Monitoring and identif ication of spoilage-related microorganisms in braised chicken with modif ied atmosphere packaging during refrigerated storage
Yng Lei, Yli Zhng, Yiqun Cheng, Jiho Hung, Ming Hung,*
a Key Laboratory of Meat Processing and Quality Control, Ministry of Education; College of Food Science and Technology, Nanjing Agricultural University, Nanjing 210095, China
b National R&D Center for Poultry Processing, Jiangsu Research Center for Livestock and Poultry Products Processing Engineering Technology,Nanjing Huangjiaoshou Food Science and Technology Co., Ltd., Nanjing 211225, China
c College of Environmental Science & Engineering, Institute of Functional Food, Anhui Normal University, Wuhu 241000, China
d College of Engineering, Nanjing Agricultural University, Nanjing 210031, China
Keywords:Braised chicken Modif ied atmosphere packaging (MAP)16S rDNA identif ication Shelf-life
A B S T R A C T This study mainly monitored the dominant bacterial populations and identified the spoilage-related microorganisms of braised chicken meat stored under different CO2-modifi ed atmosphere packaging (MAP)during refrigerated storage using a culture-dependent method and 16S rDNA identification. The quality changes and shelf life of the meat were also measured. The growth rate of total viable count (TVC) in braised chicken was slower with an increase of CO2 content in MAP, which also occurred in the remaining bacterial species monitored (lactic acid bacteria, Pseudomonas spp., Brochothrix thermosphacta). The MAP exerted beneficial effects on the quality of braised chicken, as demonstrated by retarding the production of total volatile basic nitrogen (TVB-N) and delaying lipid oxidation (TBARS test). A total of 14 isolates were identifi ed from braised chickens with different packaging at the end of storage, these included P. fragi(6 isolates), P. psychrophila (2 isolates), Enterococcus faecalis (3 isolates), B. thermosphacta (2 isolates),Staphylococcus equorum (1 isolate).
1. Introduction
Braised chicken as a traditional Chinese ready-to-eat chicken meat product is popular among consumers for its attractive color, delicious taste and unique flavor, which has greatly promoted the growth of the braised chicken industry [1,2]. In recent years, with the improvement of people’s living standards, consumers have increased their requirements for the quality and safety of braised chicken. Therefore,it has been a challenge for the poultry industry to prolong the shelf life of braised chicken while ensuring good quality and safety. The shelf life of meat products is determined by microbial growth [3].Due to the high nutrient content of chicken meat, microorganisms can grow and cause spoilage even under chilled conditions [4]. Therefore,inhibiting the growth of microorganisms is the main preservation measure for braised chicken on supermarket retail counters.
Meat and meat products are contaminated by a variety of microorganisms during processing, but only a small part of the microorganisms participate in the corruption during storage. These bacterial groups are called specific spoilage organisms (SSOs)because they are suitable for survival, reproduction, and gradually occupy a dominant position and produce spoilage metabolites. The types of meat, packaging methods and storage temperature will affect the differences of SSOs in the products [5]. Therefore, determining the dominant spoilage bacteria is of great significance for the targeted control of the spoilage of meat products during storage.
Modified atmosphere packaging (MAP), a modern non-thermal method of food preservation, is widely used in the packaging of meat and meat products [6,7]. The optimal mixture of gases used in MAP are carbon dioxide (to inhibit the growth of aerobic bacteria), oxygen(to retain meat color and to prevent anaerobic bacteria growth) and nitrogen (to delay oxidation of fats and to prevent package collapse).It has been reported that the minimum inhibitory concentration of CO2is 20%–30%, while higher than 50% can lead to packaging collapse [8-10]. However, few studies have reported on the effects of different carbon dioxide MAP concentrations on cooked chicken meat, especially on the preservation of braised chicken.
Traditional culture-dependent methods and 16S rDNA identification have been widely used to isolate and identify microorganisms in meat products. A variety of bacteria that cause the spoilage can be found in different packaging of cooked meat products,includingEnterobacteriaceae,Pseudomonasspp. andBrochothrix thermosphacta[11,12]. However, the main spoilage bacteria that cause meat spoilage under different packaging methods are also different. In order to take effective measures to extend the shelf life of braised chickens, it is necessary to isolate and identify the dominant spoilage bacteria.
Therefore, this study was designed to investigate the effect of MAP on quality and shelf-life extension, monitor the dominant bacterial populations and identify the spoilage related microorganisms of braised chicken with different packaging during refrigerated storage. This should also provide a theoretical basis for inhibiting the dominant spoilage bacteria in braised chicken.
2. Materials and methods
2.1 Sample preparation
One hundred and fifty-two braised chicken drumsticks((90 ± 10) g each) were obtained from Nanjing Professor Huang Food Technology Co., Ltd., (Nanjing, China). The process of braised chicken is as follows: first, the whole chicken carcasses were painted with honey and fried at (175 ± 5) °C for 90 s until its surface became golden yellow. Next, the fried chickens were braised in soup with different spices (for example, fructus amomi, clove,Chinese cinnamon bark, dried tangerine peel, round cardamom,star anise, ginger, angelica dahurica, and so on) for 1.5 h [13].After cooling, the braised chicken samples were packaged in the air (A) and MAP with different gas mixtures (M20: 20% CO2/80%N2, M30: 30% CO2/70% N2and M40: 40% CO2/60% N2) using the MAP machine (SMART500, ULMA Co., Spain). For the air treatment, samples were packaged with a polyethylene film(75 μm in thickness), which has an oxygen permeability of 14 483 cm3/(m2·day·atm), CO2permeability of 63 683 cm3/(m2·day·atm),and water vapor permeability of 54 g/(m2·day·atm). For the MAP treatment, samples were packaged with 75 μm low-density barrier pouches consisting of polyethylene/polyamide/low-density polyethylene (LDPE/PA/LDPE) barrier pouches (2 drumsticks/pouch), with an oxygen permeability of 24 cm3/(m2·day·atm)at 0% relative humidity (RH)/23 °C, CO2permeability of 78 cm3/(m2·day·atm) at 0% RH/23 °C and water vapor permeability of 44 mL/(m2·day) at 100% RH/38 °C. The gas mixtures were provided by Nanjing Special Gas Co., Ltd. The volume ratio of gas to the product was 2.58. All the samples were stored at (4 ± 1) °C (Compressor-Cooled Incubator ICP260, Memmert, Germany). Physicochemical(n= 3 per group for each sampling time) and microbial (n= 5 per group for each sampling time) indicators of all samples were tested at 0, 5, 10,15 and 20 days of storage. The control group (A) and the MAP groups(M20, M30 and M40) were stored for 15 and 20 days, respectively.
2.2 Microbial enumeration and bacteria isolation
Samples (25 g) were removed aseptically using scalpels from the braised chicken drumsticks and homogenized with 225 mL sterile saline (0.85% NaCl) in a stomacher bag (HaiBo Co., Qingdao, China)for 2 min. Decimal dilutions were prepared using sterile saline. Then aliquots of 1 mL were spread on the following growth media: Plate Count Agar (PCA, Lang Bridge Co., Beijing, China) which was incubated at 37 °C for 48 h; Man Rogosa Sharpe (MRS) agar (Lang Bridge Co., Beijing, China) for lactic acid bacteria (LAB), incubated at 30 °C for 48 h under anaerobic environment; streptomycin sulphate thallous acetate cycloheximide (actidione) agar (STAA) medium(HaiBo Co., Qingdao, China) and Centrimide Fucidin-Cepha Loridine(CFC) medium (HaiBo Co., Qingdao, China) forB. thermosphactaandPseudomonasand incubated at 25 °C for 48 h, respectively. The CFC and STAA agar were prepared according to the manufacturer’s direction (HaiBo Co., Qingdao, China). At the end of storage,colonies were selected from different growth media according to the morphological features of the colonies (appearance, size, and colors)and were sub-cultured three times. A bacterial suspension of each isolate in saline (%) was mixed with 60% glycerol in a volume ratio of 1:1 and stored at –20 °C.
2.3 DNA extraction and identification of 16S rDNA
Genomic DNA of pure cultures was extracted by a TaKaRa MiniBEST Bacteria Genomic DNA Extraction Kit Ver.3.0 according to the manufacturer’s instructions(TaKaRa, Beijing, China). After amplification with the primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R(5′-AAGGAGGTGATCCAGCCGCA-3′), the purified PCR products were sent to Biozeron (Shanghai, China) for sequencing. The results of sequencing were blasted using the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to confirm the species and uploaded to obtain an accession number. All the 16S rDNA sequences of isolates and corresponding model strains were constructed the Neighbor-Joining phylogenetic tree using MEGA version 7 [14].
2.4 Headspace gas measurement
The changes of headspace gases in the modified atmosphere package (M20, M30 and M40) were measured using an Oxybaby 6.0i Gas Analyzer (Witt-gasetechnik GmbH&Co KG, Witten, Germany)and expressed as the percentage of CO2, O2and other gas before opening the package. The mean values were collected from three packages and analyzed every five days.
Kevin visited the coffee shop four times during his vacation. He always sat at a table outside by himself and read the free daily paper. Each day he was there, he read most of the newspaper articles while he enjoyed two cups of coffee.
2.5 pH measurement
The braised chicken samples without skin (5 g) were mixed with 45 mL distilled water. They were then homogenized(Ultra Turrax T25, IKA, Germany) at 5 000 r/min for 30 s(2 × 15 s with a 5 s interval). The pH values were measured using a pH meter (Hanna HI9025c, Portugal).
2.6 Color evaluation
The surface color of samples, expressed by lightness (L*), redness(a*), and yellowness (b*) were measured using a Chroma meter CR-400 (Minolta, Osaka, Japan) under an illuminantD65and 10°standard observer, an 8 mm diameter measuring area and 11 mm aperture for illumination [15]. Before taking measurements, the instrument was calibrated with a white tile for standardization.
2.7 Lipid oxidation assay
Lipid oxidation was measured using the method described by Utrera et al. [16] and expressed as a thiobarbituric acid reactive substances(TBARS) value in mg malondialdehyde (MDA)/kg sample. The MDA standard curve was prepared using 1,1,3,3-tetraethoxypropane (TEP)and used to calculate the TBARS value of the braised chicken.
2.8 Total volatile base nitrogen (TVB-N) analysis
TVB-N content in braised chicken was determined according to the Chinese standard (GB/T 5009.228-2016). Briefly, samples(10 g) were ground individually by a knife type mixing grinder(Grindomix GM 200, Retsch, Germany). Automatic Kjeldahl nitrogen analyzer (Hanon K9860, Jinan, China) was used for analysis and determination. The results were expressed as mg of TVB-N per 100 g of chicken.
2.9 Sensory evaluation
For sensory assessment, ten experienced panelists were selected among graduate students who participated in the sensory tests from the National Center of Meat Quality and Safety Control of China trained according to ISO 11037-2011. Along with the test samples, the freshly braised chicken sample was served as the reference sample.Acceptability as a composite of odor, appearance and tissue state was estimated using a scale ranging from 1 to 9, where 9 represents no foreign flavor or like extremely and 1 represents extreme foreign flavor or dislike intensely. A score of 6 was the limit of acceptability [17].
2.10 Statistical analysis
All data were calculated as the means and standard deviations and statistically analyzed using ANOVA (P< 0.05) via SAS 9.2 statistical software (SAS Institute Inc., Carry, NC, 2003). Pearson correlation coefficient was calculated by SPSS software (v.20; SPSS Inc.,Armonk, NY, USA). The heatmap of Pearson correlation coefficients was drawn by TBtools software v0.6673 [18].
3. Results and discussion
3.1 Microbiological analysis
The changes in the microflora (total viable count (TVC), LAB,Pseudomonas,B. thermosphacta) of A, M20, M30 and M40 braised chicken samples are shown in Table 1. The initial TVC of braised chicken was 2.27 lg(CFU/g) (day 0), which is a relatively high bacterial load compared to that of 1.53 lg(CFU/g) obtained for roast chicken [19] and 1.6 lg(CFU/g) was obtained for boiled salted duck [7]could probably be attributed to contamination in the cooling room.The TVC grew most rapidly in air-packaging (A), while the use of MAP was more effective for reducing the TVC population,particularly with M40 treatment (40% CO2/60% N2). TVC values of > 4 lg(CFU/g) are considered the upper microbiological limit for cooked meat products as defined by GB 2726–2016. The TVC of A,M20, M30 and M40 samples exceeded the limit after approximately 6–7, 10–11, 10–11 and 15–16 days, respectively (Table 1). This study showed that MAP could inhibit the growth of microorganisms in braised chicken samples compared to air packaging, and the growth rate of bacteria decreased gradually with the increase of carbon dioxide content in the MAP. The overall trends for TVC were also inagreement with Patsias et al. [6] and Guo et al. [19] who studied the effect of MAP on the chilled pre-cooked chicken products and roast chicken meat, respectively. However, the shelf-life of braised chicken samples packaged in the M40 sample was longer than that reported by Liu et al. [2] which might be due to the higher carbon dioxide content.
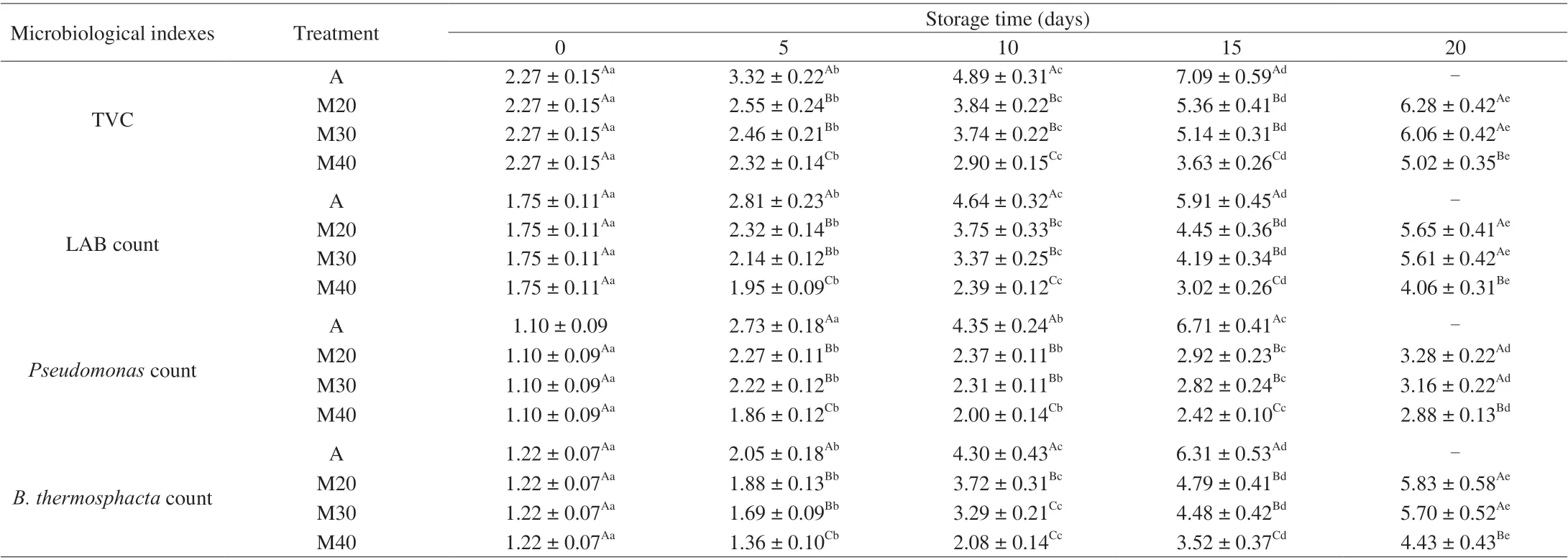
Table 1 Viable counts (lg(CFU/g)) of different spoilage-related bacteria in braised chicken during refrigerated storage.
Lactic acid caused the pH to decrease and leads to sour spoilage of poultry meat products during refrigerated storage [4,20]. The initial count of LAB was 1.75 lg(CFU/g) (Table 1) and increased to 5.91 lg(CFU/g) on day 15 (Air samples). With the increase of storage time, LAB attained final counts of approximately 4.06–5.65 lg(CFU/g)for all MAP samples (day 20). The results showed that LAB counts for M40 were significantly lower after 20 days of storage, which may be attributed to the bacteriostatic properties of carbon dioxide. This finding was also in agreement with Patsias et al. [6] and Zhai et al. [7].
BothPseudomonasandB. thermosphactawere also monitored in our study. The counts ofPseudomonasspp. in MAP samples remained low (≤ 3.28 lg(CFU/g)) throughout the 20 days storage at 4 °C (Table 1).AlthoughPseudomonaswas strictly aerobic bacteria, their growth was not inhibited by the different ratios of CO2/N2gas mixture [8,21].In our study, MAP effectively inhibits the growth ofPseudomonasspp.,with M40 (high CO2concentrations) being significantly lower than A, M20 and M30 MAP, which is in agreement with Latou et al. [22]and Hasapidou et al. [23].B. thermosphactais a facultative anaerobe bacterium, the major spoilage bacterium of meat and meat products packaged aerobically or under MAP [24]. In this study, the initial(day 0) counts ofB. thermosphactawas 1.22 lg(CFU/g) and increased counts progressively with storage attaining final counts of 6.31 lg(CFU/g) for air-packaged samples. The MAP samples attained final values of approximately 4.43–5.83 CFU/g throughout 20 days of storage with M40 being significantly lower at day 20 (Table 1).The use of carbon dioxide could effectively inhibit the growth ofB.thermosphactain braised chicken, thus delaying the deterioration process and prolonging the shelf life. This is consistent with the results of Skandamis et al. [25] and Chouliara et al. [8].
3.2 Identification of the isolates from braised chicken
A total of 14 strains were isolated from different packed braised chicken at the end of storage using the different growth media. The sequences of the 14 strains were submitted to Genebank to obtain an accession number (Table 2). The results showed the 14 isolates were divided into 5 species, sixP. fragi, twoP. psychrophila, threeEnterococcus faecalis, twoB. thermosphactaand oneStaphylococcus equorum. All of these isolates had more than 96% identity with the closest GenBank relative. Strains that generally show higher than 98.65% similarity of the 16S rDNA gene sequence are considered to be the same genus [26]. Therefore, we did physiological and biochemical identification to further confirm that the isolated strains were belongs to the genus (the data was not shown). Recent studies have found thatLactobacillus sakeiandL. curvatushave been isolated from vacuum-packed, smoked bacon at the end of storage [27]. The spoilage-related microorganisms under different packaging methods with different types of cooked meat vary greatly. A phylogenetic tree was constructed with isolate sequences and corresponding model strains (Fig. 1). The strains identified from different packaged braised chickens were clustered well with the model strains.
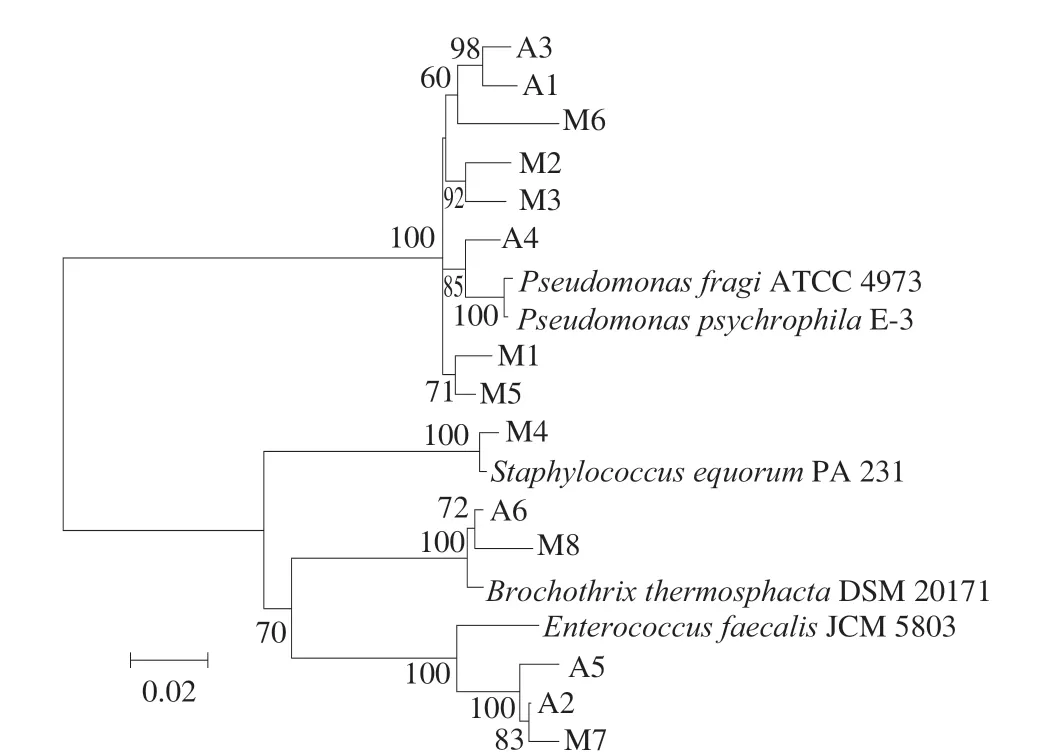
Fig. 1 Phylogenetic tree of 14 strains based on 16S rDNA sequences.
3.3 Headspace gas change
The headspace compositions of MAP treatments are shown in Fig. 2.The reduction of CO2content on day 5 was approximately 30%for all three MAP treatments. With storage time increasing, thereduction of CO2decreased gradually and tended to stabilize. Since the headspace was dynamic, this phenomenon was mainly due to the dissolution of CO2into the aqueous phase of the chicken meat and the packaging permeability [28,29]. The changes of O2content in the MAP was always between 0% and 0.05% (data not shown), and there was no significant difference during storage (P> 0.05).
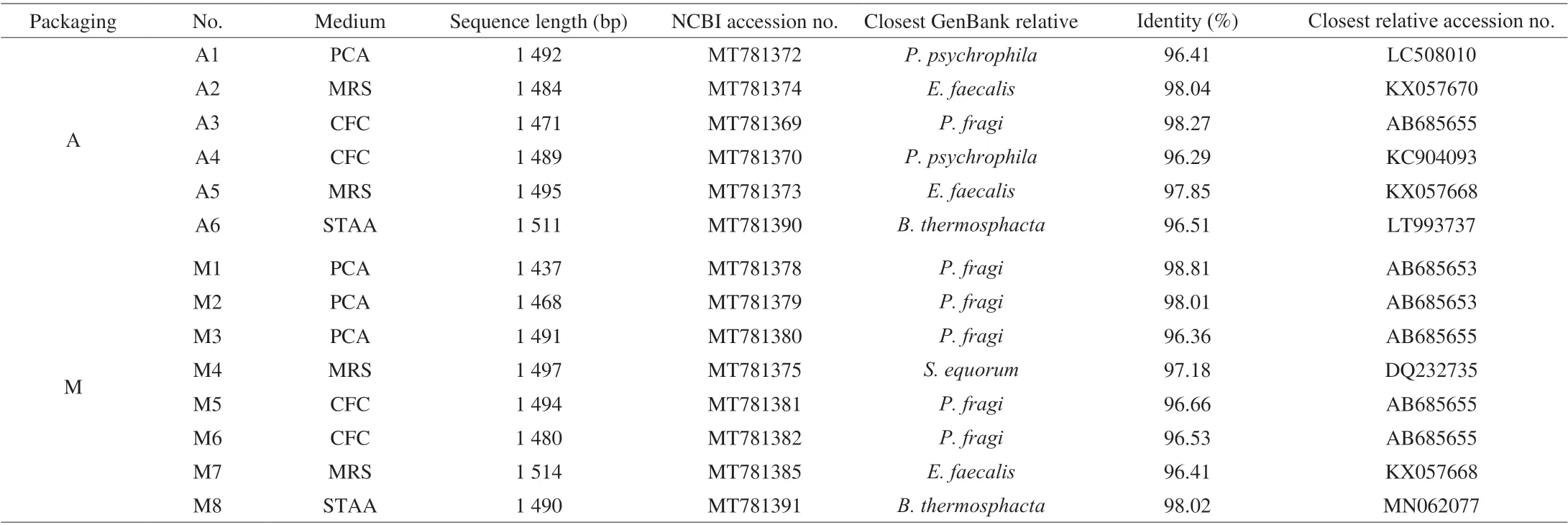
Table 2 Isolation and identification of spoilage-related microorganisms based on 16S rDNA sequence analysis of braised chicken at the end of refrigerated storage.
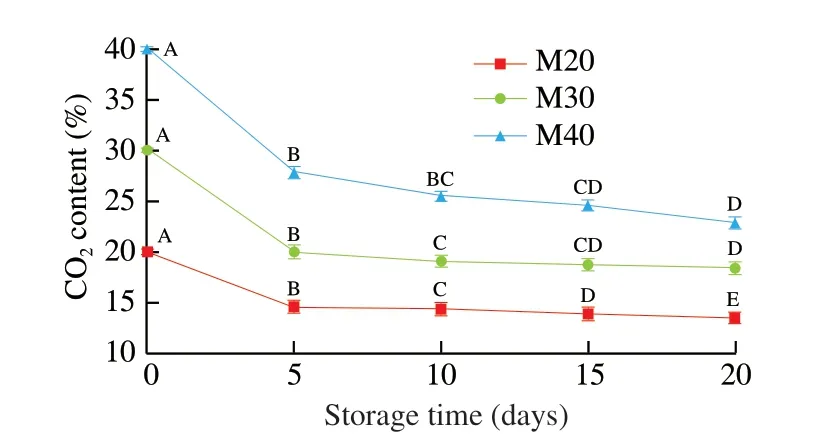
Fig. 2 Effect of storage time on CO2 content in MAP at 4 °C. A–E: values with different uppercase were significantly different (P < 0.05).
3.4 pH value
The pH values were in the range of approximately 6.63–6.86 for both air-packaged and M20, M30 and M40 chicken samples(Table 3). A small but statistically significant (P< 0.05) decrease was recorded in air-packaged samples during the first 10 days of storage. There was no significant difference (P> 0.05) in the change of pH for MAP chicken samples during the entire storage. The finding is in agreement with the results reported by Guo et al. [19]and Economou et al. [30]. It has been reported that the pH change in muscle is affected by many factors such as the dissolution of CO2into the water and fat phases of meat, the decomposition of proteins into amino acids which upon decarboxylation lead to the formation of alkaline amines, lactic acid production by lactic acid bacteria and the buffering capacity of the meat tissue [31,32]. The changes in pH showed a negative correlation with TVC, LAB,PseudomonasandB.thermosphacta(Fig. 3).
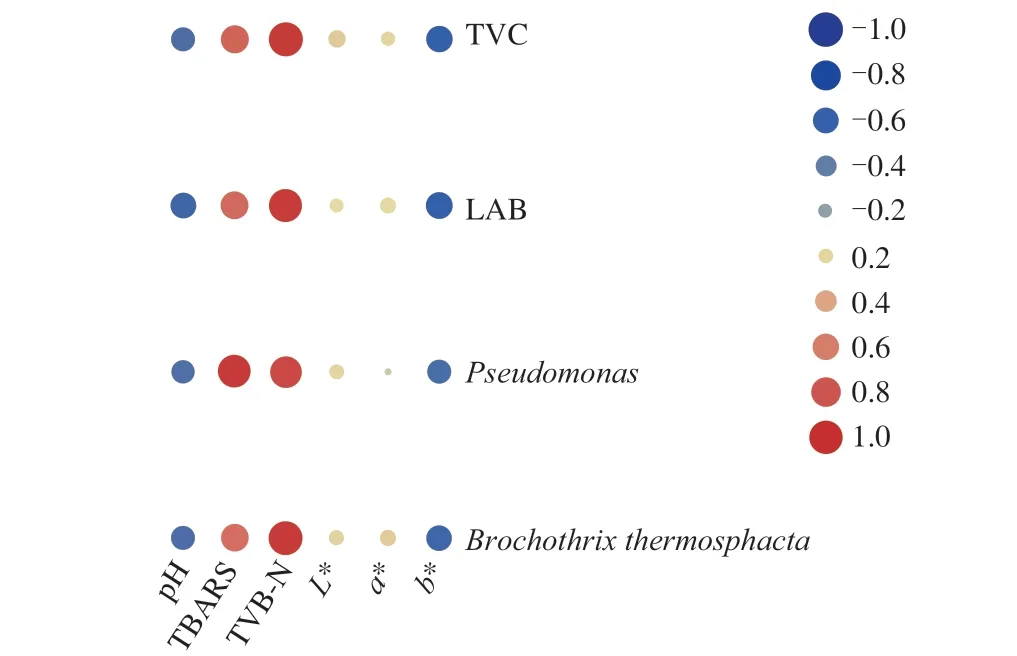
Fig. 3 Heatmap of Pearson’s correlation of microbiological and physicochemical index of braised chicken under chilled condition ((4 ± 1) °C)for up to 15 days. The area of the circle represents the absolute value of the correlation coefficient, where red represents positive correlation, and blue represents negative correlation.
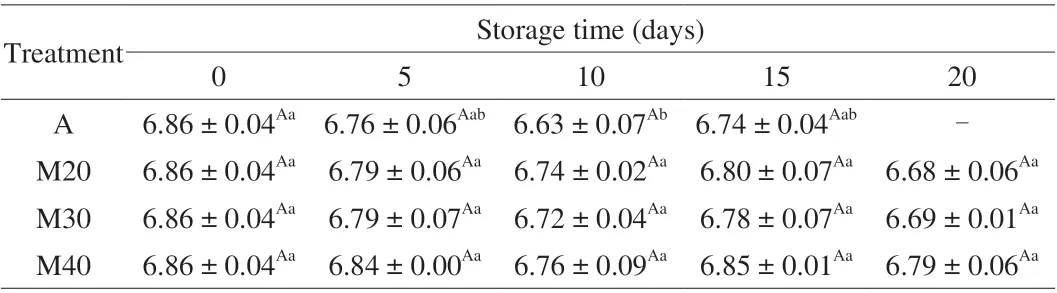
Table 3 The pH changes of braised chicken under chilled condition ((4 ± 1) °C) for up to 20 days.
3.5 Color evaluation
Color (L*,a* andb*) values for all braised chicken treatments are given in Table 4. TheL* values of all samples varied between 39.01 and 43.23 throughout the storage period and the MAP had no significant (P> 0.05) effect on lightness during the entire storage.Parametera* values ranged between 13.49 and 17.91 and had nosignificance (P> 0.05). The initial yellowness (b*) values were 21.8,and decreased to 15.74 (day 15), 15.54 (day 20), 15.74 (day 20)for A, M20 and M30, respectively. The reason for this may be attributed to the MAP effectively inhibiting the oxidation of lipids and stabilization of the yellowness of braised chicken samples, especially the M40 treatment. However, these changes in color values are hardly perceptible by the human eye [30].
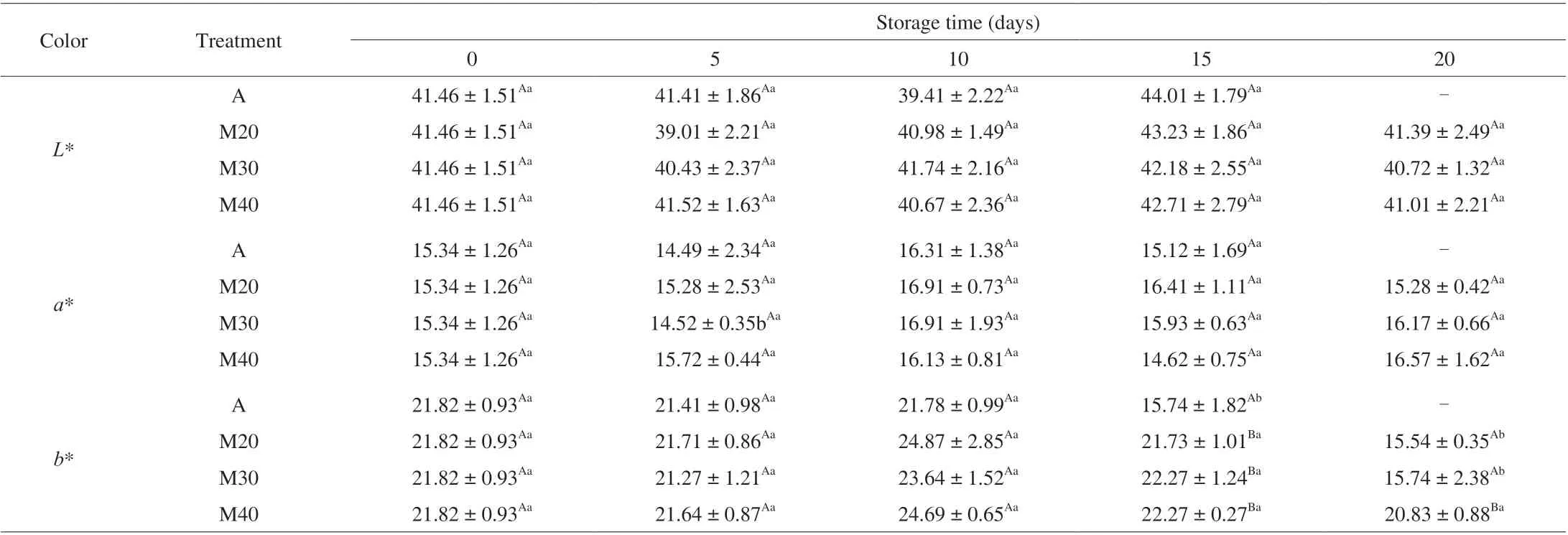
Table 4 The color changes of braised chicken under chilled condition ((4 ± 1) °C) for up to 20 days.
3.6 Lipid oxidation
TBARS is a measure of MDA, which is one of the secondary products of lipid oxidation. The TBARS values of all braised chicken samples are presented in Table 5. The initial TBARS value was 0.55 mg/kg which increased with storage time reaching a final value of 1.59 mg/kg for air-packaged samples (day 15) and approximately 0.81–0.84 mg/kg for M20, M30 and M40 samples (day 20). Compared with air packaging, MAP significantly (P> 0.05) inhibited lipid oxidation while there is no significant (P< 0.05) difference within the MAP treatments. The data show that aerobic storage enhances lipid oxidation, whereas CO2-modified atmosphere packaging has the potential to control lipid oxidation in braised chicken. The lack of oxygen appears to effectively inhibit the occurrence of fat oxidation.This finding is also supported by Patsias et al. [6] who studied the effect of MAP on lipid oxidation in the chilled pre-cooked chicken products. Remarkably, the changes in TBARS showed a high positive correlation withPseudomonas(Fig. 3). We cannot explain it by now,and further research is needed to confirm this result.
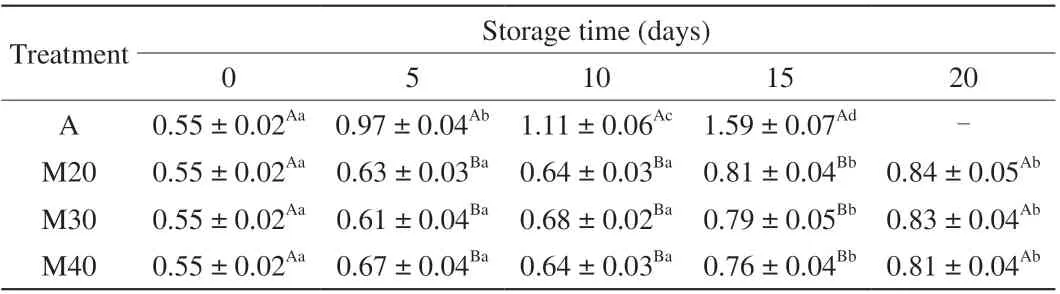
Table 5 The TBARS values (mg MDA/kg) changes of braised chicken under chilled condition ((4 ± 1) °C) for up to 20 days.
3.7 TVB-N content
Total TVB-N is widely used as an important indicator of meat spoilage due to the microbial degradation of protein and nonprotein nitrogenous compounds [33]. The TVB-N content of all samples are given in Table 6. The initial content of TVB-N was 13.48 mg/100 g and increased with storage, the TVB-N content of all A, M20, M30 and M40 samples reached 31.33, 21.39, 20.98 and 20.13 mg/100 g (day 15), respectively. TVB-N content increased sharply for the air-packaged samples but were significantly slower for the MAP samples (P< 0.05). This is also in agreement with those of Zhang et al. [3] who reported TVB-N content with MAP were lower than with air-packaging. It must be mentioned that a positive correlation was observed between microbial growth and TVB-N content in the braised chicken (Fig. 3), and they were significantly(P< 0.01) correlated with TVC, LAB,PseudomonasandB. thermosphacta. The reason is probably due to the decomposition of microorganisms in braised chickens during storage [26,34,35].
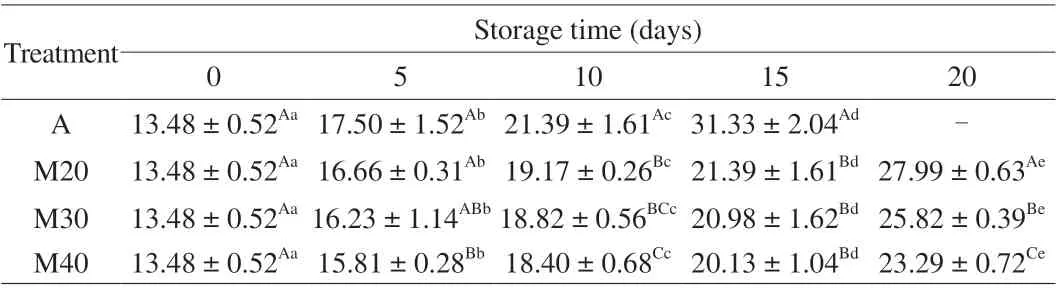
Table 6 The TVB-N content (mg/100 g) changes of braised chicken under chilled condition ((4 ± 1) °C) for up to 20 days.
3.8 Sensory evaluation
Sensory scores for overall odor and overall acceptance of all braised chicken samples decreased with time of refrigerated storage (Fig. 4). Air-packaged chicken samples received lower acceptability scores (P< 0.05) than M30 and M40 samples during the refrigerated storage. The limit (score 6) of acceptability was reached approximately on day 7 for the air-packaged samples and on day 20 for M20, M30 samples, whereas the limit of acceptability was not reached for the M40 samples throughout the entire store. However,the TVC of A, M20, M30 and M40 samples exceeded the limit after approximately 6, 10, 10 and 15 days, respectively. The shelf life of all packaging samples is not consistent with the time of reaching the limit score of acceptability. It is not the TVC but the specific spoilage microorganisms (SSO) that cause spoilage to the meat product [3,8].
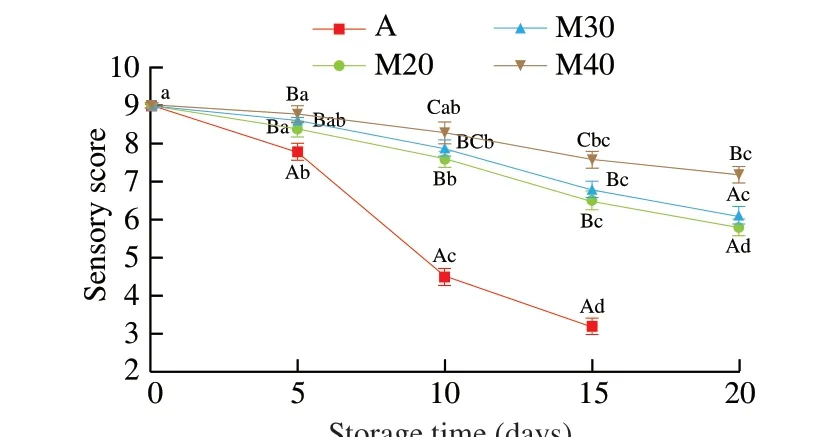
Fig. 4 Changes in sensory scores (mean values of odor and overall acceptance) of braised chicken stored at 4 °C. A–C: values with different uppercase letters in the same sampling day were significantly different(P < 0.05); a–d: values with different lowercase letters in different storage times were significantly different (P < 0.05).
4. Conclusion
In our study, MAP at 40% CO2effectively inhibited the growth of microorganisms and maintained the edible quality of braised chicken. The shelf of braised chicken was extended approximately 5 days for M20 and M30 packaging and more than 10 days for M40 packaging according to the TVC counts. This increase of microbial quality corresponded with an increase of quality indicated by the physiochemical characteristics of the braised chicken and could potentially improve the diet quality for consumers and profitability for producers. In addition, 14 strains of potential spoilage bacteria were isolated which are available for use to evaluate efficacy of food grade additives to reduce spoilage bacteria on braised chicken in vitro allowing researchers to improve the quality of braised chicken,chicken products, other fully cooked meat products, etc.
Declaration of competing interest
The authors declare no conflicts of financial interest.
Acknowledgments
This work was financially supported by China Agriculture Research System (Beijing, China, CARS-41-Z06), Nanjing Professor Huang Food Technology Co., Ltd..
- 食品科学与人类健康(英文)的其它文章
- Wine, beer and Chinese Baijiu in relation to cardiovascular health:the impact of moderate drinking
- Comparative analysis of physicochemical properties, ginsenosides content and α-amylase inhibitory effects in white ginseng and red ginsen
- Effect of cooking processes on tilapia aroma and potential umami perception
- Formation mechanisms of ethyl acetate and organic acids in Kluyveromyces marxianus L1-1 in Chinese acid rice soup
- Volatile prof ile and multivariant analysis of Sanhuang chicken breast in combination with Chinese 5-spice blend and garam masala
- Physicochemical, rheological and antioxidant prof iling of yogurt prepared from non-enzymatically and enzymatically hydrolyzed potato powder under refrigeration

