Effect of cooking processes on tilapia aroma and potential umami perception
Dnni Zhng, Chrfedinne Ayed, In D. Fisk,c,*, Yun Liu,*
a Department of Food Science & Technology, School of Agriculture & Biology, Shanghai Jiao Tong University, Shanghai 200240, China
b Division of Food, Nutrition and Dietetics, School of Biosciences, University of Nottingham, Sutton Bonington Campus, Loughborough LE12 5RD, UK
c University of Adelaide, North Terrace, Adelaide 5005, Australia
Keywords:Tilapia Thermal cooking process Aroma Off-note Umami
A B S T R A C T Tilapia is a freshwater fish group with a sustainable prospect but suffers off-notes appearing during cooking processes. To promote pleasant odorants by thermal cooking processes, tilapia fillets were cooked in different ways (roasting, microwave-heating, boiling and steaming). Their aroma profiles were analysed with special focus on off-notes and umami-enhancing odorants by principal component analysis, and correlated with the heating time, colour, moisture and water activity by partial least squares regression analysis. Results showed that the “green” and “earthy” off-notes were highly correlated with the boiling process (excess of water, short heating time), while most of the umami-enhancing odorants had a high association with the roasting process(low water content, long heating time, better Maillard reaction). This study indicated that roasting is the most adapted cooking process promoting Maillard-derived aromas, umami-enhancing aromas and meanwhile,reducing off-notes. This research helps in understanding the off-note generation in tilapia and promoting desirable umami-enhancing odorants.
1. Introduction
Tilapia is a major freshwater fish group, of which Nile tilapia(Oreochromis niloticus) is the most important commercial species. It is easy to breed with lower protein levels feeds, and can resist to poor water quality and disease [1]. Besides, it also grows very fast but has good nutrition properties and can be introduced into most countries to meet local protein needs [1]. Nowadays, the production of Nile tilapia is ranked in the fourth position in global finfish aquaculture after grass carp, silver carp and common carp [2]. As marine stocks are overfished and climate change impacts become more pronounced,Food and Agricultural Organization of the United Nation (FAO)recommend sustainably farmed species to feed the global appetite for fresh fish, such as tilapia. It was estimated that current global greenhouse gas (GHG) emission from inland fisheries was 43 million tonnes, and if inland fisheries were replaced with other forms of food production, aquaculture replacement would have the smallest net increase (22.3 million tonnes, the average value for tilapia, salmon and trout) in GHG emissions, compared with beef and rice production replacements (0.82 billion tonnes and 9.3 billion tonnes, respectively) [2].According to Fishery Commodities and Trade dataset from FAO,compared with the import production quantity of the tilapia in the United States of America (over 100 000 t), it increased very slowly and remains stable (around 1 000 t) in the United Kingdom from 2014 to 2017. This is partially due to the “muddy” and “earthy” notes or unpleasant tastes from tilapia, which have been emphasized for nearly 20 years [3], and negatively impacts consumer consumption.
The off-notes of tilapia can be due to numbers of volatile compounds, such as geosmin and 2-methylisoborneol from the breeding environment [3]. Apart from breeding factors, fish processing also affects fish aroma dramatically by various chemical reactions, such as non-enzymatic/enzymatic lipid oxidation and Maillard reaction [4]. During different cooking processes, many factors, such as the heating medium, heating time, temperature or water contents, can influence the chemical reactions leading to various aroma categories, aroma quantities and fish notes [5,6]. It can be proposed that proper thermal cooking processes can be improved to design different tilapia aroma profiles.
Food aroma perception (olfaction) starts from the binding of aroma to specific olfactory receptors in the nasal cavity, and then,the signal is transmitted and perceived by brain [6]. The fish flavour perception is formed by multiple sensory inputs, such as fish aroma and characteristic umami taste. The anterior ventral insula in brain has been strongly proposed as the first area to integrate olfactory and taste [7]. Meanwhile, specific odorants have been reported to enhance umami perception. For example, 1-octen-3-ol and 2/3-methybutanal were found to enhance the umami aftertaste and/or palatability [8,9].A savoury vegetable odour combined with monosodium glutamate(MSG) contributed to the delicious flavour of umami [10]. Moreover,mixed odorants, such as the cheese aroma mixture, also interacted with umami and enhance umami perception [11]. Given that tilapia flavour is plain, appropriate thermal cooking processes can boost the tilapia aroma, contributing to a pleasant tilapia aroma and potentially lead to enhancing umami perception.
The study on processed tilapia flavour is quite limited in literatures, and lots of research mainly focused on the sensory properties of products made from tilapia, such as croquette [12] or sausage [13]. Since thermal cooking processes play important roles on the tilapia flavour generation, a broad range of different cooking methods and parameters needs to be explored to explain the chemical reactions happening in the cooked tilapia in order to design good tilapia flavour by the cooking process.
We hypothesised that the thermal cooking process could be adapted to generate various fish aroma profiles, enhance umami perception, and meanwhile, avoid off-notes. In this study, broad thermal cooking processes (roasting, microwave-heating, boiling and steaming cooking processes) were chosen to generate different tilapia aroma profiles. Cooked tilapia odorants were analysed by gas chromatograph-mass spectrometer (GC-MS) combined with solidphase microextraction (SPME). Aroma-active and umami-enhancing odorants were screened to determine the correlation with different thermal parameters and properties, such as the heating time, water contents and colour indexes. Results can guide consumers or food companies on the most adapted cooking process techniques to create a more desirable fish aroma.
2. Material and methods
2.1 Tilapia samples and reagents
The Nile tilapia (Oreochromis niloticus) was farmed in China,specially processed and packed for Ibco Ltd., UK. Frozen tilapia fillets (Ibco brand, UK) were from the same batch, brought from local supermarket, and stored in -80 °C freezer within 2 h.
3-Heptanone (98%, ACROS OrganicsTM) and methanol(Laboratory reagent grade) were purchased from Thermo Fisher Scientific (Hemel Hempstead, UK). C8–C20alkanes dissolved in hexane were brought from Sigma-Aldrich (Analytical Standard,Dorset, UK).
2.2 Tilapia cooking processes
Four cooking processes were selected which consumers commonly use around the world (roasting, microwave-heating, boiling and steaming cooking processes). For traditional thermal cooking processes dominated by heat convection (like roasting, steaming and boiling), the heating medium conveys heat from heat sources to the surface of foods [5]. The microwave-heating process is a heat radiation technique resulting in the conversion of electromagnetic energy to thermal energy due to dipolar and ionic mechanisms,which is more efficient than traditional cooking processes because microwave directly penetrates into food materials [14].
In this study, before cooking processes, tilapia fillets were defrosted in 4 °C fridge for 20 h. and cut off small pieces from the same part to make sure the similar weight and shape of each fillet ((62.0 ± 2.0) g,length (15.2 ± 0.5) cm, width (9.2 ± 0.3) cm). Three fillets were used for each cooking process method in order to have representative results. Roasted tilapia (RT): The fish samples were covered with baking paper and put into a pre-heated hot air convention oven (Hotpoint,Peterborough, UK) at 200 °C for 25 min on each side. Microwave-heated tilapia (MT): The fish samples were put on a glass dish, and cooked in a 600 W household microwave oven (NNA773, Panasonic, Bracknell, UK)for 1.5 min. Boiled tilapia (BT): The fish samples were cooked in boiled water (fish/water, 1/4,m/m) for 2 min without the pot lid. Steamed tilapia (ST): The fish samples were steamed over boiling water (fish/water, 1/8,m/m) for 10 min with the lid.
During 4 cooking process treatments, centre temperature changes of tilapia flesh were monitored with a digital thermometer(Digitron 2022T, Rototherm Group, Port Talbot, UK) inserted in the middle of the flesh. After cooking, samples were cooled at the room temperature, and weighed to calculate remaining weight percentage(the amount after cooking divided by the amount before cooking, %).
2.3 Water and colour properties
Colour was measured on the same surface part of each fillet for 6 replicates (18 replicates per cooking treatment).L*,a* andb*values were determined by Lovibond®LC100 (Tintometer®Group,Amesbury, UK) with CieLAB system, and defined as lightness (L*),red/green (a*) and yellow/blue (b*) values. The colour difference was expressed by ∆Eand calculated by the following equation using the white colour (L* = 100,a* = 0,b* = 0) as the reference.
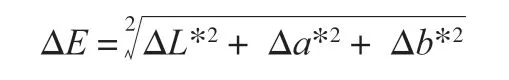
After colour analysis, samples were ground for 1 min with a cooled mortar, and weighed for moisture, water activity and volatile compounds analysis as below.
Moisture content was determined with 3 replicates per fillet (9 replicates per cooking treatment). Three grams cooked fish was dried in 120 °C drying oven for over 24 h. After drying, samples were cooled down in the desiccators and weighed. This cycle was repeated until the difference value of final weights in two continuous cycles was less than 2 mg. The moisture contents were calculated as the percentage of the mass of water (the difference of the final weight and the initial weight) divided by the initial sample weight.
Water activity was measured by AQUA LAB 4TE (METER Group, Pullman, USA) with 2 replicates per fillet (6 replicates per cooking treatment). Two grams cooked fish was weighed in a disposable cup, placed in the water activity meter, and equilibrated at room temperature. The water activity value was directly measured by the dew point sensor of the meter.
2.4 Solid-phase microextraction (SPME) and gas chromatograph-mass spectrometer (GC-MS)
2.4.1 SPME-GC-MS parameters
Tilapia is considered as an odourless and mild flavour freshwater fish. So, it is necessary to optimize the extraction parameters in order to have a higher sensitivity and accuracy. A good SPME method needs to consider fibre coating, sample volume, extraction time, etc..The previous study for the turbot [15] has determined that the optimum SPME fibre was 50/30 μm DVB/CAR/PDMS fibre (Supelco,Bellefonte, PA, US), and the best extraction temperature and the sample amount were 50 °C and 3 g, respectively. Based on previous research, we optimized the matrix modification (with 6 mL water or not) and extraction time (20, 40, 60, 80 and 120 min) without water matrix. When optimizing the matrix modification parameter, 60 min was used as the extraction time. Experiments were carried out with four replications, and samples were analysed randomly.
The grinded roasted fish (3 g) from section 2.2 and 2.3 was weighed into 20 mL SPME vials. 10 μL 0.001% (V/V) 3-heptanone(internal standard) was added into each SPME vial prior to SPME parameters development. Samples were incubated for 30 min at 50 °C.A 2 cm 50/30 μm DVB/CAR/PDMS fibre (Supelco, Sigma-Aldrich,Dorset, UK) was used to extract volatile compounds from the headspace of fish samples for specific time at 50 °C. After extraction,SPME fibre was injected into the GC-MS. GC-MS analysis was performed by Trace 1300 Gas Chromatograph combined with a TSQ series Mass Spectrometer (Thermo Scientific, Hemel Hempstead,UK). Volatile compounds absorbed on SPME fibre were desorbed at 250 °C in the GC-MS injector with a spitless mode for 0.1 min. They were further separated by a ZB-Wax column (30 m length, 0.25 mm diameter, 0.25 mm thickness, Phenomenex, Torrance, UK) with a helium carrier flow of 1 mL/min. The oven temperature was modified according to the previous report [16]. The oven temperature started from 30 °C with a holding time of 3 min, and then increased to 250 °C at a rate of 5 °C/min and held for 3 min. The MS transfer line and ion source temperature were 250 °C and 200 °C respectively. The ionization mode was EI, and MS full-scan range was 35–300m/zat 5 scans/s.
2.4.2 SPME-GC-MS parameters optimization
As shown in Fig. S1a and Table S1, except hexanal, the concentration of most aroma-active compounds decreased significantly when 6.0 g water was added into the system to cover samples in the vials. Fish aroma is trace level, and in this case,they were easy to be trapped in this amount of water matrix instead of evaporating to the headspace above the sample. Adding water into the systems changed odorant amount and might accelerate oxidation resulting in the generation of hexanal. So, water was not added into the samples for SPME extraction time optimization and later experiments.
Extraction time has an effect on the extraction equilibrium state of samples, and it has become the time-limiting step for the SPME procedure. Different volatile compounds in samples need different time to reach the equilibrium. According to Fig. S1b and Table S1, among 13 aroma-active compounds, the concentration of trimethylamine (TMA), methanethiol and acetoin decreased significantly as extraction time increased, while the significantly opposite trend showed for 2,6-dimethylpyrazine, 1-octen-3-ol,2-ethyl-3,5-dimethylpyrazine and benzaldehyde. But there was no significant increase between 40, 60 and 80 °C (P< 0.05) according to the sum concentration of all 13 aroma-active compounds. Take these facts into account, 40 °C was chosen as the best extraction time.
Moreover, the oven temperature in most previous research started at least at 40 °C, leading to occasionally detection of TMA and the failure detection of methanethiol which are typical volatile compounds in the seafood and meat products [17-20]. Concerning the initial temperature of GC-MS oven, it needs to be noted that the boiling points of TMA and methanethiol are both below 10 °C,therefore, a low initial temperature in the GC oven will improve the detection and identification. Hence, in our study, the starting oven temperature was setup at 30 °C, allowing a good detection of the selected ions. This was also proved by previous research on salteddried white herring [21].
2.4.3 Aroma analysis of four cooked tilapias by SPME-GC-MS
Three fillets were cooked for each cooking process as section 2.2. After analysing water and colour properties as section 2.3, the grinded fish (3 g) from each fillet was weighed into a 20 mL SPME vial. The vial was cooled down by liquid nitrogen immediately and stored in –80 °C freezer until use. Before SPME, 2 vials per fillet were randomly selected (6 replicates per cooking treatment). In addition, 3-heptanone was dissolved in methanol to prepare 0.001%(V/V) internal standard solution. Then, 10 μL internal standard was added into each SPME sample vial. The vial was defrosted at room temperature for 1 h before SPME-GC-MS.
The optimized SPME parameters developed in section 2.4.1 and
2.4.2 were used for cooked tilapia aroma analysis. Besides, for a slightly better peak separation, the oven temperature was set to start from 30 °C without a holding time. Other parameters were same with those in section 2.4.1 and 2.4.2. All cooked samples were analysed in random order.
2.5 Volatile compounds identification and quantification
The identification of volatile compounds relied on the comparison of MS spectra with reference database (NIST/EPA/NIH Mass Spectral library Version 2.2) and Kovats retention index [22] with C8–C20alkanes in hexane under the same experimental condition as samples.The relative quantification of volatile compounds was calculated by the internal standard (3-heptanone), comparing the ratio of the peak area (selected ions) to the concentration [16].
2.6 Statistical analysis
Data in all tables was presented as mean ± standard deviation.Independent-samplest-test and analysis of variance (ANOVA) were conducted using IBM SPSS Statistics software (Version 20) with a post-hoc Tukey test atα= 0.05. Principal component analysis (PCA)and partial least squares regression (PLS-R) analysis were determined by XLSTAT (Annual Version 2020.1.3).
3. Results and discussion
3.1 Water and colour properties of cooked tilapias
Different cooking processes lead to different temperature and moisture distributions inside the food, resulting in food properties changes, such as colour, aroma and structure [23]. The remaining weight percentage, moisture, water activity and colour changes of cooked tilapias were shown in Table 1.
The boiled tilapia (BT) gained weight (remaining weight percentage > 100%) during boiling process significantly (P< 0.05),while the roasted tilapia (RT), microwave-heated tilapia (MT) and steamed tilapia (ST) lost weight (remaining weight percentage <100%)after cooking. RT had the lowest remaining weight, moisture and water activity significantly (P< 0.05) compared with other three cooked samples. But for moisture and water activity, there was no significant difference between BT and ST. Compared MT with BT and ST, the remaining weight percentage and moisture were less than BT and ST significantly (P< 0.05), while the water activity was higher but without significant difference among MT, BT and ST. Regarding RT, roasting process lasted the longest heating time by heat conduction with hot air movement, resulting in continuous water loss from fish flesh. The supplementary analysis measuring the centre temperature inside the tilapia during cooking (Fig. 1) could give the explanation to the phenomenon. Although the heat transfer efficiency of roasting was lowest [5], the cooking process of RT led to the longest heating time and kept the longest time (about 35 min)at above 90 °C, which could lead to the lowest values for remaining weight percentage, moisture and water activity. Compared BT with ST, the water surrounding BT was much more abundant than ST,leading to the highest remaining weight percentage of BT. But the heat transfer efficiency and heating time of BT was less than ST [5](Fig, 1), so there was no significant difference for moisture and water activity. Compared MT with BT and ST, the best heating efficiency of microwave (Fig. 1) can explain the less remaining weight percentage and moisture of MT significantly (P< 0.05). But for water activity, it can be hypothesised that during the microwave-heating process, more bound-form water absorbed energy from microwave and disassociated from the matrix to give free-form water. However, the heating time was not long enough for free water evaporation during microwave-heating,therefore, that may be the reason why water activity was the highest for MT. So, heating time couldn’t be ignored during cooking.
Colour analysis results (∆E,L*, a* andb* values) varied among different cooked tilapias. RT had highest ∆E,a*,b* values and lowestL* value significantly (P< 0.05) in contrast with other three cooked samples. Among MT, BT and ST, the ∆EandL* values were not significantly different between MT and BT, but less than those of ST significantly (P< 0.05). As for the colour of RT, the highest ∆Eindicated biggest total colour difference for RT compared with the brightest white colour.L*,a* andb* values proved that the colour of the RT was darker, redder and yellower, having the trend to the browner colour. The brown colour is an important characteristic of the Maillard reaction [23], which meant the most intense Maillard reaction occurred in RT, and can be proved by supplementary analysis shown in Fig. 1. If we assume Maillard reaction started from 70 °C, the heating duration time when Maillard reaction happened for cooking RT, MT, BT and ST were 40, 1, 0.2 and 8 min, respectively.Compared MT with BT, no significant difference (P< 0.05) for the∆EandL* values can be due to the different transport direction of heat energy [24]. During the microwave heating, a large amount of water can flow from the flesh centre to the surface resulting in the surface of MT soggy [24], while the water loss during the conventional hot air heating was from the surface to the flesh centre.Compared with BT and ST, the higher ∆EandL* values of BT can be due to longer heating time. As shown in Fig. 1, ST kept longer time (over 7 min) at above 85 °C, while boiling process ended when temperature reached about 85 °C for BT.
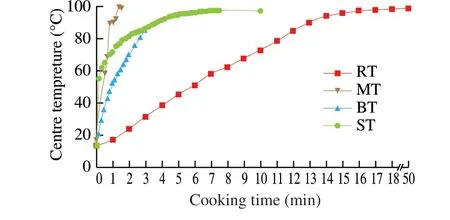
Fig. 1 Centre temperature curves of tilapia flesh during different cooking processes.
3.2 Impact of cooking processes on tilapia aroma generation
Cooking processes alter the tilapia aroma intensely by diverse chemical reactions. A total of 62 volatile compounds were detected in four cooked tilapias by SPME-GC-MS using optimized parameters.Table S2shows that62, 43, 48 and 45 odorants were identified in RT,MT, BT and ST, respectively. A summary of 23 important volatile compounds were listed in Table 2including 1 amine, 4 sulphurcontaining compounds, 1 furan, 5 ketones, 5 aldehydes, 1 terpene,3 alcohols and 3 pyrazines. Seventeen aroma-active compounds were selected from related studies on the freshwater fish aroma [17-20], whichwas similar with tilapia aroma from a biological point of view, and 11 volatiles were potential umami-enhancing odorants (UEOs) [8-11]based on the reported odorants or related descriptions.
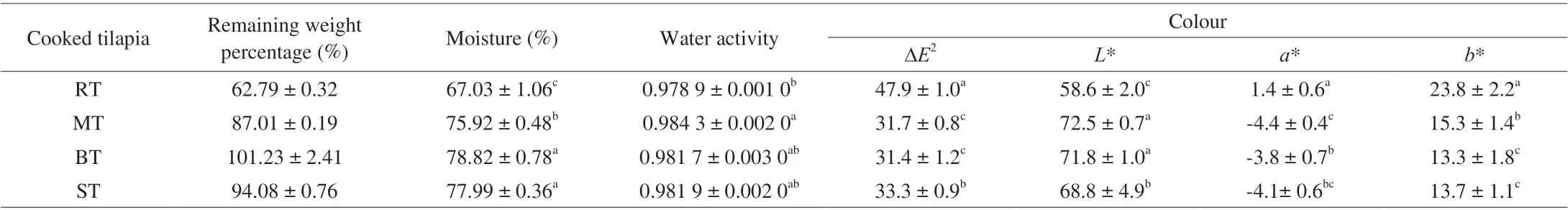
Table 1 Water and colour properties of cooked tilapias1.
3.2.1 Amine
Trimethylamine (TMA) was the most abundant volatile compound in cooked tilapias. Its concentration in RT was at least 4 times higher than that in other three cooked tilapias significantly (P< 0.05). TMA has been reported in most freshwater or marine fish, contributing to a “fishy” aroma note with a low detection threshold of 8 μg/kg in water [25]. It can be generated from trimethylamine oxide (TMAO),which is derived from soluble substances in the muscle (sarcoplasmic proteins and nucleotides) during alterations of fish post-mortem [26].During the fish storage, TMA can be formed from TMAO by microbial growth and metabolism, and has been one of important indexes for judging fish freshness and spoilage [27]. The amount of TMA also increased as the heating time increased, which matched previous report [28]. It is undesirable to have a high level of TMA for consumers. Nevertheless, it should be noted that TMA contributed to the aroma profile recombination for prawn meat [29], indicating that the contribution of TMA for the seafood aroma can’t be ignored.
3.2.2 Sulphur-containing compounds
Sulphur-containing compounds is the next important group for fish aroma generated from thermal cooking processes. Methanethiol is a common volatile compound appearing in food. It is described as sulphurous and cabbage-like smell with a low detection threshold of 0.02 μg/kg [25]. Its concentration did not have a significant difference among 4 cooked tilapias. Amino acid methionine is the main precursor of methanethiol. Methionine generates methional by Strecker degradation firstly, and converts to methanethiol during thermal cooking processes, and can finally lead to the generation of dimethyl sulphide [4]. It has been reported that the formation of methanethiol in cooked herring initially increased to a maximum, and then declined as thermal time increased [30]. It was possible that the methanethiol in RT was on the decline period, while methanethiol in MT, BT and ST were in the beginning formation period, leading tothe similar amount in four cooked tilapias. So, the heating time was one of the main factors to generate methanethiol.
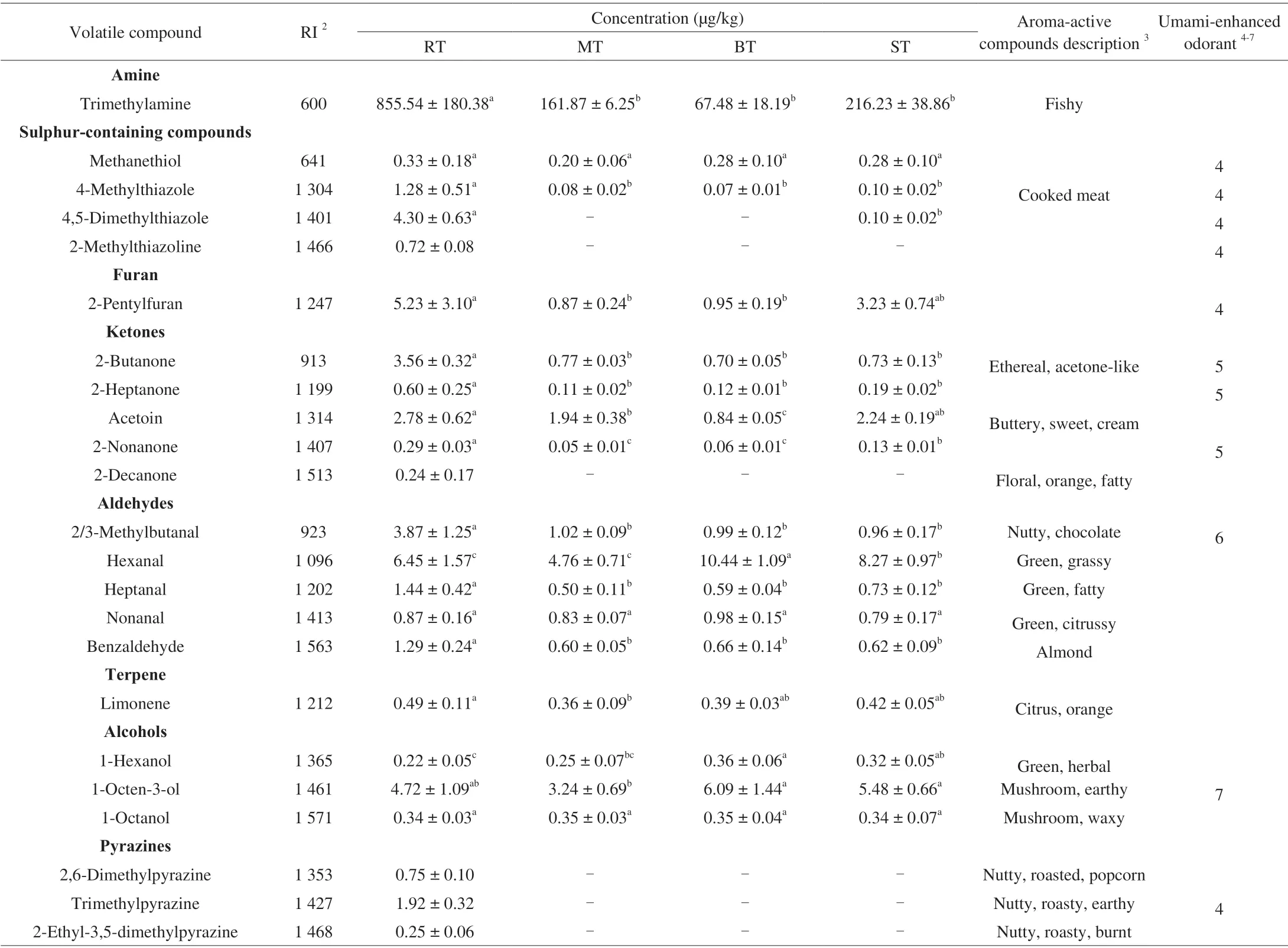
Table 2 Important volatile compounds of cooked tilapias1.
All concentrations of thiazoles, tiazolines and thiophenecarboxaldehyde were the most abundant in RT (Table S2),of which only 4-methylthiazole was reported as aroma-active compound in freshwater fish, contributing to a cooked-meat smell [20].But 4-methylthiazole, 4,5-dimethylthiazole and 2-methylthiazoline were reported as “vegetable”, “savoury” or “sulphurous” notes, having the potential to enhance umami perception (Table 2) [10]. Thiazoles are the major heterocyclic sulphur-containing compounds formed via the final stage of Maillard reaction [6]. But their intermediate reaction compounds, such as carbonyl containing compounds, are formed from Strecker degradation or lipid oxidation. In this study,the concentrations of 4-methylthiazole and 4,5-dimethylthiazole were dominate in RT significantly (P< 0.05), at least over 10 times bigger than those of other three cooked tilapias, while 2-methylthiazoline was only found in RT. The reason might come from the longest time or higher heating temperature [31] during the roasting process compared with microwave-heating, boiling and steaming processes.
3.2.3 Furans
Only 4 furans, including 2-methylfuran, 2-ethylfuran,2-pentylfuran and 2-furanmethanol were detected in the study, and their concentrations were the highest in RT (Table S2). Although 2-ethylfuran was sniffed by gas chromatography-olfactometry (GC-O)in grilled eel [16], furans do not contribute a lot to overall aroma in most fish species according to most research on fish, which is due to the concentration below the detection threshold. 2-Alkylfurans can be formed by lipid oxidation [6], and their formation from the correspondingα,β-unsaturated aldehydes can be catalysed by amino acid [32]. So, during roasting tilapia, there were more amino acids generated from protein degradation over time, which can lead to more 2-alkylfurans in RT than other three cooked tilapias.
3.2.4 Ketones
Ten ketones were detected in four cooked tilapias, including 6 methyl ketones, 2 hydroxy ketones, 1 diketone and 1 lactone (Table S2), and three of them have been reported as aroma-active compounds in freshwater fish (Table 2) [17,20]. These 3 aroma-active compounds(2-butanone, acetoin and 2-decanone) were most abundant in RT significantly (P< 0.05). Methyl ketones in Table 2were most abundant (5 times higher) in RT significantly (P< 0.05), while acetoin in RT only had significant difference compared with that in MT and BT (P< 0.05). Methyl ketones were generated from long-chain(n-3) fatty acid which generatedβ-ketoacids byβ-oxidation firstly,and decarboxylation to produce the corresponding methyl ketones under thermal cooking process [33]. Their formation pathway was dominated by longer roasting time in this study. Acetoin is a kind of hydroxyketone, and is considered to be derived by the decomposition of aliphatic carbonyl compounds from the final stage of Maillard reaction [4]. So, there was abundant acetoin in RT because the longer Maillard reaction happened.
3.2.5 Aldehydes
The low detection thresholds of 5 aroma-active aldehydes contributed a lot to freshwater fish aroma, such as hexanal with a detection threshold of 5 μg/kg [25]. Among the 5 aroma-active aldehydes, the concentration of hexanal showed the opposite trend compared with others (Table 2). Hexanal, heptanal and nonanal were lipid oxidation derivatives with typical “green” smell [17].2/3-Methylbutanal were generated from Strecker degradation giving “nutty” or “chocolate” notes [18], while benzaldehyde was reported to be generated from Strecker degradation or lipid oxidation,contributing to a pleasant “almond”, “nutty” and “fruity” aroma [4].Hexanal was considered as an indicator for meat deterioration, whose concentration increased more quickly than other aldehydes at the early stage of storage and then declined over time [34], which might be explained by the irreversible chemical reaction with protein [35]or the reversible binding with protein [36]. Given the heating time,although RT lasted the longest time followed by ST, the concentration of hexanal was less than that in BT, which indicated the hexanal generation had reached the maximum and were in decline period in the system of RT and ST. BT and MT maintained the similar heating time, but the concentration of hexanal had significant difference,revealing that the generation pathway of hexanal was quite sensitive to the water environment. Considering that hexanal contributes to a typical “green” note, it is suggested to control the water content during tilapia cooking processes.
3.2.6 Alcohols
Alcohols are generated from lipid oxidation whose thresholds are normally high and do not make great contributions to the aroma [6].For example, the detection threshold of 1-hexanol was reported over 100 μg/kg [25]. But there were 3 aroma-active alcohols reported in freshwater fish [19]. As shown in Table 2, 1-hexanol and 1-octen-3-ol were the most abundant in BT followed by ST, which showed the same trend as hexanal. 1-Octen-3-ol is an unsaturated alcohol with a low detection threshold of 1.5 μg/kg [25] contributing to mushroom aroma [17], and it was one of the main volatiles detected in fresh fish together with hexanal [4]. Although different fatty acid has been reported as the precursor of hexanal, linoleic acid was reported as the same precursor for hexanal and 1-octen-3-ol [4]. As was shown in this study, their generation pathway might be dominated by water contents. Therefore, controlling water contents should be an important criterion during fish cooking processes.
3.2.7 Pyrazines
Pyrazines are a typical chemical group compounds detected in roasted meat, contributing to the majority of “nutty” or “roasted”notes. They are one of the major nitrogen-containing volatile groups generated from the final stage of Maillard reaction. There were 11 pyrazines found in RT, of which 10 pyrazines can’t be identified in MT, BT and ST (Table S2). Only 3 of them have been reported as aroma-active compounds in freshwater fish (Table 2) [17,19]. It may be because the cooking process conditions in previous research were not as intense as what we used in this study. There are different unclear ways to form pyrazines. For example, the carbonyl-amine condensation occurred between two amino ketones from Strecker degradation and further oxidised to pyrazine [37]. However,more highly substituted pyrazines with lower thresholds are more possible to be generated from alternative pathways, of which one of substituents are often Strecker aldehydes [37]. As pyrazines are relatively stable compounds, their formation increased as heating time increased [6]. Therefore, the roasting process kept the longest time,leading to more pyrazines generated in RT. Pyrazines and thiazoles somewhat contribute to similar sensory properties [6], hence, it is necessary to guarantee the heating time during the cooking process for their generation.
3.3 Aroma profiles of cooked tilapias
To visualize the cooking process effect on the aroma-active compound and aroma profiles, PCA was employed to classify the difference among cooked tilapias according to their concentrations(Fig. 2a) and the sum of concentrations based on their aroma similarities (Fig. 2b). Only 15 aroma-active compounds were chosen for the PCA due to their concentrations with significant difference(P< 0.05). The two principal components (PCs) represented over 90% of the dataset variance, illustrating the clear separation for different cooked tilapias according to the aroma-active compounds.From the PC1 of Fig. 2a, only RT located on the positive side of the axis, while others located on the negative side of the axis.Meanwhile, BT, ST and MT also separated clearly from PC2, of which BT was on the positive side of the axis, ST was near the axis,and MT was on the negative side of the axis. 1-Hexanol, hexanal,1-octen-3-ol and nonanal had a high correlation with BT, which indeed, their concentrations showed significant differences in Table 2(P< 0.05). Except 3 aroma-active compounds highly correlated with BT, other 12 odorants highly correlated with RT due to the hot air heat convection with longer time. There was no volatile compound around MT, indicating the aroma of MT was quite plain.
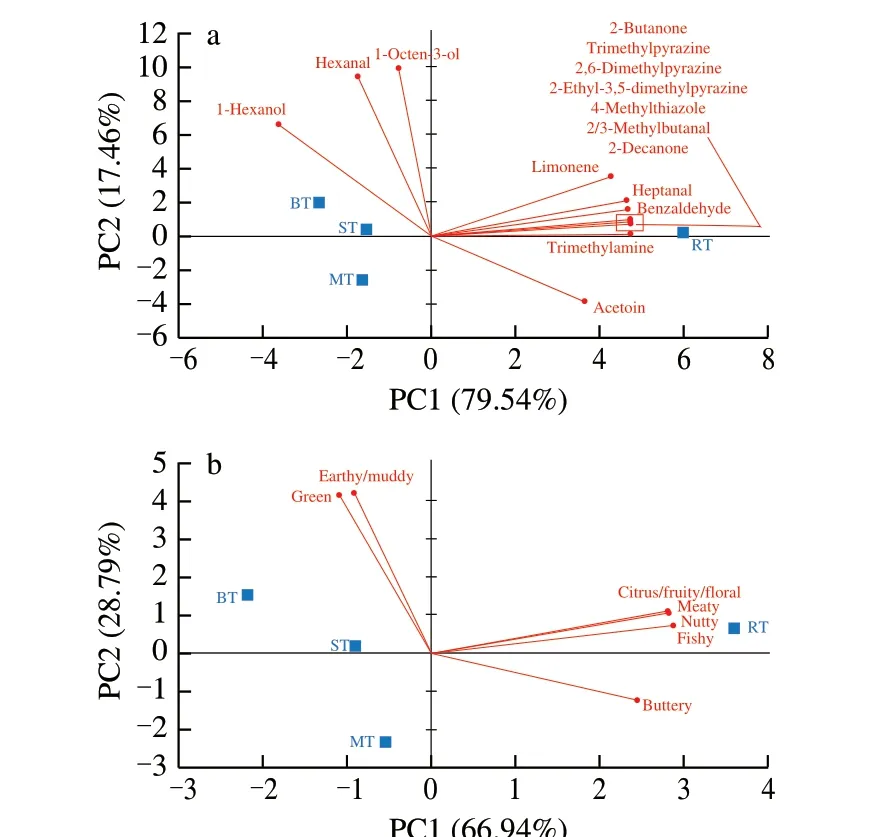
Fig. 2 Principal component analysis of (a) aroma-active compounds in four cooked tilapias; (b) sum of aroma-active compounds based on aroma similarities.
Based on the sum concentration of odorants with similar aroma category, another PCA was performed and showed in Fig. 2b. Earthy,green, citrus, meaty, nutty, fishy and buttery characters were main contributors for cooked tilapias, and the relative aroma strength of these 7 characters varied among them. RT relatively contained higher aroma intensities in the citrus, meaty, nutty and fishy categories,while aroma intensities of earthy and green categories were higher correlated with BT. Meanwhile, all odorants looked less concentrated in MT compared with other cooked tilapias. Yeo et al. [38] showed that microwave-heated food contained less nutty odorants, leading to the low flavour unacceptability. The authors demonstrated that the food temperature, the length of time and water content were all important for the development of flavour compounds in microwave systems. Recently, microwave combination heating has been developed not only to improve heat efficiency but also to achieve desired temperature or moisture profile to improve the quality of cooked food [24]. It is worth mentioning that earthy and green odours are normally not accepted by the consumer, and are associated with bad quality of fish. This work revealed that the water cooking condition with short heating time were both highly related to inducing“earthy” and “green” notes.
3.4 Umami-enhancing odorants in cooked tilapias
To determine the potential umami enhancing properties of generated odorants in cooked tilapias, 10 volatile compounds, whose concentration showed significant difference, were chosen according to the literatures [8-11] and analysed by PCA (Fig. 3). The sum of PC1 and PC2 accounted for over 95% of the variance for each PCA.As shown in Fig. 3a based on UEOs in four cooked tilapias, RT is projected on the positive side of axis PC1, clearly separated from ST, BT and MT. Among all UEOs, 1-octen-3-ol, 2-pentylfuran,2-nonanone, 2-heptanone and 2-butanone were generated from lipid oxidation [6,33], while trimethylpyrazine, 4-methylthiazole,4,5-dimethylthiazole, 2-methylthiazoline, 2/3-methylbutanal were from Maillard reaction or Strecker degradation [6,18,37]. Except 1-octen-3-ol, other UEOs were highly correlated with RT resulted from longer Maillard reaction. Moreover, according to its PC1 of Fig. 3a, ST located between MT/BT and RT, and could be the next cooking process method to generate more UEOs. This was further illustrated by Fig. 3b comparing UEOs among ST, BT and MT. These three cooked tilapias were totally separated according to 7 UEOs, of which ST highly correlated with 4 UEOs. It indicated the possibility to have the similar number of UEOs by modifying different cooking process parameters such as water, heating time, etc.
Indeed, Maillard reaction has been reported to modify food flavour. Hexanal, the compound contributing to a “green” note in Fig. 2b, were reported to form pyrazines (a “roasted” note) in model systems of acetol and ammonium acetate at 100 °C for 4 h [35]. In our study, compared RT with other three cooked tilapias, it might be possible that some aldehydes in the roasting system, like hexanal, can be involved in the reaction to generate roasted fish aroma or UEOs under the longer heating time, leading to a better Maillard reaction,and meanwhile, the “green” note can be reduced.
Furthermore, except UEOs in this study, other odours such as fishy, broth-like, meaty or garlic-like odours, might also relate with umami perception because of flavour learning through experience over time [10]. The research on cheese odorants and umami interactions proved that cheese odorants enhanced the umami intensity and cheese flavour intensity [11]. This may be because umami is an important taste characteristic in cheese flavour [39], which trained consumers associate the umami perception with cheese aroma, indicating the possibility to enhance umami intensity only by improving the aroma property. More research on the interaction between umami and odours need to be carried out in the future. In this part, we highlighted the correlation between UEOs and umami perception. Sensory analysis is being performed to confirm the potentiality.
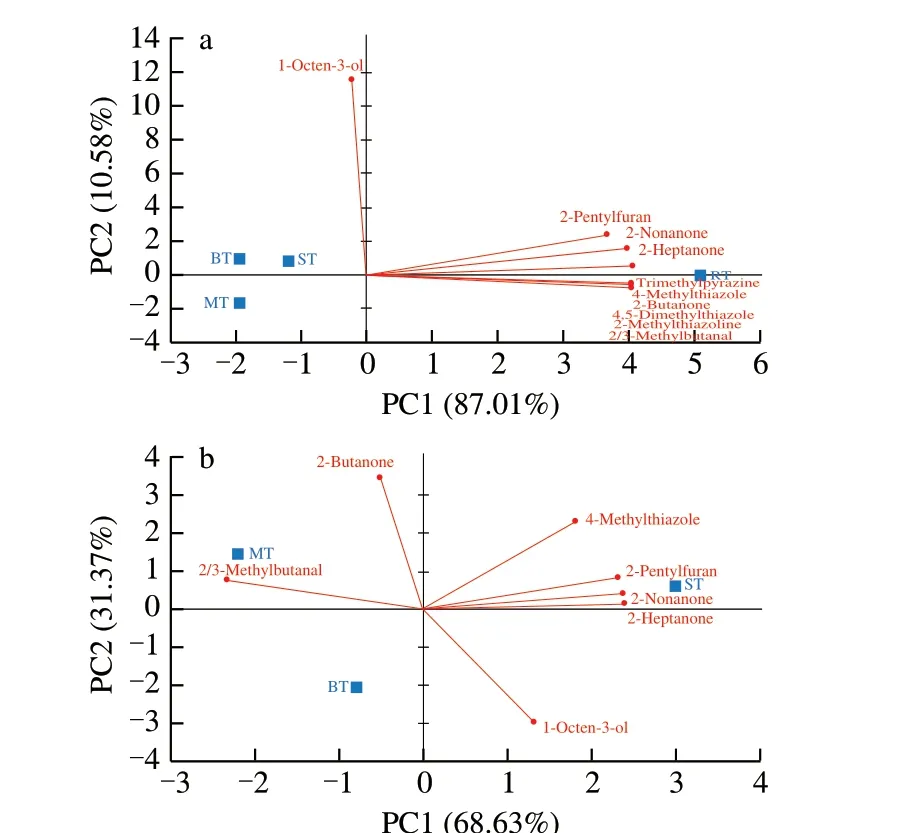
Fig. 3 Principal component analysis based on the umami-enhancing odorants(a) in four cooked tilapias; (b) in three cooked tilapias except roasted tilapia.
3.5 Correlation between the heating time, physical-chemical properties and important odorants of cooked tilapias
To investigate the link between the heating time, basic physicalchemical properties with aroma-active compounds/UEOs, PLS-R analysis was applied to establish the correlation in cooked tilapias(Fig.4). Heating time (1.5, 2.0, 10.0 and 50.0 min), moisture, water activity and colour (L*,a*,b* and ∆E) values (Table 1) were set asXquantitative explanatory variables. The concentration of 23 important odorants (Table 2) were set asYdependent variables. Different observation labels represented RT, MT, BT and ST. Four components were set when performing PLS-R, but only first two representative components was shown in Fig. 4.
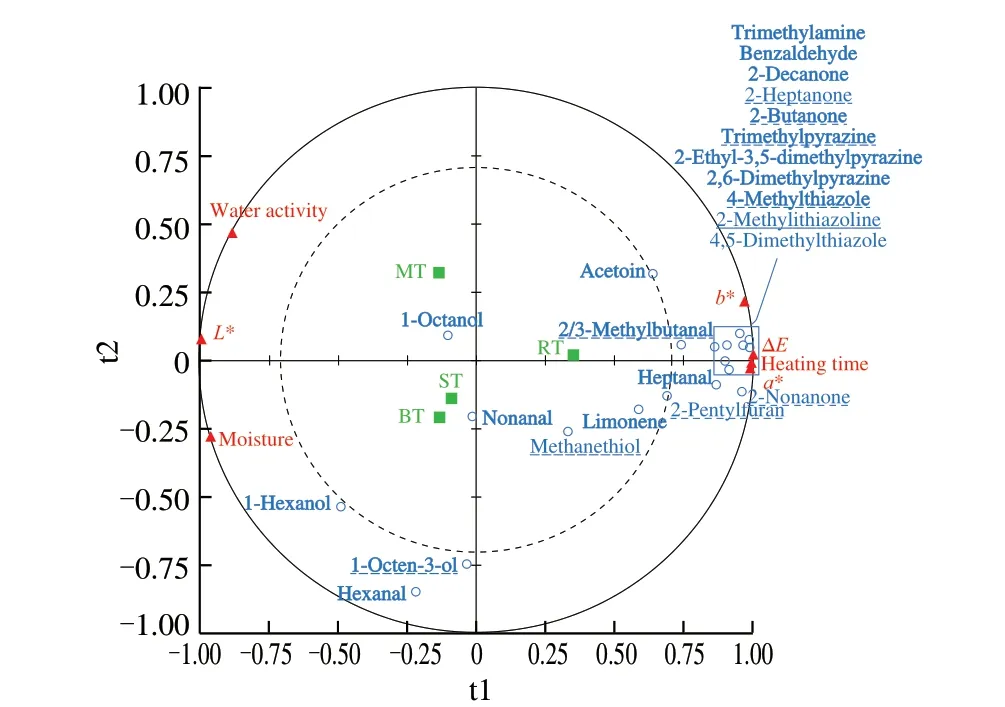
Fig. 4 Partial least squares regression (PLS-R) analysis. Bold mark for aroma-active compounds; Underline mark for umami-enhancing odorants. The first two components: Q2 cum = 0.625, R2Y cum = 0.685, R2X cum = 0.994.
The first two components ofQ2cumulated index were 0.625,which meant the overall goodness of fit and the good predictive quality. The model in Fig. 4 showed the first two components of the cumulatedR2YandR2Xcum were 0.685 and 0.994, respectively,which meant 68.5% of Y variables and 99.4% ofXvariables were explained. RT, MT, BT and ST were projected into three different quadrants, while BT and ST were in the same quadrants. The outer and inner circles represent 100% and 50% of explained variance for different variables. As shown in Fig. 4, except limonene,methanethiol, nonanal and 1-octanol, other variables were between two circles, indicating the good correlation between physical-chemical properties and aroma-active compounds/UEOs. Compared with the other three cooking processes, RT was correlated with the heating time, ∆Evalue,a* value,b* value and most of odorants with high concentration in the headspace. Therefore, this indicated that there was more Maillard reaction resulting in brown colour and intense aroma in RT. Besides, BT and ST seemed correlated with 1-hexanol,hexanal, 1-octen-3-ol and moisture. Meanwhile, the factors projected on the negative part of axis t1 (moisture,L* value and water activity)were negatively correlated with the factors highly correlated with RT (most of the odorants, heating time, ∆Evalue,a* value andb*value), which confirmed the impact of an efficient Maillard reaction on the physical-chemical properties of cooked tilapias. Moreover,the moisture had significantly positive association with 1-hexanol,hexanal and 1-octen-3-ol from lipid oxidation.
For wet fish sample with high moisture, lipid oxidation can increase as water activity increased, while the opposite trend shows for Maillard reaction [23], leading to a whiter or brown colour for different cooked tilapias. Meanwhile, too many water trapped aroma(Fig. S1), and similar result was also observed when compared the aroma of boiled sea bass (2 L water, 100 °C, 10 min) with steamed sea bass (100 °C, 10 min) [40]. Heating time also plays an important role in the cooking process. This was confirmed by previous research showing that similar aroma, taste, flavour and texture profiles were determined by the sensory analysis if similar heating time was kept between steaming (100 °C, 20 min) and roasting (180 °C, 15 min)processes within one fish species [41].
From the view of the “green” note caused by hexanal and pleasant perception caused by UEOs, the water content and heating time need to be combined together to discuss. On the one hand, compared the concentration of hexanal in BT with MT, more water might increase the generation of hexanal from lipid oxidation which was also observed in the matrix modification result of Table S1. Meanwhile, as water increased, the generation of pyrazines (roasted aroma or umami flavour) decreased [6], which was due to a less intense Maillard reaction [41]. On the other hand, the concentration of hexanal might decrease as heating time increased compared BT (2 min) with RT(50 min). This phenomenon may have two possible explanation,including reversible binding with protein or the irreversible chemical reaction. The reversible binding between hexanal with protein has been determined in fish system [36]. Xu et al. [36]reported that the protein adsorption capacity of hexanal increased in the first 5 min of heating time and then declined significantly(P< 0.05). The irreversible chemical reaction between hexanal and protein or intermediate reaction compounds has been reported [35],but still remains unconfirmed within the complex fish system. So,to some extent, less water and longer heating time can lead to the decrease of “green” note and the increase of “roasted” notes linked with UEOs.
Overall, the cooking method is the first step to be considered to optimise the aroma generation. Based on the observation presented here, it is better to avoid a cooking environment with too much water (such as boiling) during the tilapia cooking process to reduce the concentration of unpleasant “green” and “earthy” note in the headspace. Meanwhile, a sufficient heating time for an efficient Maillard reaction is also needed to boost tilapia’s UEOs and potentially enhance umami perception.
4. Conclusion
Roasting, microwave-heating, boiling and steaming processes have different significant effects on tilapia aroma due to various chemical reactions. In this study, the roasting process was the best process to generate brown colour and pleasant aroma due to the longer Maillard reaction, followed by steaming. The boiling process generated more lipid-derived aldehydes and unsaturated alcohols,such as hexanal and 1-octen-3-ol, contributing to “green” and “earthy”notes, which were significantly correlated with the high moisture content. Moreover, based on literature, the roasting process in this study promoted the generation of umami-enhancing odorants. These odorants were highly related to the heating duration time of Maillard reaction. So, the cooking method with low water content and longer heating time, is a good way to drive the generation of pleasant tilapia aroma, and meanwhile, reduce the “green” note, which can be due to the odorant protein interaction. Controlling the cooking process can regulate the chemical reactions leading to the generation of different volatile molecules and aroma profiles. However, it is still necessary to confirm the aroma-active compounds contained in tilapia fish by GC-O/MS and to explore more in detail the impact of cooking time/temperature and water content on the chemical reactions leading to fewer off-notes and more umami correlated ones. It also would be interesting to explore other strategies such as pre-soaking methods as adding compounds in the matrix such as salt or sugar, will potentially enhance the generation of desirable aroma while reducing the unpleasant off-notes.
Declaration of competing interest
The authors declare no conflicts of financial interest.
Acknowledgements
This study was supported in part by the China Scholarship Council Fund.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.07.016.
- 食品科学与人类健康(英文)的其它文章
- Wine, beer and Chinese Baijiu in relation to cardiovascular health:the impact of moderate drinking
- Comparative analysis of physicochemical properties, ginsenosides content and α-amylase inhibitory effects in white ginseng and red ginsen
- Monitoring and identif ication of spoilage-related microorganisms in braised chicken with modif ied atmosphere packaging during refrigerated storage
- Formation mechanisms of ethyl acetate and organic acids in Kluyveromyces marxianus L1-1 in Chinese acid rice soup
- Volatile prof ile and multivariant analysis of Sanhuang chicken breast in combination with Chinese 5-spice blend and garam masala
- Physicochemical, rheological and antioxidant prof iling of yogurt prepared from non-enzymatically and enzymatically hydrolyzed potato powder under refrigeration

