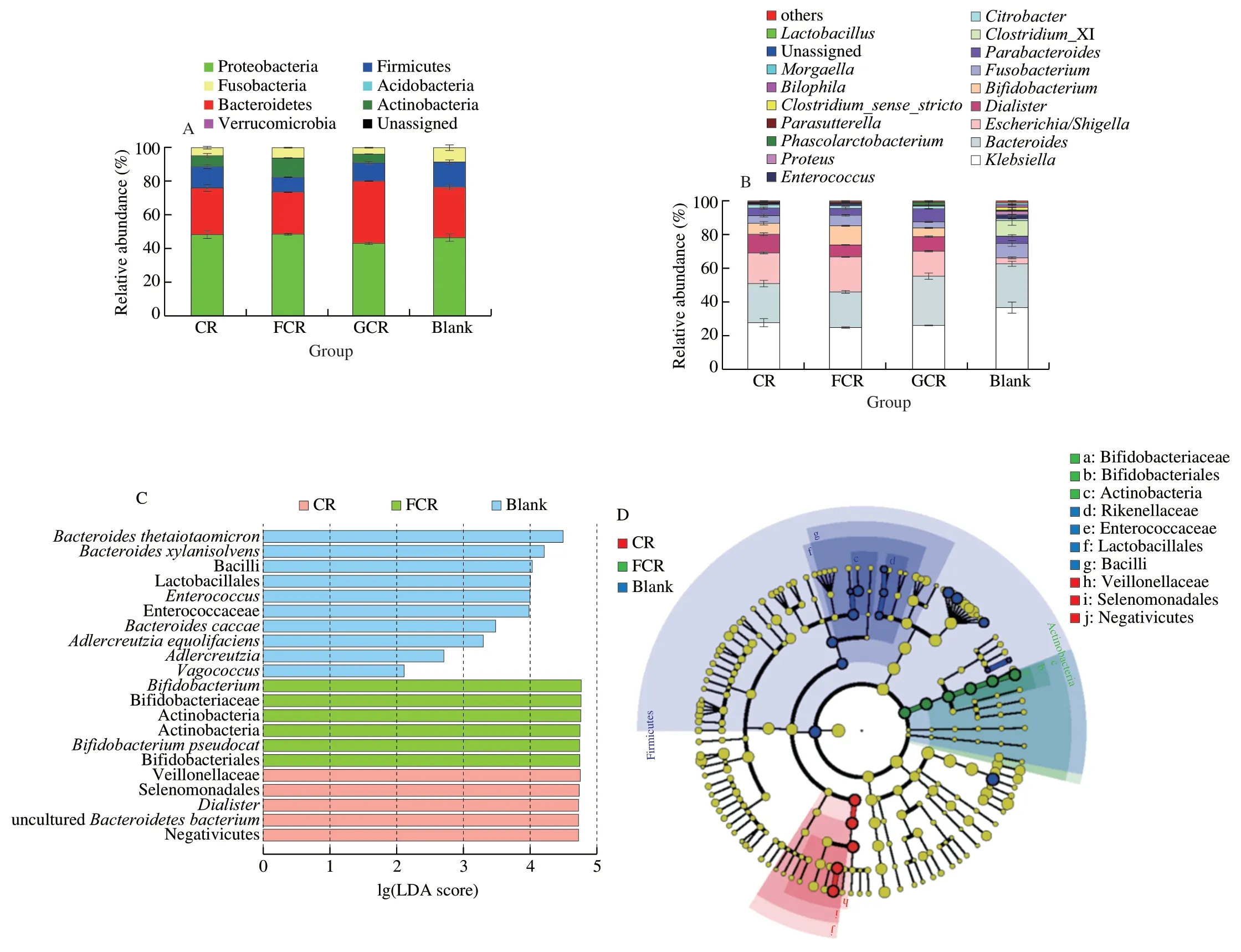
Fig.4 Bacterial taxonomic profiling in the phylum (A) and genus (B) level of fermentation liquor from different treatment groups.(C) LDA score.Enriched taxa with an LDA score >2 were shown in the histogram.(D) LEfSe taxonomic cladogram.
Moreover,the FCR showed a strong advantage in promoting beneficial bacteria and inhibiting pathogens.The reason for the increase in the activity of the FCR was that the FOS could selectively stimulate the growth and maintain the colonization of probiotics bacteria,particularlyBifidobacteriumandLactobacillus[43].However,the GCR was significantly (P<0.05) higher than the CR and the FCR at the relative abundance ofBacteroidesandParabacteroides(Figs.5E and 5F).TheBacteroidesandParabacteroidesin the genus belonging to theBacteroidetesphylum are important opportunistic anaerobic pathogens that are highly infectious [44].This result is in correspondence with those of Demirci et al.[45],according to which the relative abundance ofBacteroidetesin the faeces of individuals with high sugar intake was significantly higher than those of healthy controls.This suggests that the addition of glucose stimulates the growth of Bacteroidetes, such asBacteroidesandParabacteroides.Briefly,the FCR showed the advantages of promoting beneficial bacteria,such asBifidobacteriumandLactobacillus,and inhibiting harmful bacteria,such asBacteroidesandKlebsiella.Concurrently,compared to the traditional CR,the FCR decreased the pH and increased the SCFAs concentration significantly duringin vitrofermentation.Therefore,FOS has a high potential for developing high-quality healthy rice foods.
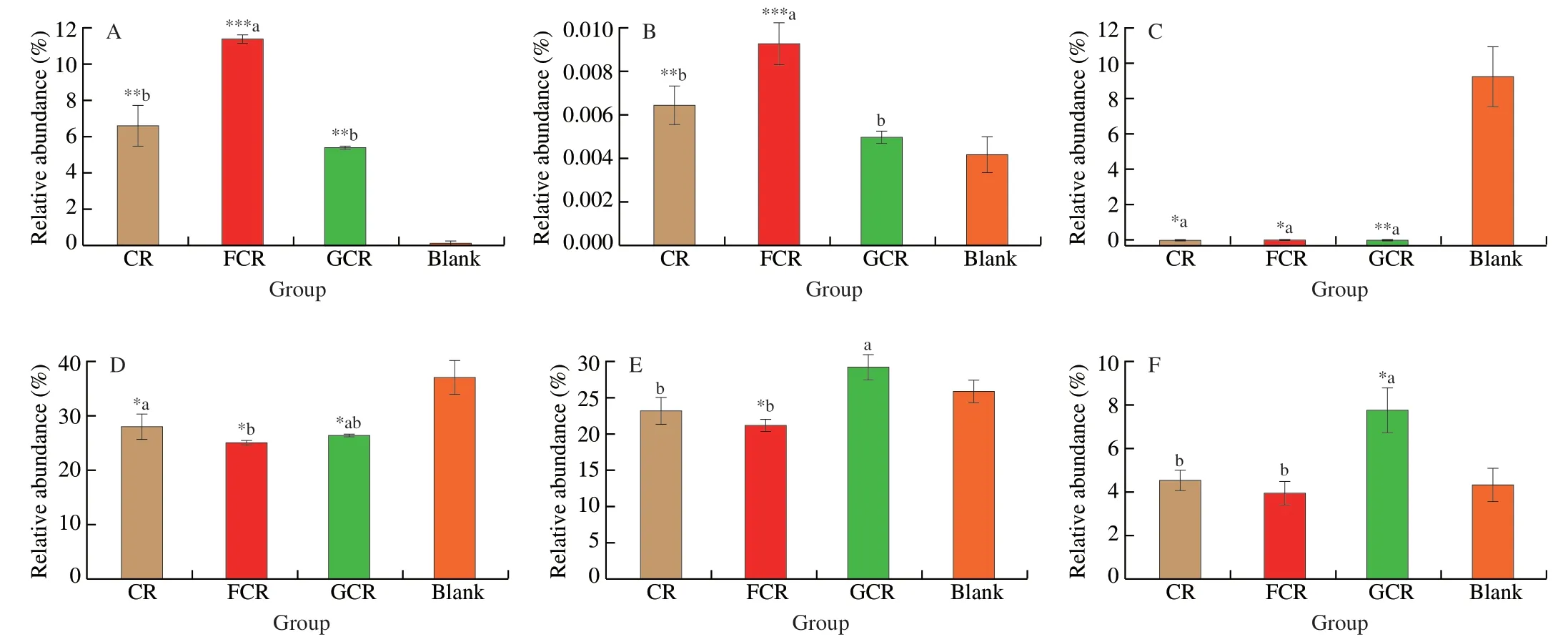
Fig.5 Relative abundance values in the genus level of fermentation liquor from different treatment groups.Different lowercase letters mean significantly different (P <0.05).The three groups were respectively compared to the Blank,and the symbols *,** and *** respectively mean P <0.05,P <0.01 and P <0.001.(A) Bifidobacterium.(B) Lactobacillus.(C) Clostridium_XI.(D) Klebsiella.(E) Bacteroides.(F) Parabacteroides.
To identify the key phylotypes of gut microbiota in different groups,LEfSe analysis among the CR,the FCR and the Blank(Fig.4C) were performed.LDA results showed the mian dominant microbiota in the FCR (LDA >4,P<0.05) were Actinobacteria,Bifidobacteriales and Bifidobacteriaceae.The CR showed the major microbiota (LDA >4,P<0.05) were Firmicutes,Negativicutes,Selenomonadales,Veillonellaceae andDialister.Then,an evolutionary clustering analysis diagram was delivered to identify major microflora by taxonomy (Fig.4D).In cladogram,Bifidobacteriaceae had the highest flora abundance in the green parts and Veillonellaceae was the richest in the red area,which represented the FCR and the CR,respectively.Overall,these results indicated that FOS treatment altered the key distribution of gut microbiota and promoted the multiplication of specific bacteria.
4.Conclusions
This work revealed that cooked rice with an addition of FOS significantly improved the faecal flora structure by simulating thein vitrodigestion and fermentation.This provided a theoretical basis for deeply proceeding the healthy and convenient rice products with a good quality.Our results showed that the concentration of total carbohydrate in FCR was significantly lower than that in cooked rice with added glucose (GCR) and cooked rice (CR) duringin vitrodigestion.This is due to a slowerin vitrohydrolysis rate for the rice that is induced by FOS.No significant difference was seen for the content of FOS in digestive fluids that is prepared at various digestion stages.This indicates that FOS is not consumed during the digestion,while it behaves as a probiotic in the intestine.Duringin vitrofermentation,compared to the concentration of CR that did not have a significant change,the pH of FCR decreased and the concentration of short-chain fatty acids (SCFAs) increased.This demonstrates that the metabolic regulation is enhanced due to the treatment of FCR.The FCR showed the advantages for promoting the growth of beneficial bacteria,i.e.,BifidobacteriumandLactobacillus,and inhibiting that of harmful bacteria,i.e.,BacteroidesandKlebsiella.Therefore,FCR is a novel processing strategy for manufacturing the high-quality and healthy rice products with a better taste and higher content of probiotics.
Conflict of interest
There are no conflicts of interests to declare.
Acknowledgements
The authors acknowledge financial support from the National Key R&D Program of China (2018YFD0400500),the Key Research and Development Program of Jiangsu Province (BE2018323),Qing Lan Project of Jiangsu Province and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
- 食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Biological factors controlling starch digestibility in human digestive system
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
- Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil

